Retroviral transduction of tumor antigen-specific T-cell receptor (TCR) genes into lymphocytes redirects T cells to lyse tumors. Furthermore, adoptive transfer of these lymphocytes has mediated objective responses in patients with metastatic cancer. From 2004 to 2006, more than 40 patients were treated with autologous gene-modified lymphocytes expressing a melanoma antigen-specific TCR at the National Cancer Institute. Eighteen such patients were analyzed for persistence and gene expression in vivo. In addition, the impact of epigenetic silencing and of lymphocyte restimulation was studied. Although gene-modified lymphocytes persisted in vivo, the shutdown of TCR transgene expression was observed. Bisulfite sequencing analysis and ex vivo DNA methyltransferase inhibition demonstrated that the decrease in gene expression did not result from DNA methylation. Surprisingly, down-regulation of vector-driven transgene transcriptional activity was not vector specific but mimicked that of endogenous genes. The decrease in TCR transgene expression, however, was reversed upon lymphocyte stimulation. These data demonstrate a lack of γ-retroviral promoter-specific gene silencing in adoptively transferred human lymphocytes and support that transgene expression is largely affected by global cellular mechanisms. The use of immunomodulatory adjuvants, eg, vaccination or cytokine therapy, for in vivo T-cell activation may help overcome this metabolic quiescence and thus augment cellular immunotherapy-based cancer therapy.
Introduction
Strategies that use cell-based immunotherapy have successfully treated patients with advanced malignancies,1 and perhaps the most effective has been autologous tumor-infiltrating lymphocytes (TILs). When large numbers of melanoma-reactive TILs were generated ex vivo from metastatic tumor deposits and administered with lymphodepletion and high-dose interleukin-2 (IL-2), roughly half of those treated experienced objective tumor regression.2,3 This potent therapy, however, has been limited by the requisite surgery to procure tumor-reactive TIL and by ex vivo identification and expansion of these cells. Recent advances in human gene therapy may obviate these limitations.
Current human gene therapy techniques enable the efficient transfer of T-cell receptor (TCR) genes into peripheral-blood lymphocytes (PBLs). Retrovirus-mediated introduction of tumor antigen-specific TCRs into PBLs has resulted in gene-modified lymphocytes capable of activation, cytokine secretion, and targeted lysis.4,–6 With the use of autologous PBLs modified to express a TCR specific for melanoma antigen recognized by T cell-1 (MART-1), we treated patients with progressive, metastatic melanoma.7 After a lymphodepleting regimen, patients received large numbers of cells in combination with high-dose IL-2 (similar to TIL protocols). Durable, objective tumor regression was observed in 2 of the initial 17 patients treated,7 with another 2 responders in the final 14 patients; the overall response rate was 13%. (S.A.R., unpublished observation, January 2009).
Preliminary characterization of circulating cells in patients on this trial indicated a loss of the MART-1–specific TCR transgene expression, measured by MART-1 tetramer or appropriate Vβ staining, soon after adoptive transfer.7 These findings were striking because the percentage of CD8+ cells binding the MART-1 tetramer was less than 1% (and often < 0.1%) as early as 4 weeks after adoptive transfer. In contrast, the persistence of transferred cells, measured by quantitative polymerase chain reaction (PCR) was evident even beyond 6 months for some patients. Not surprisingly, this decrease in MART-1–specific TCR expression was associated with a profound loss of lymphocyte function when tested ex vivo. The level and duration of the transgenic TCR expression may be important for clinical efficacy because the initial patients who responded to treatment had sustained expression, which was likely a key factor in their therapy. Therefore, we sought to more thoroughly evaluate this phenomenon, anticipating that better understanding of the mechanism(s) involved in transgene shutdown will result in protocol modifications to minimize this limitation.
Human gene therapy protocols often rely on γ-retrovirus–based platforms for efficient, stable integration into the genome of host cells. Early work with γ-retroviruses, however, revealed marked silencing, with a lack of retroviral transcriptional activity in cells containing proviral DNA early after transduction.8,9 These studies used the Moloney murine leukemia virus (MMLV) to transduce embryonic stem cells and embryonal carcinoma cells, which were thought to possess efficient mechanisms limiting the expression of foreign DNA. Optimized γ-retroviral vectors, such as the MFG vector, augmented transgene expression but remained susceptible to transgene silencing.10,11 Generation of the murine stem cell virus (MSCV) vector, however, enabled efficient transgene expression in hematopoietic stem cells.12 Unfortunately, this system was prone to extinction, defined as a gradual decrease in gene expression upon differentiation or proliferation of transduced cells.
Most studies13,14 of mechanisms regulating γ-retroviral gene silencing and/or extinction have focused on epigenetic processes, that is, DNA methylation and histone modification. Although initial reports8,9 associated transgene silencing with de novo cytosine methylation at cytosine-guanine (CpG) dinucleotides, later reports15,–17 demonstrated silencing in the absence of de novo methylation and implicated a repressive histone code as the critical factor in transgene silencing. In a study by Swindle et al,18 they explored the process of transgene silencing using a γ-retroviral system similar to the one used in our most recent clinical trial. Their investigation demonstrated in vitro silencing of the MSCV long terminal repeat (LTR), which was diminished by mutational elimination of the CpG sites within the LTR.18 Given these reports, we hypothesized that the lack of transgene expression in vivo observed in our clinical trial may have resulted from DNA methylation (specifically, the clustered CpG residues within the γ-retroviral LTR) or from histone modification.
In this study, therefore, we sought to further characterize the persistence of gene-modified cells and to analyze TCR transgene expression in patients treated on adoptive immunotherapy protocols for metastatic melanoma. On the basis of the extensive literature linking classical epigenetic alterations with transgene shutdown, our second objective was to assess the level of CpG methylation within proviral integrants in vivo and the impact of pharmacologic inhibitors of DNA methylation and histone deacetylation on transgene expression in vitro. Furthermore, we sought to assess the impact of lymphocyte stimulation on transgene expression, both ex vivo and in vivo.
Methods
Lymphocytes and patient treatments
Peripheral blood mononuclear cells were collected via leukaphereses from patients with metastatic melanoma treated at the Surgery Branch, National Cancer Institute. PBLs were then separated via centrifugation on a Ficoll-Hypaque cushion, as previously described.7 Patients with metastatic melanoma that progressed after IL-2 therapy were treated under institutional review board-approved protocols at the Clinical Center, National Institutes of Health. Patient informed consent was obtained in accordance with the Declaration of Helsinki.
In 1 protocol, cells were modified with an MSCV-based vector encoding the α-chain and β-chain of a MART-1–specific TCR (Figure 1A, top),19,20 whereas in another protocol, cells were modified with an MMLV-based vector encoding the α-chain and β-chain of a gp100-specific TCR (Figure 1A, bottom).21 In brief, patient PBLs were stimulated for 2 days with 50 ng/mL OKT-3 (Ortho Biotech) in the presence of 300 IU/mL IL-2 (Chiron Corporation), then transduced with 1 of the γ-retroviral vectors, and expanded in vitro for up to 18 days (Figure 1B). These autologous gene-modified lymphocytes were administered to lymphodepleted patients in combination with high-dose IL-2 and vaccination. Patients in the MART-1–specific TCR protocol (Figure 1C top) received an anchor-modified MART-1:26-35 (27L) peptide vaccine.22,23 Similarly, patients in the first cohort of the gp100-specific TCR protocol received an anchor-modified gp100:209-217 (210M) peptide vaccine.23,24 Those in the second cohort of the gp100-specific TCR protocol (Figure 1C bottom) received a recombinant fowlpox virus encoding the 210M peptide preceded by an endoplasmic reticulum signal sequence25 ; these patients were retreated with vaccine and IL-2 after 1 month.
Melanoma antigen-specific TCR-encoding γ-retroviral vectors, production of autologous TCR gene-modified lymphocytes, and treatment protocols. (A) Diagram of an MSCV-based γ-retroviral vector encoding the α-chain and β-chain of a MART-1–specific TCR (top) and of an MMLV-based γ-retroviral vector encoding the α-chain and β-chain of a gp100-specific TCR (bottom). (B) Timeline depicting production of autologous TCR gene-modified lymphocytes. The infusion product was composed of cells after short-term ex vivo culture (day 6-7) with or without a portion of cells exposed to an OKT-3-based rapid expansion protocol.20 (C) Summary of TCR gene-modified lymphocyte protocols with administration of peptide vaccine (top) or fowlpox viral vaccine (bottom). All patients received nonmyeloablative lymphodepleting chemotherapy consisting of 2 days of cyclophosphamide (Cy) at 60 mg/kg followed by 5 days of fludarabine (Flu) at 25 mg/m2.
Melanoma antigen-specific TCR-encoding γ-retroviral vectors, production of autologous TCR gene-modified lymphocytes, and treatment protocols. (A) Diagram of an MSCV-based γ-retroviral vector encoding the α-chain and β-chain of a MART-1–specific TCR (top) and of an MMLV-based γ-retroviral vector encoding the α-chain and β-chain of a gp100-specific TCR (bottom). (B) Timeline depicting production of autologous TCR gene-modified lymphocytes. The infusion product was composed of cells after short-term ex vivo culture (day 6-7) with or without a portion of cells exposed to an OKT-3-based rapid expansion protocol.20 (C) Summary of TCR gene-modified lymphocyte protocols with administration of peptide vaccine (top) or fowlpox viral vaccine (bottom). All patients received nonmyeloablative lymphodepleting chemotherapy consisting of 2 days of cyclophosphamide (Cy) at 60 mg/kg followed by 5 days of fludarabine (Flu) at 25 mg/m2.
Ex vivo human lymphocyte culture, drug treatment, and restimulation
Peripheral blood, cryopreserved at time points greater than 8 weeks after adoptive transfer, from 4 patients was thawed and cultured in complete medium for up to 3 days. In the epigenetic studies, cells were treated with low-dose IL-2 (60 IU/mL) and a DNA methyltransferase inhibitor decitabine (5-aza-2-deoxycytidine; Sigma-Aldrich) at 0.3 μmol/L daily, a histone deacetylase inhibitor trichostatin A (Sigma-Aldrich) at 0.3 μmol/L for the final day, or a combination of both inhibitors. For the restimulation studies, cells were treated with IL-2 (600 IU/mL) alone or in combination with anti-CD3/anti-CD28 Dynabeads (Invitrogen). Cytokine-release assays using peptide-pulsed T2 cells were performed as previously described.7
Molecular analyses
Genomic DNA and total RNA were isolated concurrently from cryopreserved leukapheresis, infusion, or peripheral-blood specimens. Reverse transcription (RT) was performed by use of the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) with random octamer primers. Quantitative PCR was performed with the TaqMan Fast Universal PCR Master Mix (Applied Biosystems) by the use of a 7500 Fast Real-Time PCR System (Applied Biosystems).
Bisulfite conversion of genomic DNA was performed with the EpiTect Bisulfite Kit (QIAGEN). A 594-bp region of the LTR was amplified via PCR and cloned into the pCR4 TOPO vector (Invitrogen). Plasmid DNA from randomly selected bacterial clones was subjected to DNA sequencing. Primer and probe sequences are available upon request.
Statistics
Relative, corrected expression level of the TCR transgene and relative, corrected expression level of β-actin were evaluated in a paired fashion by use of the Wilcoxon signed-rank test at each time interval (1 week, 1 month, and > 2 months). Analysis also was performed for the TCR transgene and both the endogenous TCR α-chain and CD3ϵ. Reported P values are 2-sided.
Results
Kinetics of TCR vector persistence and expression after adoptive cell transfer in melanoma patients
Patients with progressive metastatic melanoma were treated with autologous PBL that were transduced with an MSCV-based, γ-retroviral vector to express a melanoma-specific TCR (Figure 1A top).19,20 We previously reported the treatment of the initial 17 patients7 and continued to treat an additional 14 patients with a cell product enriched for CD8+ cells. Therefore, a total of 31 patients were treated with this regimen of T cells targeting the MART-1 antigen, high-dose IL-2, and a peptide-based vaccine after lymphodepleting chemotherapy (Figure 1C top). A total of 10 of these patients (Table 1)26 had PBL samples available from time points greater than 2 months after treatment and were analyzed for persistence of gene-modified lymphocytes and for expression of the transgenic TCR after adoptive transfer.
Patient characteristics, description of treatments, and clinical outcomes
| ID . | Patient . | Cell treatment . | Response (duration in months)* . | |||
|---|---|---|---|---|---|---|
| Age/sex . | Evaluable disease sites . | TCR antigen . | Infusion no. (×109) . | Vaccine type . | ||
| 4 | 52/M | Li, Ly | MART-1 | 1.0 | p-27L | PR (34) |
| 5 | 50/M | Sq | MART-1 | 13 | p-27L | NR |
| 8 | 37/M | Lu, Ly | MART-1 | 6.1 | p-27L | NR |
| 9 | 53/M | Ad, Ly, Sq | MART-1 | 4.2 | p-27L | MR |
| 10 | 45/M | Ad, Ki, Ly, Sq | MART-1 | 8.6 | p-27L | NR |
| 11 | 45/M | Lu, Ly, Pa | MART-1 | 6.3 | p-27L | NR |
| 14 | 30/M | Ly | MART-1 | 86 | p-27L | CR (52+) |
| 17 | 20/F | Im, Lu, Ly, Sq | MART-1 | 23 | p-27L | NR |
| 19† | 52/M | Ly, Rp | MART-1 | 40 | p-27L | NR |
| 20† | 48/M | Lu | MART-1 | 37 | p-27L | NR |
| A | 42/F | Ly, Sq | gp100 | 24 | p-210M | NR |
| B | 44/F | Cu | gp100 | 3.8 | p-210M | NR |
| D | 52/F | Lu, Ly | gp100 | 11 | rFP-210M‡ | NR |
| E | 61/M | Ly | gp100 | 48 | rFP-210M‡ | NR |
| F | 27/M | Ad, Li, Lu, Rp | gp100 | 22 | rFP-210M‡ | NR |
| G | 44/M | Ad | gp100 | 40 | rFP-210M‡ | NR |
| H | 52/F | Lu, Ly, Sq | gp100 | 34 | rFP-210M‡ | NR |
| I | 46/F | Ip, Li, Ly | gp100 | 32 | rFP-210M‡ | NR |
| ID . | Patient . | Cell treatment . | Response (duration in months)* . | |||
|---|---|---|---|---|---|---|
| Age/sex . | Evaluable disease sites . | TCR antigen . | Infusion no. (×109) . | Vaccine type . | ||
| 4 | 52/M | Li, Ly | MART-1 | 1.0 | p-27L | PR (34) |
| 5 | 50/M | Sq | MART-1 | 13 | p-27L | NR |
| 8 | 37/M | Lu, Ly | MART-1 | 6.1 | p-27L | NR |
| 9 | 53/M | Ad, Ly, Sq | MART-1 | 4.2 | p-27L | MR |
| 10 | 45/M | Ad, Ki, Ly, Sq | MART-1 | 8.6 | p-27L | NR |
| 11 | 45/M | Lu, Ly, Pa | MART-1 | 6.3 | p-27L | NR |
| 14 | 30/M | Ly | MART-1 | 86 | p-27L | CR (52+) |
| 17 | 20/F | Im, Lu, Ly, Sq | MART-1 | 23 | p-27L | NR |
| 19† | 52/M | Ly, Rp | MART-1 | 40 | p-27L | NR |
| 20† | 48/M | Lu | MART-1 | 37 | p-27L | NR |
| A | 42/F | Ly, Sq | gp100 | 24 | p-210M | NR |
| B | 44/F | Cu | gp100 | 3.8 | p-210M | NR |
| D | 52/F | Lu, Ly | gp100 | 11 | rFP-210M‡ | NR |
| E | 61/M | Ly | gp100 | 48 | rFP-210M‡ | NR |
| F | 27/M | Ad, Li, Lu, Rp | gp100 | 22 | rFP-210M‡ | NR |
| G | 44/M | Ad | gp100 | 40 | rFP-210M‡ | NR |
| H | 52/F | Lu, Ly, Sq | gp100 | 34 | rFP-210M‡ | NR |
| I | 46/F | Ip, Li, Ly | gp100 | 32 | rFP-210M‡ | NR |
See Figure 1 for protocol description.
Ad indicates adrenal; CR, complete response; Cu, cutaneous; Im, intramuscular; Ip, intraperitoneal; Ki, kidney; Li, liver; Lu, lung; Ly, lymphatic; MR, mixed or minor response; NR, no response; p-210M, anchor-modified gp100 peptide vaccine; p-27L, anchor-modified MART-1 peptide vaccine; Pa, pancreas; PR, partial response; rFP-210M, recombinant fowlpox anchor-modified gp100 vaccine; Rp, retroperitoneal; and Sq, subcutaneous.
Based on Response Evaluation Criteria in Solid Tumors classification.26
These patients received CD8-enriched cells.
Vaccine, in combination with high-dose IL-2, was readministered at 1 month after cell infusion.
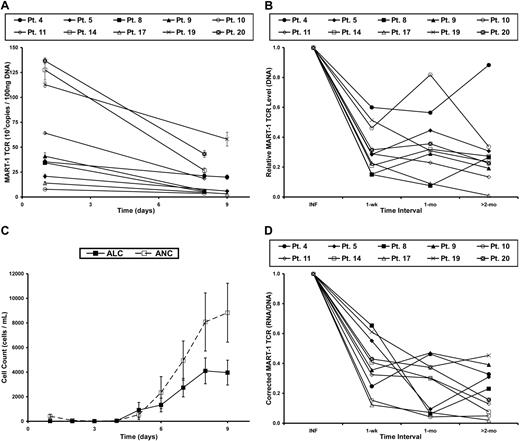
Genomic DNA, which was isolated from the infused cells and subsequent peripheral-blood samples, was used to quantify the level of proviral DNA in samples from each time point. The level of proviral DNA decreased the most during the first week after infusion (Figure 2A-B), which correlated with the recovery of endogenous immune cells (Figure 2C). When the level of transgene DNA at each time point was normalized to that of the patient's infusion sample, an acute decrease in the persistence of the gene-marked cells over the first week was observed, followed by a slower loss of these cells in the months to follow (Figure 2B). As previously described,7 kinetic analysis of TCR transgene expression based on flow cytometric analysis was severely limited at later time points. Therefore, we chose to perform expression analyses using a more sensitive technique, quantitative RT-PCR.
Levels of gene-modified cells and transgene RNA expression decrease in PBL after adoptive cell transfer in patients with metastatic melanoma. (A) DNA was isolated from cells in the treatment infusion (day 1) and subsequent peripheral blood samples of patients receiving autologous MART-1–specific TCR-transduced lymphocytes. Real-time PCR was performed to quantify the amount of transgene DNA present at each time point. Each line represents a single patient, and data points represent the mean of 3 replicates ± SD. (B) Persistence of MART-1 TCR transgene, normalized to the infusion sample (INF) at various time intervals after infusion. (C) The kinetics of immune reconstitution in the peripheral blood after the nonmyeloablative, lymphodepleting chemotherapy regimen. Adoptive cell transfer, IL-2 administration, and peptide vaccinations were initiated on day 1. Data points represent the mean of all patients in this report ± SEM. ALC indicates absolute lymphocyte count; and ANC, absolute neutrophil count. (D) MART-1–specific TCR transgene RNA expression, corrected for the level of transgene DNA, at each time interval and normalized to the infusion value (INF).
Levels of gene-modified cells and transgene RNA expression decrease in PBL after adoptive cell transfer in patients with metastatic melanoma. (A) DNA was isolated from cells in the treatment infusion (day 1) and subsequent peripheral blood samples of patients receiving autologous MART-1–specific TCR-transduced lymphocytes. Real-time PCR was performed to quantify the amount of transgene DNA present at each time point. Each line represents a single patient, and data points represent the mean of 3 replicates ± SD. (B) Persistence of MART-1 TCR transgene, normalized to the infusion sample (INF) at various time intervals after infusion. (C) The kinetics of immune reconstitution in the peripheral blood after the nonmyeloablative, lymphodepleting chemotherapy regimen. Adoptive cell transfer, IL-2 administration, and peptide vaccinations were initiated on day 1. Data points represent the mean of all patients in this report ± SEM. ALC indicates absolute lymphocyte count; and ANC, absolute neutrophil count. (D) MART-1–specific TCR transgene RNA expression, corrected for the level of transgene DNA, at each time interval and normalized to the infusion value (INF).
Total RNA was isolated from cells in the treatment infusion and subsequent blood samples and was subjected to quantitative RT-PCR. To minimize the confounding factors in the relationship between the level of the transgene RNA expression and proviral DNA, the TCR copy number from the RNA sample was corrected for the number of copies in the DNA sample at each time point. This ratio (corrected expression level), therefore, assessed the RNA expression level in the specific context of the number of gene-modified cells present at each time point. The corrected expression level was then normalized to the value of the infusion sample. This process enabled the kinetic analysis of transgene expression as it related to the persistence of TCR-transduced lymphocytes; in other words, it assessed the transcriptional activity of each integrated copy of LTR-driven, proviral transgene DNA. There was an acute decrease in corrected RNA expression during the first week in vivo (Figure 2D). This observation was noted in all patients, including the 2 exhibiting tumor regression. Beyond the first week, there was greater variability in the change of the RNA/DNA ratio among the different patients (patients 5, 8, and 19). In general, however, the TCR transgene expression decreased at a slower rate after the first week, with a consistent level less than 50% of the infusion sample after 2 months in vivo.
Low-level cytosine methylation of the γ-retroviral LTR in vivo
The impact of epigenetic silencing mechanisms was assessed in a short-term ex vivo model. Cryopreserved human PBL samples from patients 2 months after treatment with gene-modified lymphocytes expressing the MART-1–specific TCR were thawed and cultured in the presence of low-dose IL-2. These cells were used to evaluate the role of DNA methylation and histone deacetylation on transgene expression. During the course of 3 days, these lymphocytes were treated with the DNA methyltransferase inhibitor dacitabine and/or the histone deacetylase inhibitor trichostatin A. Dacitabine treatment did not augment transgene expression in terms of RNA expression (Figure 3A left); cyclin-dependent kinase inhibitor 1A (p21) expression, on the other hand, increased as expected (Figure 3A right).27,28 The use of trichostatin A alone or in combination with dacitabine had no attributable effect on human lymphocytes (data not shown). These experiments demonstrated that the in vivo shutdown of transgene expression in human PBL could not be reversed by ex vivo inhibition of DNA methyltransferases.
Low-level methylation of CpG residues within the LTR of proviral integrants. (A) Peripheral blood from 4 patients who received MART-1–specific TCR-transduced lymphocytes was harvested 2 months after adoptive transfer. Lymphocytes were isolated and cultured in complete media with low-dose IL-2 (60 IU/mL; MOCK) or with the addition of daily dacitabine (0.3 μmol/L; DAC). Total RNA was isolated after 3 days of ex vivo culture, reverse transcribed, and subjected to real-time PCR for the MART-1 TCR (left panel) or p21 (right panel). Each bar represents an individual patient. (B) Clustering of CpG dinucleotides within the MSCV LTR, where each oval depicts a single CpG site. Numbering is relative to the transcription start site. (C) Genomic DNA from retrovirally transduced lymphocytes of 2 donors was isolated 7 and 21 days after stimulation and bisulfite conversion was performed. A region of clustered CpG residues within the MSCV LTR were amplified by PCR and cloned. Each row represents sequencing of an individual clone, with methylated cytosine residues shown by shaded circles and nonmethylated residues by unshaded circles. (D) Bisulfite sequencing results of lymphocytes from 4 patients at early time points (1-4 weeks) and late time points (2-12 months) in vivo.
Low-level methylation of CpG residues within the LTR of proviral integrants. (A) Peripheral blood from 4 patients who received MART-1–specific TCR-transduced lymphocytes was harvested 2 months after adoptive transfer. Lymphocytes were isolated and cultured in complete media with low-dose IL-2 (60 IU/mL; MOCK) or with the addition of daily dacitabine (0.3 μmol/L; DAC). Total RNA was isolated after 3 days of ex vivo culture, reverse transcribed, and subjected to real-time PCR for the MART-1 TCR (left panel) or p21 (right panel). Each bar represents an individual patient. (B) Clustering of CpG dinucleotides within the MSCV LTR, where each oval depicts a single CpG site. Numbering is relative to the transcription start site. (C) Genomic DNA from retrovirally transduced lymphocytes of 2 donors was isolated 7 and 21 days after stimulation and bisulfite conversion was performed. A region of clustered CpG residues within the MSCV LTR were amplified by PCR and cloned. Each row represents sequencing of an individual clone, with methylated cytosine residues shown by shaded circles and nonmethylated residues by unshaded circles. (D) Bisulfite sequencing results of lymphocytes from 4 patients at early time points (1-4 weeks) and late time points (2-12 months) in vivo.
Surprised by the lack of improvement in LTR-driven transgene expression after treatment with a DNA methyltransferase inhibitor, we assessed the level of DNA methylation within the viral LTR promoter in vivo. The region containing the greatest density of CpG dinucleotides (Figure 3B) was therefore analyzed by the use of bisulfite sequencing. To determine the methylation status of ex vivo–engineered cells, PBLs were transduced with γ-retroviral supernatant encoding the MART-1–specific TCR, and the level of cytosine methylation at early (day 7) and late (day 21) time points was analyzed. The sequencing results from 8 representative proviral integrants for each donor at each time point are displayed in Figure 3C. This analysis demonstrated a lack of robust methylation in clustered CpG dinucleotides within the γ-retroviral promoter of transduced T cells in vitro and served as a baseline for in vivo studies.
To assess the level of cytosine methylation in vivo, we isolated DNA from the PBL of patients treated with gene-modified lymphocytes expressing the MART-1–specific TCR. The data from bisulfite sequencing of 4 patients (up to 16 clones per time point) at early (1-4 weeks) and late (2-12 months) time points after adoptive transfer are displayed in Figure 3D. There was a minimal level of cytosine methylation within this region of the LTR, which did not appear to increase with time. These experiments demonstrated that γ-retroviral–based gene transfer into human PBL did not result in significant DNA methylation of the LTR promoter either in vitro or in vivo. This observation may help to explain the unexpected findings that inhibition of DNA methylation did not improve expression of the MART-1-specific TCR in human PBL.
Down-regulation of vector LTR-driven TCR expression is nonspecific
We next sought to evaluate the in vivo down-regulation of the TCR transgene in the context of endogenous genes. As detailed previously, there was an acute decrease in RNA expression during the first week in vivo, even when correcting for the loss and/or dilution of transferred cells (Figure 2D). To compare the down-regulation of the TCR transgene with that of other genes, the level of the housekeeping gene β-actin and 2 T cell–specific genes, the endogenous TCR α-chain and CD3ϵ, also were analyzed (Figure 4). With respect to β-actin, the expression level was maximal in the infusion for 6 of the 8 patients (Figure 4A). The increase in endogenous gene expression comparing the preinfusion to infusion samples was expected by the ex vivo culture conditions, which induced T-cell activation and resulted in a more than 95% CD3+ cell population at infusion. On average, β-actin expression decreased to a level of approximately 50% of the infusion sample at 1 month and remained approximately 50% of the infusion sample at the latest time point analyzed (Figure 4A).
Down-regulation of transgene expression is similar to that of endogenous genes. (A) β-actin RNA expression, corrected for the level of β-actin DNA, before (PRE) and at time intervals after cell infusion were normalized to the infusion value (INF). Corrected RNA expression of the endogenous TCR α-chain (B) and CD3ϵ (C) before (PRE) and at time intervals after cell infusion were normalized to the infusion values (INF). (D) The relative, corrected MART-1–specific TCR transgene expression and the relative, corrective expression of β-actin, the endogenous TCR α-chain, and CD3ϵ at each time interval after treatment. Values are the mean of the analyzed patients ± SEM.
Down-regulation of transgene expression is similar to that of endogenous genes. (A) β-actin RNA expression, corrected for the level of β-actin DNA, before (PRE) and at time intervals after cell infusion were normalized to the infusion value (INF). Corrected RNA expression of the endogenous TCR α-chain (B) and CD3ϵ (C) before (PRE) and at time intervals after cell infusion were normalized to the infusion values (INF). (D) The relative, corrected MART-1–specific TCR transgene expression and the relative, corrective expression of β-actin, the endogenous TCR α-chain, and CD3ϵ at each time interval after treatment. Values are the mean of the analyzed patients ± SEM.
The expression of TCR α-chain (Figure 4B) and CD3ϵ (Figure 4C) also was increased by ex vivo activation, being maximal at the time of cell infusion in all 8 patients. These levels then decreased after treatment to levels fewer than 50% of the infusion sample after 2 months in vivo. The average values (± SEM) for all 8 patients analyzed were then determined (Figure 4D). It should be noted that expression of the T-cell–specific transcripts TCR α-chain and CD3ϵ were determined from bulk PBL and not corrected for the percentage of T cells in these samples. This step was taken because of the limited number of cells in these samples and because of an effort to minimize spurious changes in gene expression resulting from even a few hours of processing. Given this caveat, the expression kinetics of the endogenous TCR α-chain were similar to that of the MART-1–specific TCR transgene, as analyzed by the Wilcoxon signed-rank test at each time interval (1 week: P = .84, 1 month: P = .84, >2 months: P = .46). Likewise, the change in relative expression of CD3ϵ showed no trend toward a difference from the expression of the MART-1–specific TCR transgene (1 week: P = .84, 1 month: P = .74, >2 months: P = .74). Because these 2 T-cell–specific gene products mimicked the corrected expression of the vector LTR-driven transgenic TCR, this finding suggested that the decrease in vector-derived gene expression was nonspecific.
Ex vivo restimulation up-regulates transgenic TCR expression and cell function
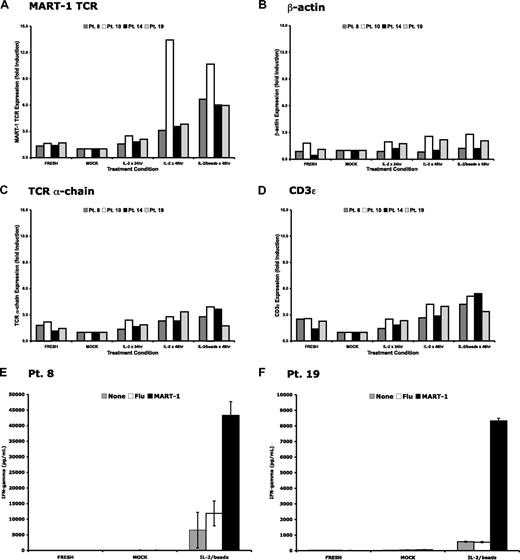
Because our data supported the premise that the down-regulation of the MART-1–specific TCR transgene in transduced lymphocytes was nonspecific and that cytosine methylation did not appear to play a significant role, we sought to reactivate transgene expression in gene-modified lymphocytes ex vivo. PBLs were isolated at late time points (2-10 months) after cell infusion from 4 patients treated on the MART-1–specific TCR protocol. These cells were then cultured for 48 hours in the presence of media alone, media with IL-2, or media with IL-2 and anti-CD3/anti-CD28 beads. RNA was then isolated from these cells, and gene expression levels were determined (Figure 5A-D). Vector-specific RNA levels were induced greater than 3-fold in the presence of IL-2 and up to 10-fold in the presence of IL-2 and anti-CD3/anti-CD28 beads. Control genes, including TCR α-chain and CD3ϵ, were up-regulated although to a lesser extent than the TCR transgene. These data demonstrated that nonspecific, short-term ex vivo stimulation of human T cells was sufficient to up-regulate transgene expression in circulating cells derived from patients months after treatment.
Up-regulation of transgenic TCR and other genes after ex vivo restimulation. Peripheral blood from 4 patients who received MART-1–specific TCR-transduced lymphocytes was harvested 2 to 10 months after adoptive transfer. Lymphocytes were isolated and cultured in complete media alone (MOCK), with exogenous IL-2 (600 IU/mL), or with IL-2 and anti-CD3/anti-CD28 beads (1 bead/cell). Total RNA was isolated at the time of in vitro culture (FRESH) or after 48 hours of ex vivo culture, reverse transcribed, and subjected to real-time PCR for (A) the MART-1–specific TCR, (B) β-actin, (C) the endogenous TCR α-chain, and (D) CD3ϵ. Each bar represents an individual patient. Peripheral blood from 2 of the 4 patients were maintained in ex vivo culture for 3 days with or without stimulation conditions and compared with the freshly thawed peripheral blood. Only those cells receiving ex vivo restimulation secreted interferon-γ and demonstrated specific recognition of MART-1 peptide-pulsed target cells (E-F).
Up-regulation of transgenic TCR and other genes after ex vivo restimulation. Peripheral blood from 4 patients who received MART-1–specific TCR-transduced lymphocytes was harvested 2 to 10 months after adoptive transfer. Lymphocytes were isolated and cultured in complete media alone (MOCK), with exogenous IL-2 (600 IU/mL), or with IL-2 and anti-CD3/anti-CD28 beads (1 bead/cell). Total RNA was isolated at the time of in vitro culture (FRESH) or after 48 hours of ex vivo culture, reverse transcribed, and subjected to real-time PCR for (A) the MART-1–specific TCR, (B) β-actin, (C) the endogenous TCR α-chain, and (D) CD3ϵ. Each bar represents an individual patient. Peripheral blood from 2 of the 4 patients were maintained in ex vivo culture for 3 days with or without stimulation conditions and compared with the freshly thawed peripheral blood. Only those cells receiving ex vivo restimulation secreted interferon-γ and demonstrated specific recognition of MART-1 peptide-pulsed target cells (E-F).
There were sufficient cells from 2 patients to evaluate the effect of ex vivo stimulation on lymphocyte function. Freshly thawed cells from these patients 2 months after cell infusion were compared with the same cells cultured for 3 days in the presence of low-dose IL-2 or IL-2 and anti-CD3/anti-CD28 beads. Lymphocyte function was assessed by interferon-γ production in a coculture assay. Only those lymphocytes restimulated with IL-2 and anti-CD3/anti-CD28 beads demonstrated specific reactivity against T2 cells pulsed with MART-1 peptide. Taken together, these data suggest that restimulation of gene-modified lymphocytes at time points months after infusion can induce LTR-driven transgene expression and specific TCR reactivity.
In vivo T-lymphocyte restimulation up-regulates transgenic TCR expression
A second group of patients with progressive metastatic melanoma was treated with autologous PBL genetically engineered to express a different melanoma antigen-specific TCR. In this protocol, an MMLV-based retroviral system was used to transduce lymphocytes with a TCR targeting the gp100 antigen (Figure 1A bottom).21 Similar to the MART-1–specific TCR protocol, cells were adoptively transferred to lymphodepleted patients in combination with high-dose IL-2 and vaccination. A total of 11 patients were treated, and there were no objective responses to therapy; all 8 who had sufficient PBL samples were used in the analysis (Table 1).
Patients receiving this protocol were treated in 2 distinct cohorts. The initial cohort consisted of 3 patients and followed a treatment regimen very similar to the patients on the MART-1–specific TCR protocol (Figure 1C top). Two of these 3 patients had archived samples sufficient for molecular analysis. Gene-modified cells from both patients persisted in the peripheral blood for more than 1 month but demonstrated rapid down-regulation of the TCR transgene, corrected for cell persistence (Figure 6A shaded squares). Other cellular gene products (including β-actin, TCR α-chain, and CD3ϵ) exhibited similar down-regulation over time (Figure 6A: filled diamonds, open triangles, and open circles, respectively). These expression profiles were consistent with those of the patients treated with the MART-1–specific transgenic TCR detailed in Figure 4D.
Up-regulation of gene expression in patients after in vivo restimulation. The gp100-specific TCR transgene RNA level was corrected for the DNA level at each time interval and normalized to the infusion values (INF). RNA expression of β-actin, the endogenous TCR α-chain, and CD3ϵ before (PRE) and at time intervals after cell infusion was normalized to the infusion value (INF). (A) Patients A and B from the first cohort of this trial received IL-2 and peptide vaccination on the day of adoptive cell transfer. (B) Patients C through H from the second cohort of this trial received IL-2 with recombinant fowlpox vaccination on the day of adoptive cell transfer, as well as repeat administration of IL-2 with recombinant fowlpox vaccination approximately 1 month after adoptive cell transfer (indicated by  ).
).
Up-regulation of gene expression in patients after in vivo restimulation. The gp100-specific TCR transgene RNA level was corrected for the DNA level at each time interval and normalized to the infusion values (INF). RNA expression of β-actin, the endogenous TCR α-chain, and CD3ϵ before (PRE) and at time intervals after cell infusion was normalized to the infusion value (INF). (A) Patients A and B from the first cohort of this trial received IL-2 and peptide vaccination on the day of adoptive cell transfer. (B) Patients C through H from the second cohort of this trial received IL-2 with recombinant fowlpox vaccination on the day of adoptive cell transfer, as well as repeat administration of IL-2 with recombinant fowlpox vaccination approximately 1 month after adoptive cell transfer (indicated by  ).
).
The 8 patients comprising the remaining portion of this protocol were treated in the same manner except for 2 specific modifications (Figure 1C bottom). First, these patients received a gp100-specific fowlpox vaccination instead of peptide vaccination. Second, repeat vaccination was performed in conjunction with high-dose IL-2 approximately 1 month after cell infusion. Of these 8 patients, 6 had sufficient PBL samples and were analyzed for expression of the transgene as well as endogenous genes. None of the patients in either protocol arm experience objective tumor regression. After a second vaccination and IL-2 treatment, quantification of RNA expression demonstrated up-regulated or persistent expression of the TCR transgene (as well as β-actin, TCR α-chain, and CD3ϵ) in all patients (Figure 6B). Together, these data suggested that adjuvants that stimulate lymphocytes in vivo could enhance vector LTR-mediated expression of exogenous transgenes in genetically engineered PBL after adoptive transfer.
Discussion
Modern gene-transfer techniques have translated conceptual strategies into realistic approaches for the treatment of human disease. Of the human gene therapy trials, the first approved clinical protocols targeted T cells to correct severe combined immunodeficiency caused by adenosine deaminase deficiency.29 This group transduced autologous lymphocytes with full-length adenosine deaminase cDNA under the control of a γ-retroviral LTR, and readministered these cells to 2 affected children after ex vivo expansion. These authors demonstrated that treatment with gene-modified lymphocytes could be safe and that long-term engraftment could be maintained. Despite routine adenosine deaminase enzyme replacement, 1 patient had long-term persistence of the transferred cells, which likely possessed a survival advantage over endogenous cells and those transferred with low or silenced expression.30 Another group31 performed similar trials, modified by coadministration of transduced T cells and bone marrow–derived cells and then followed up on the initial treatments by withdrawal of enzyme replacement therapy.32 These early gene therapy trials provided detailed analysis of lymphocyte persistence but did not perform systematic analysis of transgene expression, let alone LTR-mediated transcriptional activity.
Gene transfer with γ-retroviral vectors also has been used to treat graft-versus-host disease, HIV infection, and a variety of malignancies. Graft-versus-host disease often is found in patients with hematologic malignancies who undergo allogeneic hematopoietic stem cell transplantation. This potentially severe morbidity may be limited by inserting a suicide gene, most commonly the herpes simplex virus thymidine kinase, into the transferred cells and thereby conferring sensitivity to ganciclovir.33,34 In a study by Ciceri et al,35 which reported long-term follow-up, the gene-modified T cells were detected in the circulation of all evaluable patients (14 by flow cytometry and 3 by PCR only) by 6 weeks after adoptive transfer. Furthermore, elimination of transferred cells and clinical improvement was noted in the 3 patients who received ganciclovir to treat graft-versus-host disease, suggesting that the alloreactive T cells continue to express the thymidine kinase transgene.
In the treatment of HIV-seropositive patients, 2 groups examined the ability of CD4+ T cells, engineered to express a γ-retroviral LTR-driven mutant inhibitory form of Rev (an essential viral protein). Ranga et al,36 in a pilot study, treated 3 patients and detected cells up to 9 months after infusion. These cells had enhanced in vivo persistence compared with cells transduced with a similar vector not encoding the transgene. Expression, however, was low and required ex vivo expansion to detect the transgene by RT-PCR. Another trial treated patients with lymphocytes donated by their unaffected twin.37 Cells modified with a mutant Rev transgene again demonstrated prolonged survival but poor transgene expression.
Most of the cancer immunotherapy trials that use gene-modified lymphocytes, on the other hand, have been hindered by low cell persistence. In 1 protocol,38 lymphocytes from ovarian cancer patients were transduced to express a chimeric antigen receptor targeting α-folate receptor, an ovarian cancer-associated antigen. Of the 12 patients treated, only 1 had detectable gene-modified cells in the circulation after 1 month. Another group sought to treat patients with renal cell carcinoma via adoptive transfer of autologous lymphocytes redirected against carbonic anhydrase IX.39 Unfortunately, this trial was ended after 3 patients were treated because 2 patients developed significant biliary toxicity. Again, transferred cells did not persist longer than 1 month, and expression of the receptor transgene was not detected after 1 week in vivo.40 Despite the number of human gene therapy clinical trials, none has provided a detailed, longitudinal analysis of cell persistence and transgene expression in vivo.
In this report, we presented the detailed analysis of 18 separate patient treatments from 2 gene therapy clinical trials. Our studies demonstrated a loss of LTR-driven TCR transgene transcriptional activity in patients shortly after they received lymphocyte that was genetically engineered to target melanoma antigens. These data recapitulated our previous study, which revealed a low level of MART-1 tetramer-positive, CD8+ cells in the peripheral blood of patients within days of adoptive transfer.7 The inability to detect this gene-transferred TCR by tetramer analysis was in part hampered by the relatively low avidity41 of the specific TCR (termed DMF4) used in this initial trial. Similarly, the percentage of Vβ12-positive T cells detected in peripheral blood decreased during the initial week after cell infusion. Within a month, it was only slightly greater than the endogenous Vβ12-positive T cells detected before therapy. Using highly sensitive molecular assays, we now show that this decrease in expression also was observed at the level of transcription. An additional finding was that transgene down-regulation was independent of the γ-retroviral system (MSCV versus MMLV) used to generate the gene-modified lymphocytes. Therefore, although the rapid decrease in LTR-driven transgene expression in vivo resembled classic silencing or extinction of γ-retroviral transgenes, the concomitant down-regulation of endogenous genes was inconsistent with a mechanism distinctly affecting the transcription of proviral DNA.
Additional data supported the conclusion that LTR-driven transgenes in human lymphocytes were not silenced in vivo. First, bisulfite sequencing analysis of CpG sites within the MSCV LTR revealed only low levels of DNA methylation. Seemingly random sites in occasional clones were methylated at early time points in vivo (when transgene expression was highest), as well as at time points later than 10 months in vivo (when transgene expression was lowest). There have been a limited number of reports detailing the level of DNA methylation in γ-retroviral systems, but 1 group42 found methylated CpG residues within the LTR of proviral integrants in embryonic stem cells, but not fibroblasts, in vitro. It appears that primary lymphocytes (like fibroblasts), therefore, differ from embryonic stem cells in terms of de novo DNA methylation within the LTR of proviral integrants. Second, the ex vivo use of a DNA methyltransferase inhibitor failed to augment expression of the MART-1–specific TCR transgene, which had decreased during 2 months in vivo. These results were surprising given the reports that these epigenetic mechanisms are involved in transgene silencing.13,,,–17 Again, this may represent an inherent difference in transduced mature human lymphocytes, compared with embryonic stem cells.
We further demonstrated that ex vivo restimulation of PBLs derived from patients more than 2 months after cell infusion was sufficient to up-regulate TCR transgene expression. Other cellular genes, including β-actin, TCR α-chain, and CD3ϵ, were also up-regulated by restimulation but to a lesser degree than the TCR transgene. These data support the conclusion that lymphocyte activation may be a key determinant of transgene expression, even at late time points after gene transfer. Our findings were consistent with those of Pollok and colleagues,43 who transduced primary human T lymphocytes with an MMLV-based retrovirus encoding murine B7-1 and found that although transgene expression decreased rapidly in the presence of IL-2 in vitro, it was up-regulated upon short-term exposure to plates coated with both anti-CD3 and anti-CD28 monoclonal antibodies. A subsequent report44 by this group demonstrated that this transgene up-regulation was not affected by treatment with the protein synthesis inhibitor cyclohexamide (ie, does not require new protein synthesis) but was abrogated by treatment with multiple protein kinase inhibitors. Quinn et al45 have also reported on T-cell activation as it relates to transgene expression. Using an MFG-based γ-retroviral system to express IL-1β in PBL, this group treated transduced cells with IL-2 plus insoluble OKT-3 or with anti-CD3/anti-CD28 beads. They noted significant increases in transgene, as well as interferon-γ, expression within 72 hours. Similarly, in a nonhuman primate model, the expression of γ-retroviral vector-derived sequences was only detected after cells were cultured in the presence of IL-2.46 In this report, we extended these findings by demonstrating rapid ex vivo reinduction of LTR-driven transgenes in PBL derived from patients enrolled in gene therapy trials. Furthermore, short-term restimulation also induced MART-1-specific activity in the circulating lymphocytes of these patients.
Finally, we assessed the impact of lymphocyte restimulation on TCR transgene expression in vivo through a unique clinical trial. In this study, 11 patients were treated with autologous PBL engineered to express gp100-specific TCR via an MFG-like retrovirus in 2 distinct cohorts. The first cohort received gene-modified cells after nonmyeloablative chemotherapy in the traditional manner (with high-dose IL-2 and peptide vaccination), whereas the second cohort received the same TCR but with high-dose IL-2 and fowlpox vaccination. Both patients analyzed from the first cohort exhibited progressive down-regulation of TCR transgenes and endogenous genes, similar to patients analyzed in the larger MART-1–specific TCR trial. All 6 patients analyzed in the second cohort demonstrated a markedly different pattern in gene expression after IL-2 and vaccination. In stark contrast, there was an up-regulation of TCR transgenes and endogenous genes in these patients after receiving an additional cycle of IL-2 with repeat vaccination (at 1 month). This enhancement of TCR transgene expression, however, was not sufficient to mediate any objective clinical response.
Together, these data suggest that expression of an LTR-driven transgene was subject to the global cellular mechanisms, which control endogenous gene expression in activated or quiescent lymphocytes. Using an in vitro model for activated and quiescent T cells, Cooper et al47 demonstrated that expression of a destabilized enhanced green flourescent protein reporter gene correlated with the level of stimulation. This group also found that the viral LTR promoter provided high transgene expression in proliferating T cells and that the addition of the interferon-β scaffold attachment region enhanced transgene expression in both resting and cycling cells. A potential mechanism involved in lymphocyte quiescence may include regulation by members of the suppressor of cytokine signaling gene family.48,49 These proteins inhibit cytokine signaling pathways and regulate T-cell activation. As we gain further understanding of these pathways, we may be able to better engineer lymphocytes for adoptive transfer. Alternatively, gene transfer into virus-specific T cells might be used to limit the loss and quiescence of transferred lymphocytes. In a recent report50 the authors demonstrated that Epstein-Barr virus-specific T cells transduced to express a GD2 ganglioside-specific chimeric antigen receptor survived longer in vivo then similar gene-modified T cells lacking virus specificity. The use of virus-specific T cells, therefore, offers promise for future adoptive transfer protocols.
To our knowledge, this is the first report to characterize transgene expression because it compares with other cellular genes in a human adoptive cell transfer trial. Through molecular techniques, we demonstrated that the loss of transgene expression in human lymphocytes is nonspecific. We demonstrated that DNA methylation was not involved in this process but rather that administration of lymphocyte activation signals was sufficient to up-regulate LTR-driven expression of a MART-1–specific TCR, as well as revert patients' circulating lymphocytes into cells capable of recognizing and responding to the MART-1 antigen. We further demonstrated that the adjuvant use of cytokine therapy and vaccination was associated with sustained transgene expression in vivo. As we move forward with trials centered on the adoptive transfer of gene-modified lymphocytes, this understanding will shape future protocols and may help maintain long-term transgene expression in vivo, thereby augmenting cell-based cancer immunotherapy.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Arnold Mixon and Shawn Farid (Surgery Branch, National Cancer Institute), for their assistance with flow cytometry and cell sorting. We further thank David Schrump and Julie Hong (Surgery Branch, National Cancer Institute), for advice regarding bisulfite sequence and cell treatments with inhibitors of DNA methylation and histone deacetylation. Finally, we thank Nicholas Restifo (Surgery Branch, National Cancer Institute), for his careful review and suggestions regarding the manuscript.
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: W.R.B. designed and performed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; Z.Z. performed research; S.A.R. interpreted data; and R.A.M. designed research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard A. Morgan, Surgery Branch, NCI, NIH, Bldg 10-CRC, Rm 3W/3-5940, 10 Center Dr, Bethesda, MD 20892; e-mail: rmorgan@mail.nih.gov.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal