Abstract
Hemojuvelin (HJV) was recently identified as a critical regulator of iron homeostasis. It is either associated with cell membranes through a glycosylphosphatidylinositol anchor or released as a soluble form. Membrane-anchored HJV acts as a coreceptor for bone morphogenetic proteins and activates the transcription of hepcidin, a hormone that regulates iron efflux from cells. Soluble HJV antagonizes bone morphogenetic protein signaling and suppresses hepcidin expression. In this study, we examined the trafficking and processing of HJV. Cellular HJV reached the plasma membrane without obtaining complex oligosaccharides, indicating that HJV avoided Golgi processing. Secreted HJV, in contrast, has complex oligosaccharides and can be derived from HJV with high-mannose oligosaccharides at the plasma membrane. Our results support a model in which retrograde trafficking of HJV before cleavage is the predominant processing pathway. Release of HJV requires it to bind to the transmembrane receptor neogenin. Neogenin does not, however, play a role in HJV trafficking to the cell surface, suggesting that it could be involved either in retrograde trafficking of HJV or in cleavage leading to HJV release.
Introduction
Iron is an indispensable nutrient in most organisms but is also toxic when in excess. Iron homeostasis is maintained by an elegant control mechanism that coordinates iron absorption from the intestine, iron recycling from senescent red blood cells, and mobilization of iron stores from liver hepatocytes. Hemojuvelin (HJV) is central to this process. HJV is a glycosylphosphatidylinositol (GPI)–linked protein and has Asn-linked glycosylation sites in its extracellular domain.1 It is mainly expressed in muscle and, to a lesser extent, in the liver.1,2 Clinical studies demonstrated that homozygous or compound heterozygous mutations in the HJV gene (HFE2) lead to juvenile hemochromatosis (JH), a severe iron overload disorder, indicating that HJV plays an important role in the regulation of iron homeostasis.2
HJV regulates serum iron levels by modulating expression of hepcidin, a hepatocyte-derived peptide hormone. The marked suppression of hepcidin expression in JH patients and HJV knockout mice indicates that HJV is a critical upstream regulator of hepcidin expression.2-4 Hepcidin regulates serum iron levels by decreasing iron efflux from intestinal epithelial cells, macrophages, and hepatocytes.2-5 Thus, HJV activates transcription of hepcidin, which decreases serum iron levels by limiting iron efflux.
There are 2 forms of HJV: a membrane-anchored GPI-linked form and a secreted soluble form (sHJV) that is generated by furin-mediated cleavage of GPI-HJV.1,5-9 Both forms of HJV regulate hepcidin transcription and iron metabolism, although they have opposite effects. GPI-linked HJV increases transcription of hepcidin through the bone morphogenetic protein (BMP)–signaling pathway by acting as a coreceptor for BMP ligands.10-12 Disruption of BMP signaling by hepatocyte-specific knockout of Smad4, a central mediator of the BMP-signaling pathway, results in decreased hepcidin expression and iron overload in mice.13 Conversely, sHJV decreases the level of hepcidin mRNA in primary human hepatocytes.10 Moreover, injection of sHJV into mice decreases BMP signaling and hepcidin expression and increases the amount of serum and liver iron.14 sHJV could antagonize BMP signaling by competing with membrane-associated HJV for binding to BMP ligands, preventing them from interacting with cell-associated HJV and therefore inhibiting hepcidin expression.10,14 Because the GPI-linked and soluble forms of HJV have opposing roles, regulation of HJV processing is important for the control of iron homeostasis.
Generation of sHJV requires neogenin, a transmembrane receptor in the immunoglobulin superfamily.15 HJV binds to neogenin,7,16,17 specifically to the membrane-proximal fifth and sixth fibronectin type III (FNIII) domains.16 Knockdown of neogenin blocks HJV release but does not affect trafficking of HJV to the plasma membrane.18 Neogenin is unable to interact with the G320V mutant form of HJV, the most common disease-causing mutation in type 2A JH patients.2,7 Although neogenin is necessary for HJV release, the role it plays in this process is not known.
HJV is endocytosed through a cholesterol-dependent and dynamin-independent pathway.18 Endocytosis of HJV is blocked by filipin, which depletes cholesterol and has been shown to block the endocytosis of other GPI-linked proteins.18-20 Filipin also blocks generation of sHJV.18
In the current study, we sought to understand how HJV trafficking leads to its release and investigate how neogenin affects this process. Using a hepatic cell line as a model system, we showed that HJV trafficked to the plasma membrane without acquiring complex oligosaccharides and that neogenin was not required for this process. Moreover, cell-surface HJV acquired complex oligosaccharides before it was released into the media. Furthermore, blocking HJV cleavage using a furin inhibitor did not lead to a buildup of Endo H–resistant HJV in the cell, suggesting that cleavage precedes complex glycosylation.
Methods
Cell culture
HepG2 and Hep3B cells were purchased from ATCC (Manassas, VA) and maintained in minimum essential medium (MEM)/10% fetal calf serum (FCS)/1 mM pyruvate/1× nonessential amino acids (complete medium). Human embryonic kidney 293 (HEK293) cells (ATCC) were grown in Dulbecco modified Eagle medium/10% FCS/1 mM pyruvate. HepG2 cells stably transfected with wild-type HFE2 (HJV), G320V mutant HFE2 (G320V), or pcDNA3 empty vector (control) were generated previously.15 The stably transfected cells were maintained in complete medium with 800 μg/mL G418. tTA-HJV-HepG2 cells were generated by cloning HFE2 into a tetracycline-inducible pcDNA4 vector, which was then stably transfected into tTA-HepG2 cells.21 They were maintained in complete medium with 800 μg/mL G418 and 5 μg/mL blasticidin and induced to express HJV using 2 μg/mL doxycycline. Neogenin/HJV-HEK293 cells, stably expressing both neogenin and HJV, were maintained in Dulbecco modified Eagle medium with 10% FCS and 800 μg/mL G418.
Endo H and PNGase F digestion
Endo H and PNGase F were used to analyze the Asn-linked oligosaccharides on HJV and neogenin protein in cell lysate, conditioned medium, and streptavidin bead eluate. Briefly, samples prepared were incubated with Endo H or PNGase F (New England Biolabs, Ipswich, MA) according to the manufacturer's instruction. After 4 hours of incubation at 37°C, the digested samples were subjected to Western blot analysis of HJV and neogenin as described for immunodetection.
Immunodetection
Cell lysates, conditioned medium, or streptavidin eluates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, followed by transfer onto a nitrocellulose membrane. Membranes were probed with affinity-purified rabbit anti-HJV antibody (0.22 μg/mL; generated against residues 1-401 of HJV as described previously18 ), rabbit anti-neogenin antibody (0.4 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-Tf serum (generated against purified human Tf, 1:10 000), or mouse anti–β-actin antibody (1:10 000; Chemicon International, Temecula, CA), followed by immunodetection using a corresponding horseradish peroxidase–conjugated secondary antibody (Chemicon International) and chemiluminescence (Super Signal; Pierce Chemical, Rockford, IL). Alternatively, HJV was detected using an Alexa Fluor 680 goat anti-rabbit secondary antibody (1:10 000; Invitrogen, Carlsbad, CA) and visualized using an Odyssey Infrared Imaging System (Licor, Lincoln, NE).
Results
Release of sHJV constitutes the major pathway of cellular HJV turnover
HJV exists in either cell-associated or soluble forms. Soluble HJV is generated by furin-mediated cleavage of cellular HJV.8,9 Because the soluble and cell-associated forms of HJV have opposing effects on iron homeostasis, we analyzed the trafficking and processing of HJV. HepG2 cells stably expressing human HJV (HJV-HepG2) were used as a model system. HepG2 cells are a human hepatoma cell line, which express many proteins involved in iron metabolism but do not express HJV detectably by immunoblot.15,22 HJV-HepG2 cells express HJV, which undergoes active release.15
The kinetics of the loss of cell-associated HJV and the appearance of HJV in the media was examined. HJV-HepG2 cells were treated with cycloheximide (100 μg/mL) to block protein synthesis, and HJV levels in the cell lysate or one-third of conditioned medium at 0, 1, 2, 4, and 6 hours were detected by immunoblot. HepG2 cells transfected with an empty vector were used as a control for the specificity of the antibody against HJV. The amount of HJV in the cell lysate exhibited a rapid decrease over time (Figure 1A). Conversely, sHJV in the media was detected starting at 1 hour and continued to accumulate for at least 6 hours implicating a precursor-product relationship. The 2 bands seen for secreted HJV are consistent with the literature.6,15
Release of cellular HJV. (A) Analysis of cell-associated and secreted HJV after blocking protein synthesis. HJV-HepG2 cells in 12-well plates were incubated in 400 μL complete medium with 100 μg/mL cycloheximide (CHX) to block protein synthesis. After 0, 1, 2, 4, and 6 hours of incubation, conditioned medium (CM) was collected, and cell lysate (L) was prepared using 100 μL NET-Triton buffer (150 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 10 mM Tris, pH 7.4, and 1% Triton X-100) with 1× Protease inhibitors cocktail (Roche Diagnostics, Indianapolis, IN). Each time point was performed in duplicate. Western blotting was performed on the total lysates and one-third of conditioned medium using rabbit anti-HJV (0.22 μg/mL) and mouse anti–beta-actin (1:10 000) (lysates only). Control-HepG2 cells (Ctrl) were used as a negative control. HJV is approximately 50 kDa in the cell lysates and 38 kDa in the CM. β-Actin was used as a loading control for the cell lysates. (B) Pulse-chase analysis of cellular HJV. Cells were metabolically labeled with 35S-(Met/Cys) (PerkinElmer Life and Analytical Sciences, Waltham, MA) at 100 μCi/mL in minimum essential medium without met/cys for 30 minutes, washed and then incubated in regular growth medium for 0, 0.5, 1, 2, 3, 4, 5, and 6 hours. Cell lysates were then collected and immunoprecipiated using rabbit anti-HJV 18745 antibody. Immunoprecipiated proteins were washed and separated by SDS-PAGE. Image was obtained by exposure to x-ray film. (C) Analysis of cellular and secreted HJV after induction of HJV synthesis. Expression of HJV in tTA-HJV-HepG2 was induced by addition of 2 μg/mL doxycycline into the culture medium. The entire cell lysate (L) and one-third of CM after 0, 1, 2, 4, 6, 8, and 10 hours were subjected to Western blots for HJV in cell lysates and medium and β-actin in lysates. Dox indicates doxycycline treated. (D) Contribution of lysosomal degradation to HJV turnover. HJV-HepG2 cells in 12-well plates were incubated in presence of 100 μg/mL cycloheximide with or without addition of 100 nM bafilomycin A (Baf) for 0, 1, 2, 3, 4, 5, and 6 hours. Proteins from whole-cell lysates (L) and media (CM) precipitated with 6% trichloroacetic acid (TCA) were subjected to immunodetection. Experiments were repeated 3 times with consistent results.
Release of cellular HJV. (A) Analysis of cell-associated and secreted HJV after blocking protein synthesis. HJV-HepG2 cells in 12-well plates were incubated in 400 μL complete medium with 100 μg/mL cycloheximide (CHX) to block protein synthesis. After 0, 1, 2, 4, and 6 hours of incubation, conditioned medium (CM) was collected, and cell lysate (L) was prepared using 100 μL NET-Triton buffer (150 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 10 mM Tris, pH 7.4, and 1% Triton X-100) with 1× Protease inhibitors cocktail (Roche Diagnostics, Indianapolis, IN). Each time point was performed in duplicate. Western blotting was performed on the total lysates and one-third of conditioned medium using rabbit anti-HJV (0.22 μg/mL) and mouse anti–beta-actin (1:10 000) (lysates only). Control-HepG2 cells (Ctrl) were used as a negative control. HJV is approximately 50 kDa in the cell lysates and 38 kDa in the CM. β-Actin was used as a loading control for the cell lysates. (B) Pulse-chase analysis of cellular HJV. Cells were metabolically labeled with 35S-(Met/Cys) (PerkinElmer Life and Analytical Sciences, Waltham, MA) at 100 μCi/mL in minimum essential medium without met/cys for 30 minutes, washed and then incubated in regular growth medium for 0, 0.5, 1, 2, 3, 4, 5, and 6 hours. Cell lysates were then collected and immunoprecipiated using rabbit anti-HJV 18745 antibody. Immunoprecipiated proteins were washed and separated by SDS-PAGE. Image was obtained by exposure to x-ray film. (C) Analysis of cellular and secreted HJV after induction of HJV synthesis. Expression of HJV in tTA-HJV-HepG2 was induced by addition of 2 μg/mL doxycycline into the culture medium. The entire cell lysate (L) and one-third of CM after 0, 1, 2, 4, 6, 8, and 10 hours were subjected to Western blots for HJV in cell lysates and medium and β-actin in lysates. Dox indicates doxycycline treated. (D) Contribution of lysosomal degradation to HJV turnover. HJV-HepG2 cells in 12-well plates were incubated in presence of 100 μg/mL cycloheximide with or without addition of 100 nM bafilomycin A (Baf) for 0, 1, 2, 3, 4, 5, and 6 hours. Proteins from whole-cell lysates (L) and media (CM) precipitated with 6% trichloroacetic acid (TCA) were subjected to immunodetection. Experiments were repeated 3 times with consistent results.
To more precisely determine the rate of disappearance of HJV from the cell, a pulse-chase experiment was performed. HJV-HepG2 cells were metabolically labeled with 35S-(Met/Cys) for 30 minutes, then incubated with complete media for 0, 0.5, 1, 2, 3, 4, 5, and 6 hours (Figure 1B). HJV was then immunoprecipitated from cell lysates and analyzed by SDS-PAGE. HJV disappeared from the cell lysate quickly with a turnover of approximately 1 hour, consistent with the cycloheximide results (Figure 1A).
As an independent estimate of the approximate time interval between HJV synthesis in the endoplasmic reticulum (ER) and HJV release into the media, we generated a tTA-HJV-HepG2 cell line in which the expression of HJV is under the tight control of the tTA-inducible promoter. HJV expression was induced using doxycycline. Cellular HJV was detectable by Western blot at 4 hours after induction, whereas secreted HJV was evident in the medium at 6 hours after induction (Figure 1C). These results indicate that HJV release into the media occurs within 2 hours of synthesis.
Because cellular HJV turnover could also result from lysosomal degradation of HJV, HJV-HepG2 cells were treated with 100 μg/mL cycloheximide with or without 100 nM bafilomycin A to determine the relative contributions of sHJV release and intracellular degradation to the disappearance of HJV from cells. Bafilomycin A is an inhibitor of the vacuolar H+-ATPase, which dissipates the pH gradient in the intracellular organelles and blocks protein degradation in lysosomes.23 After 0, 1, 2, 3, 4, 5, and 6 hours of incubation at 37°C, HJV in the cell lysate and in the media was detected by Western blot. Only a slight increase in the accumulation of cellular HJV was noticeable at 3 and 4 hours when bafilomycin A was present (Figure 1D). Bafilomycin A did not significantly alter HJV release. These results suggest that, although lysosomal turnover of HJV is detectable, release of sHJV is the major mechanism of HJV loss from cells.
Distinct processing of HJV and neogenin
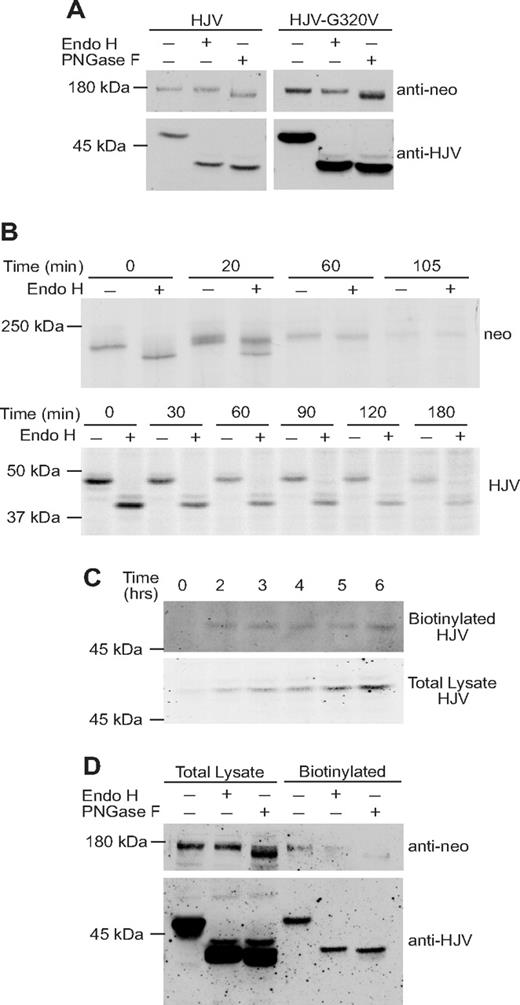
HJV is modified after synthesis by addition of a GPI anchor. GPI-linked proteins are synthesized in the ER where they are linked to a GPI anchor before being targeted to the plasma membrane. In addition to acquiring a GPI anchor, HJV is a glycoprotein with 3 potential Asn-linked glycosylation sites. To investigate the routes of HJV and neogenin trafficking, we analyzed the maturation of Asn-linked oligosaccharides on both HJV and neogenin by digestion with either Endo H, which cleaves high mannose oligosaccharides added cotranslationally in the ER, or PNGase F, which cleaves both high-mannose and Golgi-modified complex oligosaccharides. In these experiments, extracts from HJV-HepG2 cells were digested with Endo H or with PNGase F (Figure 2A). A shift in the molecular weight of the HJV and neogenin, as detected by Western blot analysis, indicates that oligosaccharides have been removed. Neogenin was sensitive to PNGase F but resistant to Endo H, indicating that it obtained complex oligosaccharides during its transit through the Golgi (Figure 2A). Cellular HJV was unexpectedly found to be sensitive to both Endo H and PNGase F, indicating that it has high mannose oligosaccharides. The pattern of glycosylation of wild-type HJV was identical to the G320V mutant HJV, which does not associate with neogenin and remains primarily in the ER.7,24 These results suggest that cellular HJV has high-mannose oligosaccharides, which have not been processed in the Golgi, whereas cellular neogenin obtains complex oligosaccharides in the Golgi.
Cell-associated HJV has high-mannose oligosaccharides, whereas cellular neogenin has complex oligosaccharides. (A) Endo H and PNGase F sensitivity of cellular HJV and cellular neogenin. Cell lysate was collected from HJV-HepG2 (HJV) cells (transfected with a nonspecific siRNA) or HJV-G320V-HepG2 (HJV-G320V) cells. Lysates were subjected to Endo H and PNGase F digestion. Immunoblots were performed using anti-neogenin (0.4 μg/mL; Santa Cruz Biotechnology) and anti-HJV antibodies (0.22 μg/mL). (B) Analysis of Endo H sensitivity of neogenin and HJV by pulse chase (in neogenin/HJV-HEK293 and HJV-HepG2 cells, respectively). Metabolic labeling and immunoprecipitations were performed as described previously39 with the following modifications: HJV-HepG2 (bottom panel) or neogenin/HJV-HEK293 (top panel) cells in 35-mm dishes were labeled in 1 mL Met/Cys-free media with 100 μCi 35S-(Met/Cys) for 30 minutes. Cells were then washed and incubated in unlabeled medium for the time points indicated. Immunoprecipitations were performed using 2 μL rabbit anti-HJV antibody, 18745 (generated against residues 1-401 of HJV as described previously18 ), or rabbit anti-neogenin 21567 antibody, which was generated using the neogenin ectodomain as an antigen (purified as described previously16 ) to generate a polyclonal antibody in rabbits (Pocono Rabbit Farm & Laboratory, Canadensis, PA). Immunoprecipitated proteins were subjected to control (mock) or Endo H digestion and separated by SDS-PAGE, followed by soaking of the gel in Amplify (GE Healthcare, Chalfont St Giles, United Kingdom) and drying of the gels before exposure to film. (C) Analysis of time taken for HJV to traffic to the cell surface after induction of HJV expression. tTA-HJV-HepG2 cells in 60-mm dishes were induced to express HJV by addition of 2 μg/mL doxycycline for 0, 2, 3, 4, 5, and 6 hours. Cell-surface proteins were biotinylated at 4°C and pulled down using streptavidin agarose. A total of 100% of the total biotinylated proteins and 15% of the internal (nonbiotinylated) proteins were subjected to immunoblotting for HJV using a rabbit anti-HJV 18746 antibody. This experiment was repeated once with similar results. (D) Cell-surface HJV has high-mannose oligosaccharides. Biotinylation of cell-surface proteins was conducted as described previously.40 Briefly, HJV-HepG2 cells in a 6-well plate at approximately 80% confluence were biotinylated with 0.25 mg/mL Sulfo-NHS-Biotin (Thermo Electron, Waltham, MA) at 4°C for 30 minutes. Cells were immediately solubilized in NET-Triton/1× Protease inhibitor cocktail; then biotinylated proteins were isolated using streptavidin agarose beads (Thermo Electron). Bound proteins were eluted with NET-Triton/1% β-mercaptoethanol/0.5% SDS and subjected to digestion with Endo H and PNGase F (New England Biolabs), followed by immunodetection of HJV and neogenin. Data are representative of 3 independent experiments.
Cell-associated HJV has high-mannose oligosaccharides, whereas cellular neogenin has complex oligosaccharides. (A) Endo H and PNGase F sensitivity of cellular HJV and cellular neogenin. Cell lysate was collected from HJV-HepG2 (HJV) cells (transfected with a nonspecific siRNA) or HJV-G320V-HepG2 (HJV-G320V) cells. Lysates were subjected to Endo H and PNGase F digestion. Immunoblots were performed using anti-neogenin (0.4 μg/mL; Santa Cruz Biotechnology) and anti-HJV antibodies (0.22 μg/mL). (B) Analysis of Endo H sensitivity of neogenin and HJV by pulse chase (in neogenin/HJV-HEK293 and HJV-HepG2 cells, respectively). Metabolic labeling and immunoprecipitations were performed as described previously39 with the following modifications: HJV-HepG2 (bottom panel) or neogenin/HJV-HEK293 (top panel) cells in 35-mm dishes were labeled in 1 mL Met/Cys-free media with 100 μCi 35S-(Met/Cys) for 30 minutes. Cells were then washed and incubated in unlabeled medium for the time points indicated. Immunoprecipitations were performed using 2 μL rabbit anti-HJV antibody, 18745 (generated against residues 1-401 of HJV as described previously18 ), or rabbit anti-neogenin 21567 antibody, which was generated using the neogenin ectodomain as an antigen (purified as described previously16 ) to generate a polyclonal antibody in rabbits (Pocono Rabbit Farm & Laboratory, Canadensis, PA). Immunoprecipitated proteins were subjected to control (mock) or Endo H digestion and separated by SDS-PAGE, followed by soaking of the gel in Amplify (GE Healthcare, Chalfont St Giles, United Kingdom) and drying of the gels before exposure to film. (C) Analysis of time taken for HJV to traffic to the cell surface after induction of HJV expression. tTA-HJV-HepG2 cells in 60-mm dishes were induced to express HJV by addition of 2 μg/mL doxycycline for 0, 2, 3, 4, 5, and 6 hours. Cell-surface proteins were biotinylated at 4°C and pulled down using streptavidin agarose. A total of 100% of the total biotinylated proteins and 15% of the internal (nonbiotinylated) proteins were subjected to immunoblotting for HJV using a rabbit anti-HJV 18746 antibody. This experiment was repeated once with similar results. (D) Cell-surface HJV has high-mannose oligosaccharides. Biotinylation of cell-surface proteins was conducted as described previously.40 Briefly, HJV-HepG2 cells in a 6-well plate at approximately 80% confluence were biotinylated with 0.25 mg/mL Sulfo-NHS-Biotin (Thermo Electron, Waltham, MA) at 4°C for 30 minutes. Cells were immediately solubilized in NET-Triton/1× Protease inhibitor cocktail; then biotinylated proteins were isolated using streptavidin agarose beads (Thermo Electron). Bound proteins were eluted with NET-Triton/1% β-mercaptoethanol/0.5% SDS and subjected to digestion with Endo H and PNGase F (New England Biolabs), followed by immunodetection of HJV and neogenin. Data are representative of 3 independent experiments.
To compare the kinetics of neogenin and HJV trafficking through the biosynthetic pathway, pulse-chase experiments were performed for neogenin and HJV. Neogenin/HJV-HEK293 cells, which stably express neogenin, were used because the level of endogenous neogenin in HJV-HepG2 cells is insufficient for visualization by pulse chase. Neogenin/HJV-HEK293 cells were metabolically labeled for 30 minutes and then incubated in complete medium for 0, 20, 60, and 105 minutes. The samples were immunoprecipitated with an anti-neogenin antibody and then digested with Endo H or mock-digested. Neogenin was completely Endo H–sensitive at the conclusion of the 30-minute pulse, after which a chase was initiated. At 20 minutes after the chase, some of the neogenin is Endo H–sensitive and some is resistant, and by 60 minutes it is completely Endo H–resistant (Figure 2B top panel), indicating that it has progressed through the Golgi. To analyze the Endo H sensitivity of HJV, HJV-HepG2 cells were labeled for 30 minutes and then chased for 0, 30, 60, 90, 120, and 180 minutes and immunoprecipitated. HJV remained Endo H–sensitive in the cell lysate for at least 3 hours after the 30-minute labeling (Figure 2B bottom panel).
To determine whether HJV reaches the cell surface within the time period in which it remains Endo H–sensitive, we induced HJV expression in tTA-HJV-HepG2 cells for 0, 2, 3, 4, 5, and 6 hours and then detected biotinylated cell-surface HJV by Western blot (Figure 2C). HJV reaches the cell surface within 2 hours of synthesis and did not further accumulate on the cell surface. These results indicate that HJV remains Endo H–sensitive throughout its biosynthetic pathway to the cell surface (Figure 2B bottom panel).
Because HJV remains Endo H–sensitive for at least 3 hours and HJV reaches the cell surface within 2 hours, we next examined the Asn-linked glycosylation status of cell-surface HJV and neogenin. Cell-surface proteins were biotinylated at 4°C followed by a streptavidin pull-down and Endo H or PNGase F digestion (Figure 2D). Cell-surface HJV and neogenin had similar Asn-linked glycosylation patterns as they did in the whole-cell lysates (Figure 2B). HJV therefore did not obtain complex oligosaccharides in the Golgi, indicating that it is inaccessible to Golgi-resident glycosylases en route to the cell surface.
Cell-surface HJV undergoes retrograde trafficking before being secreted
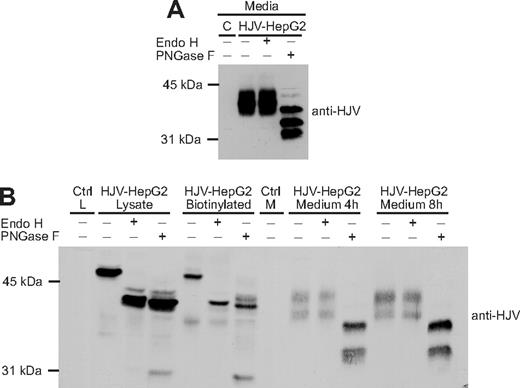
Because our studies indicated that the oligosaccharides on the GPI-linked form of HJV are not processed to a complex form, we examined the glycosylation pattern of the secreted form of HJV. The secreted HJV in the media was subjected to Endo H and PNGase F digestion, followed by detection of HJV by Western blot. Interestingly, the secreted HJV was resistant to Endo H but sensitive to PNGase F (Figure 3A), indicating that the secreted form of HJV acquired complex oligosaccharides before it was released. These results raised 2 possibilities. HJV may obtain complex oligosaccharides before being secreted by undergoing retrograde trafficking to the trans-Golgi network (TGN) and Golgi, where its oligosaccharides would be processed to a complex form and HJV could be cleaved by furin. Alternatively, secreted HJV may represent a subset of HJV that obtains complex oligosaccharides and is cleaved in the Golgi en route to the cell surface.
Secreted HJV has complex oligosaccharides and is derived from the pool of HJV at the plasma membrane. (A) Secreted HJV has complex oligosaccharides. Conditioned medium was collected from HJV-HepG2 cells and subjected to Endo H and PNGase F digestion, followed by a Western blot using a rabbit anti-HJV antibody. Conditioned medium from control-HepG2 cells (C) was included as a negative control. (B) Chasing the release of biotinylated cell surface HJV. Cell-surface HJV in HJV-HepG2 cells was biotinylated at 4°C, followed by incubation at 37°C for 4 or 8 hours in complete medium. The total biotinylated HJV in the cell lysate (HJV-HepG2 biotinylated) and the biotinylated HJV released into the medium were isolated using streptavidin-agarose beads. The eluates were digested with Endo H and PNGase F. Endo H and PNGase F digestion of one-third of cell lysates are also included. Lysate (Ctrl L) or medium (Ctrl M) from Control-HepG2 cells were used as negative controls. Experiments were repeated 3 times.
Secreted HJV has complex oligosaccharides and is derived from the pool of HJV at the plasma membrane. (A) Secreted HJV has complex oligosaccharides. Conditioned medium was collected from HJV-HepG2 cells and subjected to Endo H and PNGase F digestion, followed by a Western blot using a rabbit anti-HJV antibody. Conditioned medium from control-HepG2 cells (C) was included as a negative control. (B) Chasing the release of biotinylated cell surface HJV. Cell-surface HJV in HJV-HepG2 cells was biotinylated at 4°C, followed by incubation at 37°C for 4 or 8 hours in complete medium. The total biotinylated HJV in the cell lysate (HJV-HepG2 biotinylated) and the biotinylated HJV released into the medium were isolated using streptavidin-agarose beads. The eluates were digested with Endo H and PNGase F. Endo H and PNGase F digestion of one-third of cell lysates are also included. Lysate (Ctrl L) or medium (Ctrl M) from Control-HepG2 cells were used as negative controls. Experiments were repeated 3 times.
To determine whether retrograde trafficking occurs, cell-surface proteins were labeled with sulfo-NHS-biotin, a cell-impermeant form of biotin. After 0, 4, and 8 hours of incubation at 37°C, the conditioned medium and cell lysates were collected. The biotinylated proteins in the cell lysate at 0 hours (total biotinylated) and in the media at 4 and 8 hours were isolated with streptavidin beads and subjected to Endo H or PNGase F digestion, followed by immunodetection of HJV. Biotinylated cell-surface HJV was subsequently detected in the media, indicating that secreted HJV can be derived from the pool of HJV at the cell-surface. The secreted HJV derived from the cell surface was Endo H–resistant (Figure 3B).
This finding suggests that cell-surface HJV undergoes retrograde transport to an intracellular compartment where the oligosaccharides are modified. Furthermore, the change in size from 50 kDa in the cell lysates to 38 kDa in the conditioned medium indicates that HJV is cleaved before being released. The 2 species of secreted HJV are not caused by differential N-linked glycosylation because both species are still present after PNGase F digestion, indicating that the difference between the species is the result of either another posttranslational modification or to cleavage at more than one site. There is variability in the number of bands seen for secreted HJV. Multiple cleaved bands in the conditioned medium have previously been shown.6,15,24 In addition, HJV has an acidic autocatalytic cleavage site, which could account for the lowest molecular mass of the multiple cleavage products seen in Figure 3A.1,7
Because the formation of complex oligosaccharides occurs in the Golgi/TGN compartment, these results suggest that HJV release requires retrograde trafficking of HJV from the cell surface to the Golgi/TGN where it acquires complex oligosaccharides and is exposed to furin cleavage before it is secreted. Because no HJV with complex oligosaccharides is detected in the cell lysate, we hypothesize that once HJV obtains complex oligosaccharides, it is rapidly secreted.
Secreted HJV has complex oligosaccharides in multiple cell types
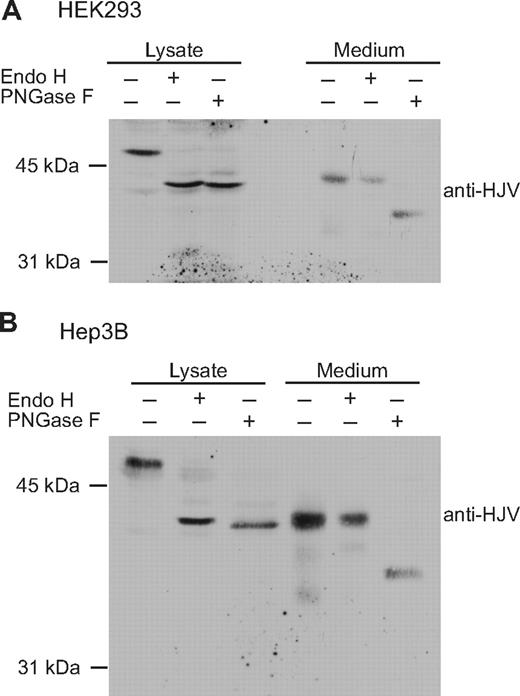
The release of sHJV with complex oligosaccharides could be specific to HepG2 cells. To test this possibility, the glycosylation status of HJV was analyzed in HEK293 and Hep3B cells. HEK293 cells are derived from human embryonic kidney, and Hep3B is a relatively undifferentiated human hepatoma cell line. Neither endogenously expresses detectable HJV by immunoblot analysis. HJV was transiently transfected into both cell lines. Proteins from cell lysates and media were subjected to Endo H and PNGase F digestions. Consistent with the findings in HepG2 cells (Figure 2A), cellular HJV in both Hep3B and HEK293 cells was Endo H–sensitive, whereas the secreted HJV was Endo H–resistant (Figure 4). Thus, complex oligosaccharides on secreted HJV occur in several cell types.
The glycosylation patterns of cellular and secreted HJV in HEK293 and Hep3B cells. (A) HEK293 cells were transiently transfected with wild-type HFE2-pcDNA3 using Lipofectamine 2000 (Invitrogen) in 6-well plates and incubated in complete medium. Approximately 48 hours after transfection, serum-free medium (1 mL) was added. After an additional 24 hours of incubation, conditioned medium was collected and cell lysate was prepared using 300 μL NET-Triton buffer with Protease inhibitor cocktail. Proteins in the medium (1 mL) were precipitated using 6% TCA. Proteins from both the cell lysate (Lysate) and medium were equally divided into 3 parts and were subjected to Endo H and PNGase F digestion followed by detection of HJV by immunoblot. (B) Hep3B cells were transiently transfected with HFE2-pcDNA3 using LipoFectamine 2000. The transfection of HFE2 into Hep3B cells, Endo H and PNGase F digestion, and immunodetection were performed as described for HEK293 cells. There was a better separation on the gel for Hep3B cells, which accounts for the apparent increased separation between the control and digested samples in this cell type. The data are representative of 3 experiments for each cell type.
The glycosylation patterns of cellular and secreted HJV in HEK293 and Hep3B cells. (A) HEK293 cells were transiently transfected with wild-type HFE2-pcDNA3 using Lipofectamine 2000 (Invitrogen) in 6-well plates and incubated in complete medium. Approximately 48 hours after transfection, serum-free medium (1 mL) was added. After an additional 24 hours of incubation, conditioned medium was collected and cell lysate was prepared using 300 μL NET-Triton buffer with Protease inhibitor cocktail. Proteins in the medium (1 mL) were precipitated using 6% TCA. Proteins from both the cell lysate (Lysate) and medium were equally divided into 3 parts and were subjected to Endo H and PNGase F digestion followed by detection of HJV by immunoblot. (B) Hep3B cells were transiently transfected with HFE2-pcDNA3 using LipoFectamine 2000. The transfection of HFE2 into Hep3B cells, Endo H and PNGase F digestion, and immunodetection were performed as described for HEK293 cells. There was a better separation on the gel for Hep3B cells, which accounts for the apparent increased separation between the control and digested samples in this cell type. The data are representative of 3 experiments for each cell type.
Blocking the interaction of HJV with neogenin does not alter the glycosylation status of cellular HJV
HJV could traffic through the Golgi and be protected from oligosaccharide modification and furin cleavage by binding to a chaperone protein. Because neogenin is known to bind to HJV, we sought to determine whether neogenin was necessary for HJV to maintain high-mannose oligosaccharides in the cell lysates. Endogenously expressed neogenin in HJV-HepG2 cells was knocked down using a small-interfering RNA (siRNA) specific for neogenin. After knockdown, neogenin was not detectable by Western blot (Figure 5A). For the parallel control transfected with scrambled siRNA, see Figure 2A, which is from the same blot and exposure. Endo H and PNGase F digestions revealed that knockdown of neogenin does not affect the glycosylation pattern of HJV. This result as well as the finding that knockdown of neogenin had no effect on HJV trafficking to the plasma membrane18 ruled out the possibility that neogenin serves as a chaperone to protect HJV from modification in the Golgi/TGN in the biosynthetic pathway.
Neogenin is necessary for sHJV release but not for the retention of high-mannose oligosaccharides by HJV. (A) Knockdown of neogenin does not alter the glycosylation of cellular HJV. Endogenous neogenin in HJV-HepG2 cells was knocked down using a siRNA specific for neogenin (for the control HJV-HepG2 cells transfected with a nonspecific control siRNA; see Figure 2A). siRNA specific for human neogenin (25 nM; Dharmacon RNA Technologies, Lafayette, CO) or scrambled control siRNA was transfected twice (once on day 1 and once on day 3) using the RNAiMAX transfection reagent (Invitrogen). Seventy-two hours after the second transfection, cell lysates were subjected to Endo H and PNGase F digestion. Both neogenin and HJV were detected by immunoblot using antineogenin and anti-HJV antibodies, respectively. (B) Disruption of the HJV/neogenin interaction inhibits release of HJV but does not perturb the high-mannose glycosylation of HJV. HJV-HepG2 cells were treated with soluble neogenin ectodomain fragments consisting of the FNIII repeats 1-6 (1 μM) or a smaller fragment of only repeats 5 and 6 (40 nM) in serum-free medium overnight. Soluble neogenin FNIII 1-6 and FNIII 5-6 as well as the whole neogenin extracellular domain (ectodomain) were generated as previously described16 and were a gift from F. Yang and P. J. Bjorkman at CalTech. Conditioned medium and cell lysates were collected, followed by Endo H and PNGase F digestion and immunodetection of HJV. Untreated HJV-HepG2 cells were used as a control (C). 1-6 indicates neogenin FNIII 1-6 fragment; 5-6, neogenin FNIII 5-6 fragment. *A nonspecific band resulting from cross reaction of either the primary or secondary antibodies with the FNIII 1-6 fragment. (C) Neogenin ectodomain inhibits HJV release and results in HJV accumulation within cells. HJV-HepG2 cells in 12-well plate were incubated in complete medium with or without addition of soluble neogenin ectodomain at 1 μM. After 24 hours of incubation, the total cell lysate (L) and 20% of conditioned medium (CM) were subjected to detection of HJV by immunoblot. Actin in cell lysates was used as a loading control. These data are representative of at least 2 experiments.
Neogenin is necessary for sHJV release but not for the retention of high-mannose oligosaccharides by HJV. (A) Knockdown of neogenin does not alter the glycosylation of cellular HJV. Endogenous neogenin in HJV-HepG2 cells was knocked down using a siRNA specific for neogenin (for the control HJV-HepG2 cells transfected with a nonspecific control siRNA; see Figure 2A). siRNA specific for human neogenin (25 nM; Dharmacon RNA Technologies, Lafayette, CO) or scrambled control siRNA was transfected twice (once on day 1 and once on day 3) using the RNAiMAX transfection reagent (Invitrogen). Seventy-two hours after the second transfection, cell lysates were subjected to Endo H and PNGase F digestion. Both neogenin and HJV were detected by immunoblot using antineogenin and anti-HJV antibodies, respectively. (B) Disruption of the HJV/neogenin interaction inhibits release of HJV but does not perturb the high-mannose glycosylation of HJV. HJV-HepG2 cells were treated with soluble neogenin ectodomain fragments consisting of the FNIII repeats 1-6 (1 μM) or a smaller fragment of only repeats 5 and 6 (40 nM) in serum-free medium overnight. Soluble neogenin FNIII 1-6 and FNIII 5-6 as well as the whole neogenin extracellular domain (ectodomain) were generated as previously described16 and were a gift from F. Yang and P. J. Bjorkman at CalTech. Conditioned medium and cell lysates were collected, followed by Endo H and PNGase F digestion and immunodetection of HJV. Untreated HJV-HepG2 cells were used as a control (C). 1-6 indicates neogenin FNIII 1-6 fragment; 5-6, neogenin FNIII 5-6 fragment. *A nonspecific band resulting from cross reaction of either the primary or secondary antibodies with the FNIII 1-6 fragment. (C) Neogenin ectodomain inhibits HJV release and results in HJV accumulation within cells. HJV-HepG2 cells in 12-well plate were incubated in complete medium with or without addition of soluble neogenin ectodomain at 1 μM. After 24 hours of incubation, the total cell lysate (L) and 20% of conditioned medium (CM) were subjected to detection of HJV by immunoblot. Actin in cell lysates was used as a loading control. These data are representative of at least 2 experiments.
To determine whether disruption of the interaction between HJV and neogenin at the cell surface affects the processing of HJV, HJV-HepG2 cells were treated with a soluble portion of the neogenin ectodomain. These soluble portions of the ectodomain compete with endogenous neogenin for binding to HJV and consequently block HJV release.18 Initially, 2 neogenin fragments were used to compete with endogenous neogenin for binding to HJV, a larger fragment comprising the FNIII repeats 1 to 6, or a smaller fragment consisting of only repeats 5 and 6. These fragments bind to HJV with 500 and 5 nM affinity, respectively.16 HJV-HepG2 cells were treated with either form of soluble neogenin overnight and conditioned medium was collected to verify that soluble neogenin blocked HJV release (Figure 5B). Cell lysates were collected and subjected to Endo H or PNGase F digestion. Cell-associated HJV was both Endo H– and PNGase F–sensitive with or without soluble neogenin (Figure 5B). Thus, cellular HJV retains high-mannose oligosaccharides independent of neogenin. When cells were incubated with the full-length ectodomain of neogenin, not only was release of sHJV blocked, but the level of cell-associated HJV also increased, indicating that generation of sHJV is a major pathway of HJV turnover in cells (Figure 5C). The interaction of HJV with full-length neogenin appears to be necessary for the processing of HJV after HJV reaches the cell surface and is consistent with the hypothesis that only HJV complexed to full-length neogenin is capable of undergoing retrograde transport for processing of HJV to the secreted form.
Filipin increases the amount of HJV in cell lysates and at the cell surface but does not affect the glycosylation status
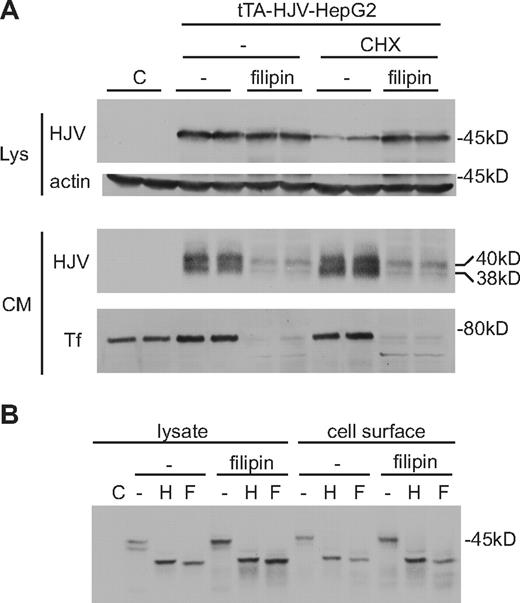
Filipin, which binds to cholesterol, has previously been shown to inhibit endocytosis of HJV and other GPI-linked proteins.18,20,25 Filipin leads to an increase in cellular HJV when protein synthesis is blocked using cycloheximide and also inhibits release of sHJV (Figure 6A). Blocking endocytosis of HJV appears to be specific as it was shown previously that filipin does not affect the endocytosis of Tf through the TfR1-mediated dynamin-dependent process.18 Blocking endocytosis of HJV using filipin leads to a mild increase in HJV on the cell surface, but it does not alter the Endo H sensitivity of HJV, indicating that filipin does not affect trafficking of HJV to the cell surface (Figure 6B).
Filipin blocks generation of sHJV but does not alter the glycosylation of cell-surface HJV. (A) Levels of cellular HJV were measured in tTA-HJV-HepG2 cells in the presence or absence of filipin and cycloheximide (CHX). Cells were grown in 6-well plates with 2 μg/mL doxycycline to induce HJV expression. Cells were treated for 2 hours in serum-free medium with or without cycloheximide (100 μg/mL) and filipin (10 μg/mL). One-half of the cell lysates (Lys) and one-fourth of the conditioned medium (CM) were subjected to Western analysis with anti-HJV and anti–β-actin or anti-Tf antibodies. Cells transfected with an empty vector (C) were used as a control for antibody specificity. (B) Analysis of cellular and cell-surface HJV by Endo H and PNGase F digestion in cells treated with filipin. Cells were treated as in panel A. For the cell lysate, 20% of a 60-mm dish was used. For the cell-surface HJV, biotinylated cell surface protein from approximately two-thirds of a 60-mm dish was used for each sample.
Filipin blocks generation of sHJV but does not alter the glycosylation of cell-surface HJV. (A) Levels of cellular HJV were measured in tTA-HJV-HepG2 cells in the presence or absence of filipin and cycloheximide (CHX). Cells were grown in 6-well plates with 2 μg/mL doxycycline to induce HJV expression. Cells were treated for 2 hours in serum-free medium with or without cycloheximide (100 μg/mL) and filipin (10 μg/mL). One-half of the cell lysates (Lys) and one-fourth of the conditioned medium (CM) were subjected to Western analysis with anti-HJV and anti–β-actin or anti-Tf antibodies. Cells transfected with an empty vector (C) were used as a control for antibody specificity. (B) Analysis of cellular and cell-surface HJV by Endo H and PNGase F digestion in cells treated with filipin. Cells were treated as in panel A. For the cell lysate, 20% of a 60-mm dish was used. For the cell-surface HJV, biotinylated cell surface protein from approximately two-thirds of a 60-mm dish was used for each sample.
In addition, cholesterol depletion has been shown to block exocytosis at the plasma membrane by disrupting SNARE cluster formation.26 Here we show that filipin inhibits secretion of Tf, consistent with recent reports showing that cholesterol is also required for insulin secretion (Figure 6A).27,28 If HJV were cleaved during the biosynthetic pathway, then an accumulation of cleaved HJV would be expected on blocking exocytosis. If endocytosis is a prerequisite for cleavage, as would be expected in the proposed retrograde trafficking pathway, then no accumulation of cleaved, 38-kDa HJV, would be expected. Filipin treatment does not lead to an accumulation of the cleaved 38-kDa species of HJV, which supports the retrograde trafficking model.
Inhibition of furin cleavage does not cause a buildup of HJV with complex oligosaccharides in the cell lysates
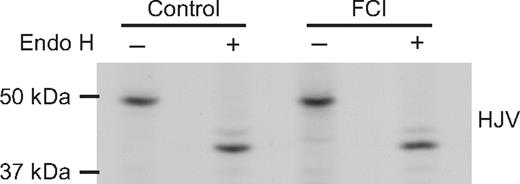
Because we hypothesize that HJV undergoes retrograde trafficking to the Golgi before being secreted, it is important to determine whether HJV obtains complex glycosylation before cleavage by furin or other proprotein convertases. To do this, we treated cells with furin convertase inhibitor (FCI), which has been shown previously to block the release of sHJV8,9 and blocks sHJV release from HJV-HepG2 cells (J.E.M. and A.-S.Z., unpublished data, 2008). Cells were treated with FCI and then metabolically labeled for 2 hours (Figure 7). Lysates were immunoprecipitated using an anti-HJV antibody and then subjected to Endo H digestion. No Endo H–resistant HJV was seen in the cell lysates, indicating that blocking furin cleavage does not lead to a buildup of complex HJV in the cell lysates. Similar results were also obtained when HJV-HepG2 cells were treated with both FCI and bafilomycin (data not shown). These data suggest that furin cleavage precedes complex glycosylation.
Addition of furin convertase inhibitor does not lead to a buildup of Endo H–resistant HJV in the cell lysate. HJV-HepG2 cells in 35-mm dishes were pretreated with no inhibitor (control) or with furin convertase inhibitor (FCI, 5 μM decanoyl-Arg-Val-Lys-Arg-chloromethylketone) for 90 minutes. Cells were then metabolically labeled for 2 hours in the presence and absence of inhibitor. Cells were washed, lysed, and immunoprecipitated using the anti-HJV antibody and subjected to Endo H digestion as described in “Methods.” These data are representative of 2 experiments.
Addition of furin convertase inhibitor does not lead to a buildup of Endo H–resistant HJV in the cell lysate. HJV-HepG2 cells in 35-mm dishes were pretreated with no inhibitor (control) or with furin convertase inhibitor (FCI, 5 μM decanoyl-Arg-Val-Lys-Arg-chloromethylketone) for 90 minutes. Cells were then metabolically labeled for 2 hours in the presence and absence of inhibitor. Cells were washed, lysed, and immunoprecipitated using the anti-HJV antibody and subjected to Endo H digestion as described in “Methods.” These data are representative of 2 experiments.
Discussion
In this study, we characterized the trafficking of HJV by analyzing the maturation of its Asn-linked oligosaccharides and its turnover. Our results indicate that the trafficking of nascent HJV from the ER to the plasma membrane avoids processing in the Golgi/TGN compartments and does not require its binding partner, neogenin. After reaching the cell surface, HJV undergoes retrograde trafficking to the Golgi/TGN where it obtains complex oligosaccharides before being released. The majority of HJV that disappeared from cells was detected in the conditioned medium, suggesting that HJV release constitutes the major pathway of cellular HJV turnover.
The release of HJV is physiologically important. Not only has it been shown to be secreted from cell lines either transfected with HJV or endogenously expressing HJV, but it can also be detected in both human and rat serum.5,6,15,24 In rats, the serum level of HJV increases in response to acute iron deprivation.15 Consistent with a role for HJV in iron homeostasis, sHJV injected into mice decreased BMP signaling and hepcidin levels.14 Both in vitro and in vivo studies demonstrate that cell-associated HJV and sHJV have opposite roles in the regulation of hepatic hepcidin expression. Cell-associated HJV is proposed to act as a coreceptor for BMPs to enhance BMP signaling and hepcidin expression,11 whereas secreted HJV may compete with cellular HJV for binding to BMPs and thereby suppresses hepcidin expression.14 Importantly, the release of sHJV is negatively regulated by iron-saturated Tf and non–Tf-bound iron.5,15,24 These data support a model in which HJV-mediated regulation of hepcidin expression is modulated by the release of HJV in response to iron loading.
Unlike many transmembrane and GPI-linked proteins, HJV appears to be capable of trafficking to the plasma membrane in the absence of Golgi processing in HepG2 cells as measured by retention of high-mannose oligosaccharides and lack of furin cleavage. Several possible pathways could be responsible for these results. First, HJV may traffic through the Golgi en route to the plasma membrane but be masked by binding to another protein and thus be inaccessible to the Golgi-resident glycosylases and proteolytic processing by furin. For example, pro–TGF-β is protected from extracellular proprotein convertase cleavage by binding to Emilin1.29 It is also possible that HJV is in a conformation that is inaccessible to glycosylases, and then it becomes accessible after it undergoes retrograde trafficking to the Golgi and is cleaved by furin, resulting in a conformational change. Alternately, HJV could bypass the Golgi altogether and traffic directly from the ER or ER Golgi intermediate compartment to the plasma membrane. Although GPI-linked proteins are known to bud from specialized transport vesicles in the ER and have specific chaperones that mediate trafficking to the plasma membrane, they are generally thought to traffic through the Golgi en route to the plasma membrane.30,31 Both GPI-linked ceruloplasmin and 5′ nucleotidase obtain complex oligosaccharides in their transit through the biosynthetic pathway.32,33 Acquisition of complex oligosaccharides by HJV therefore appears to be different from these other 2 GPI-linked proteins.
In HepG2 cells, biotinylated cell-surface HJV possesses high mannose oligosaccharides, but sHJV has complex oligosaccharides, indicating that HJV undergoes retrograde trafficking to the Golgi where its oligosaccharides are processed before release from cells. Other membrane proteins have been shown to traffic from the plasma membrane back to the TGN, including furin34 and mannose-6-phosphate receptor, as previously reviewed.35 TfR1 is capable of undergoing retrograde trafficking as far as the Golgi.36,37 Whether GPI-linked proteins are able to traffic from the plasma membrane to the Golgi is controversial. One group reported that the GPI-linked proteins, CD59 and a GPI-linked GFP, traffic from the cell surface to the Golgi; however, others reported that GPI-linked GFP traffics from the plasma membrane to recycling endosomes but not to the Golgi.19,38 Retrograde trafficking of HJV before generation of sHJV is further supported by previous data showing that cholesterol-dependent endocytosis of HJV is necessary for release.18 In the case of HJV, retrograde trafficking before the generation of sHJV may provide an opportunity for regulation of HJV release in response to extracellular factors such as transferrin.
It is possible that HJV could traffic in 2 separate pathways: one in which it undergoes retrograde trafficking before being released and another in which it is cleaved during biosynthesis. The following evidence supports that retrograde trafficking is the major, if not the only, pathway for sHJV release. First, depletion of neogenin in HepG2 cells blocks HJV release but has no effect on HJV trafficking to the cell surface.18 This suggests that HJV release does not occur on its way to the cell surface and that HJV trafficking to the plasma membrane is independent of neogenin. Second, addition of soluble neogenin ectodomain (∼ 130 kDa) to the outside of cells blocks HJV processing and results in an accumulation of cellular HJV, suggesting that HJV processing occurs after HJV reaches the cell surface. Third, disruption of the endocytosis of HJV by filipin, a cholesterol-binding agent, blocks HJV release,18 but does not block trafficking of HJV to the cell surface or alter the Endo H sensitivity of cell-surface HJV. If cleavage in the biosynthetic pathway were a major pathway of release, then a buildup of Endo H–resistant HJV would be expected when release of sHJV was blocked. This was not seen. Fourth, blocking furin cleavage does not lead to a buildup of Endo H–resistant HJV in the cell lysates. Because Endo H resistance is obtained in the medial Golgi and furin is primarily localized in the TGN and more distal compartments, this suggests that furin-mediated cleavage occurs before complex glycosylation and argues against the cleavage of HJV in the traditional biosynthetic pathway.
On the basis of the published data and the results of this study, we propose a model for HJV trafficking and processing. Nascent HJV traffics from ER to the plasma membrane and avoids modification in the Golgi/TGN in a manner that does not depend on neogenin. Upon reaching the cell surface, HJV interacts with neogenin. It then undergoes cholesterol-dependent endocytosis and retrograde trafficking to the Golgi/TGN compartments, where HJV is accessible to furin, followed by glycosylases. sHJV is then rapidly released from the cells. Trafficking of HJV to the cell surface before generation of sHJV may provide an opportunity for regulation of HJV release by iron-bound transferrin in response to changing iron levels, thus allowing HJV to modulate iron homeostasis. The details of cellular HJV trafficking and the underlying mechanism of iron-regulated HJV release remain to be determined and will be the subject of future work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Fan Yang and Pamela Bjorkman for generously providing us with neogenin ectodomain and neogenin FNIII 1-6 and FNIII 5-6 fragments, Gregory Longmore for generously giving us tTA-HepG2 cells and a tetracycline-inducible pcDNA4 plasmid, and Maja Chloupkova, Kristina Nicholson, Gary Reiness, and Juxing Chen for critical reading of this manuscript and helpful comments.
This work was supported by the National Institutes of Health (grant DK080765; A.-S.Z.). J.E.M. was partially supported by National Institutes of Health grant T32 HD049309.
National Institutes of Health
Authorship
Contribution: J.E.M. and A.-S.Z. performed experiments; and J.E.M., C.A.E., and A.-S.Z. designed the research, analyzed results, made the figures, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: An-Sheng Zhang, Department of Cell and Developmental Biology L215, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239; e-mail: zhanga@ohsu.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal