Abstract
Hereditary forms of iron-deficiency anemia, including animal models, have taught us much about the normal physiologic control of iron metabolism. However, the discovery of new informative mutants is limited by the natural mutation frequency. To address this limitation, we have developed a screen for heritable abnormalities of red blood cell morphology in mice with single-nucleotide changes induced by the chemical mutagen ethylnitrosourea (ENU). We now describe the first strain, fragile-red, with hypochromic microcytic anemia resulting from a Y228H sub-stitution in the ferrireductase Steap3 (Steap3Y288H). Analysis of the Steap3Y288H mutant identifies a conserved motif required for targeting Steap3 to internal compartments and highlights how phenotypic screens linked to mutagenesis can identify new functional variants in erythropoiesis and ascribe function to previously unidentified motifs.
Introduction
The importance of iron in normal physiology is highlighted by the evolution of a variety of highly conserved mechanisms that ensure efficient iron delivery to cells.1-3 Indeed, much understanding of these mechanisms has emerged from studying various forms of hereditary iron-deficient erythropoiesis and anemia in humans2,4 or animal models.5-7 From these studies it is known that most iron is delivered to developing erythroid cells through the transferrin (Tf) cycle. In the Tf cycle, diferric transferrin binds to the transferrin receptor (TfR) on cell membranes. The Tf-TfR complex is internalized to a unique endosomal compartment where acidification leads to the release of ferric iron. The ferric iron is then reduced to ferrous iron and transported across the endosomal membrane by the divalent metal transporter DMT1. The endosomal ferrireductase responsible for the reduction of ferric iron in erythroid cells was recently identified as the 6-transmembrane epithelial antigen of the prostate 3 (Steap3) protein. Steap3 was identified during the characterization of a mutant mouse strain that presented with severe microcytic anemia resulting from a spontaneous deletion of Steap3.2,3 Steap3 is highly expressed in hematopoietic tissues where it localizes to the specialized endosome.2,3 Three other well-conserved members of the Steap family (Steap1, Steap2, and Steap4) have been characterized, and 2 of the 3 were shown to be ferric and cupric reductases.8 The exact role of these proteins and the extent to which they may operate semiredundantly in different tissues is unknown.
To gain further insights into iron metabolism through the study of heritable defects, we have developed a program of chemical ethylnitrosourea (ENU) mutagenesis in C57BL/6 (B6) mice that is run in conjunction with a screen for anemia. This process accelerates the normal rate of single nucleotide substitutions approximately 100-fold in B6 mice, leading to approximately 1 mutation per 106 basepairs (bp).9 A significant advantage of this program is the ability to mimic natural variation in the human genome and the lack of prior assumption concerning protein function. Using this approach, we now report a novel mouse strain, fragile-red, with a hypochromic microcytic anemia, which is due to a missense mutation in the Steap3 protein (Steap3Y288H). This mutation identifies a targeting motif, essential for Steap3 function, which is highly conserved in other Steap family members and similar, but not identical, to motifs used in proteins that are targeted to endosomes, lysosomes, and lysosome-related organelles.10,11
Methods
All of the animal experiments performed in this study were approved by the Oxford University ethical review processed under United Kingdom Home Office license PPL30/2455.
ENU mutagenesis, mapping, and sequencing were as described.9 Screening for abnormal hematologic phenotypes was performed with the use of flow cytometry and May-Grünwald-Giemsa staining. Details of imaging, cell, iron, hepcidin, and red blood cell ferrireductase activity are published elsewhere12-14 ; serum and liver copper levels were assayed as described.15 FLAG epitope-tagged wild-type and mutant Steap3Y288H were expressed in human embryonic kidney (HEK)–293T cells and were localized by immunofluorescence.3
Results and discussion
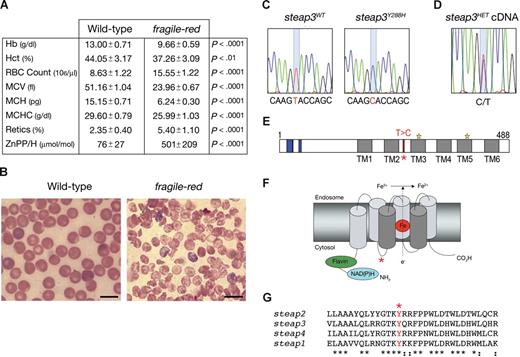
By screening for hematologic phenotypes in a library of B6 mice and segregating ENU-induced substitutions, we identified a new strain, fragile-red, with a recessively inherited hypochromic microcytic anemia. Affected mice were anemic at weaning and by 8 weeks had a mean hemoglobin level (Hb) of 9.66 g/dL plus or minus 0.59 g/dL (n = 7) compared with 13.00 g/dL plus or minus 0.71 g/dL (n = 8; P < .0001) in wild-type (WT) mice. The anemia was associated with a reticulocytosis, and the peripheral blood film showed anisopoikilocytosis, polychromasia, and target cells (Figure 1A,B; data not shown). The defect appeared restricted to the erythroid lineage, because leukocyte and platelet numbers were unaffected (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Affected male and female mice were of normal size and were able to breed normally, but they did develop cardiomegaly and splenomegaly, probably as a result of chronic anemia and extramedullary hematopoiesis, respectively (Table S2).
Identification of an ENU-induced missense substitution in Steap3 in the fragile-red mouse mutant. (A) Peripheral blood erythrocyte indices from 8-week-old wild-type (WT; n = 8) and fragile-red homozygous mice (n = 7). Data show means and SDs. Statistical analysis was performed using unpaired t test to WT controls. (B) Representative peripheral blood film from wild-type and fragile-red. Bar = 10 μm. (C) 867T → C substitution in steap3 cDNA. (D) Sequence trace of bone marrow cDNA from a Steap3Y288H/+ (steap3HET) mouse. (E-G) Location of the mutation (starred) in the domain structure of Steap3 (E) in model (F), showing putative structure of the ferrireductase in endosomal membrane (central heme iron in red), and in amino acid sequence (G) between transmembrane domains 2 and 3.
Identification of an ENU-induced missense substitution in Steap3 in the fragile-red mouse mutant. (A) Peripheral blood erythrocyte indices from 8-week-old wild-type (WT; n = 8) and fragile-red homozygous mice (n = 7). Data show means and SDs. Statistical analysis was performed using unpaired t test to WT controls. (B) Representative peripheral blood film from wild-type and fragile-red. Bar = 10 μm. (C) 867T → C substitution in steap3 cDNA. (D) Sequence trace of bone marrow cDNA from a Steap3Y288H/+ (steap3HET) mouse. (E-G) Location of the mutation (starred) in the domain structure of Steap3 (E) in model (F), showing putative structure of the ferrireductase in endosomal membrane (central heme iron in red), and in amino acid sequence (G) between transmembrane domains 2 and 3.
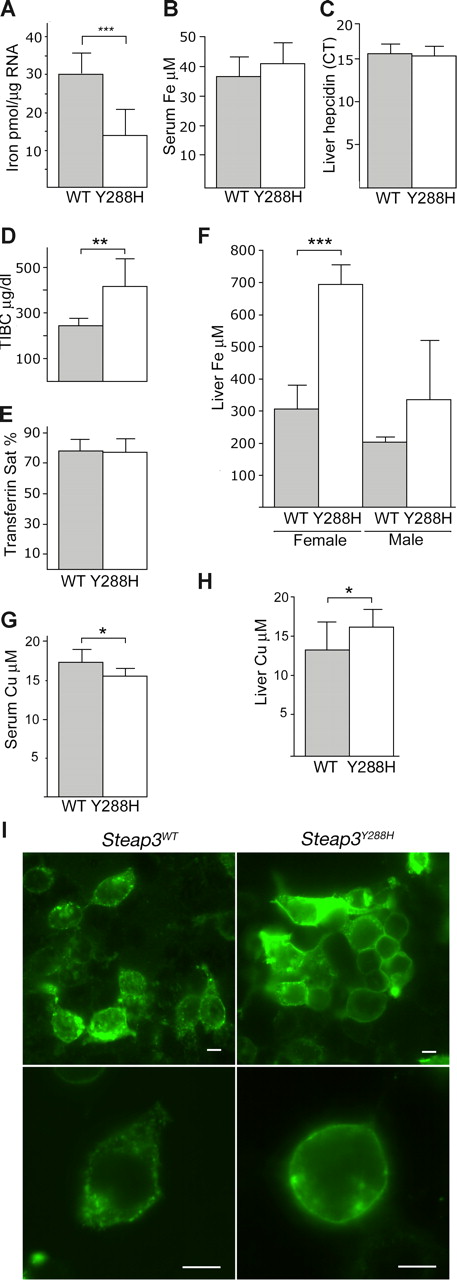
To determine whether the mutation was due to systemic iron deficiency, serum iron and hepcidin levels were measured and found to be comparable between homozygous fragile-red mutant mice and WT animals, whereas liver iron was higher in affected mice, particularly females (Figure 2B,C,F). Total iron binding capacity (TIBC) is nearly 2-fold higher in fragile-red and saturation is similar (Figure 2D,E). These findings suggest that the defective erythropoiesis occurs despite adequate iron supply and is associated with decreased intracellular iron, increased synthesis of transferrin, and increased iron deposition. The unchanged level of hepcidin is probably due to the competing effects of iron overload, anemia, and ineffective erythropoiesis.
Loss of endosomal targeting of the Steap3Y288H reduces ferrireductase activity and the uptake of iron and copper. (A) Ferrireductase activity in whole blood preparations: incubations were carried out for 30 minutes and normalized to TCA RNA (n = 11 wild-type; n = 8 mutant). (B) Serum iron (n = 11 WT; n = 9 mutant); (C) liver hepcidin, expressed as cycle threshold (Ct) (n = 8 WT; n = 12 mutant); (D) TIBC (n = 8 WT; n = 6 mutant); and (E) transferrin saturation (n = 8 WT; n = 6 mutant). (F) Liver iron in male and female WT (n = 5 or 7) and mutant mice (n = 3 or 6). (G) Serum and (H) liver copper in WT (n = 12) and mutant mice (n = 9). Graphs show means (columns) and SDs of the mean (bars). Statistical analysis was performed using unpaired t test to WT controls: *P = .01 to .05; **P = .001 to .01; ***P = < .001. (I) Localization of FLAG-tagged wild-type Steap3 (Steap3WT) and Steap3Y288H in HEK-293T cells in images taken at ×40 and ×100 magnification. Images were obtained using a Zeiss Axioplan 2 upright microscope and a 63×/1.4 NA oil-immersion objective and Zeiss Meta confocal imaging software (Zeiss, Thornwood, NY). Bars = 10 μm.
Loss of endosomal targeting of the Steap3Y288H reduces ferrireductase activity and the uptake of iron and copper. (A) Ferrireductase activity in whole blood preparations: incubations were carried out for 30 minutes and normalized to TCA RNA (n = 11 wild-type; n = 8 mutant). (B) Serum iron (n = 11 WT; n = 9 mutant); (C) liver hepcidin, expressed as cycle threshold (Ct) (n = 8 WT; n = 12 mutant); (D) TIBC (n = 8 WT; n = 6 mutant); and (E) transferrin saturation (n = 8 WT; n = 6 mutant). (F) Liver iron in male and female WT (n = 5 or 7) and mutant mice (n = 3 or 6). (G) Serum and (H) liver copper in WT (n = 12) and mutant mice (n = 9). Graphs show means (columns) and SDs of the mean (bars). Statistical analysis was performed using unpaired t test to WT controls: *P = .01 to .05; **P = .001 to .01; ***P = < .001. (I) Localization of FLAG-tagged wild-type Steap3 (Steap3WT) and Steap3Y288H in HEK-293T cells in images taken at ×40 and ×100 magnification. Images were obtained using a Zeiss Axioplan 2 upright microscope and a 63×/1.4 NA oil-immersion objective and Zeiss Meta confocal imaging software (Zeiss, Thornwood, NY). Bars = 10 μm.
To identify the causative mutation, we bred homozygous affected B6 males to nonobese diabetic (NOD) females and then established a B6 × NOD F2 intercross mapping panel. The mice were phenotyped by blood smears, and the anemia was linked to a 7.05-Mb region between 115.38 Mb and 122.43 Mb on chromosome 1, containing 25 potential genes of which 14 had known function, including Steap3. Exonic sequencing of 8 transcripts from B6 WT and fragile-red mice showed a single 867T → C substitution in the Steap3 mRNA (Figure 1C), resulting in an amino acid change of Y288H in the Steap3 protein. The mutation did not substantially affect expression or stability of Steap3Y288H mRNA, because sequencing of cDNA from Steap3WTSteap3Y288H heterozygotes showed 2 peaks of comparable size (Figure 1D); accurate measurement of protein levels in vivo depends on the future generation of a Steap3 antibody. Analysis of the protein structure showed the location of the mutation to be in a highly conserved cytoplasmic loop (Figure 1E-G).
Comparison of fragile-red with the published description of mice carrying a targeted deletion in the Steap3 protein suggests that the effect of the homozygous Steap3Y288H mutation is remarkably similar to the null allele on a mixed S129/B6 background, which leads to a Hb level of approximately 8 g/dL and mean corpuscular volume of 24 femtoliters in 8-week-old mice.3 The Steap3Y288H mutation caused a 2-fold reduction in the ferrireductase of whole blood cells (Figure 2A), which is again comparable with the targeted deletion (approximately 3-fold reduction). These similarities highlight the importance of the single amino acid change in fragile-red, and a direct comparison with the targeted deletion of Steap3 on the same genetic background will establish whether the Steap3Y288H is a true null or hypomorphic allele.
Recent data have highlighted the importance of the Steap family of proteins as copper reductases8 ; serum cupric levels were lower and liver copper was higher in affected animals (Figure 2G,H). These data suggest a possible role for Steap3 in the distribution of tissue copper stores. It will be interesting to determine whether this effect is recapitulated or more pronounced in Steap3-null animals.
The profound effect of the Steap3Y288H mutation in fragile-red was surprising given the location of the mutation and its separation from the reductase motif (Figure 1E,F). However, examination of the conserved residues surrounding the mutated tyrosine showed an YXXØ consensus sequence. This motif has been shown to target transmembrane proteins, including LAMP-1, LAMP-2, the TfR, and Nramp1,11 to endosomes, lysosomes, and lysosome-related organelles. The YXXØ motif is found in organisms as distantly related as protists and mammals11 and is conserved in the Steap family of proteins (Figure 1G), suggesting that there is stringent evolutionary pressure to conserve this consensus sequence. On this basis we hypothesized that the YXXØ consensus motif may be critical for the localization of Steap3 to the specialized endosomal compartment.
To explore this possibility and to confirm the functional effect of the point mutation, we expressed a FLAG-tagged form of WT Steap3 and Steap3Y288H in HEK-293T cells and assessed protein localization using immunofluorescence. This study showed that mutation of the YXXØ sorting motif results in retention of Steap3Y288H on the plasma membrane (Figure 2I). Interestingly, the Steap3 motif differs from the common form of lysosomal YXXØ sorting motif, which depends on a glycine residue before the tyrosine for the process of intracellular sorting but not endocytosis.10 Targeted mutagenesis will be an effective way to dissect these processes and the role of the individual amino acids in the Steap3 family.
Our analysis identifies a critical tyrosine in the targeting motif that is mandatory for the localization of Steap3 proteins to acidified endosomes1 and necessary for efficient iron transport. The study highlights the advantage of ENU in generating allelic variants for the study of biochemical and physiologic pathways in hematology and the association of function to previously unidentified motifs. Further work will investigate whether human variants affecting the YXXØ motif of Steap3 are causative in hereditary microcytic anemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Wellcome Trust (London, United Kingdom). M.D.F. is supported by R01HL074247 and R56DK080011.
Wellcome Trust
Authorship
Contribution: T.L., R.J.S., S.D., T.B-J., T.L.C., M.L., G.O.L-D., H.R., K.B.R., D.R.C., G.V., and A.T.M., performed experiments; T.L., R.J.S., A.T.M., J.C.E., C.C.G., M.D.F., and R.J.C. designed the research and wrote the paper; and all authors contributed significantly to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard J, Cornall, Henry Wellcome Building for Molecular Physiology, Wellcome Trust Centre for Human Genetics, Old Road Campus, Oxford University, Roosevelt Dr, Headington, Oxford OX3 7BN, United Kingdom; e-mail: rcornall@ccmp.ox.ac.uk.
References
Author notes
*M.D.F., A.T.M., and R.J.C. contributed equally to the study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal