In this issue of Blood, Flaumenhaft and colleagues report that circulating MVs isolated from murine blood express markers that suggest their megakaryocytic origin. They also report that MegMVs are CD62− LAMP-1− and express full length filamin in contrast to PMVs that are CD62+ LAMP-1+ filamin−.
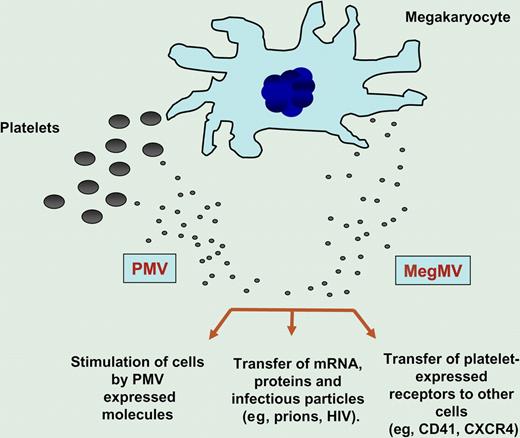
Microvesicles (MVs), small circular membrane fragments shed from the cell surface or released from the endosomal compartment, play an important and underappreciated role in cell-to-cell communication.1 This intriguing MV-mediated communication system emerged very early during evolution and served as a template for the further development of cell-to-cell interaction mechanisms involving soluble bioactive mediators and fine-tuned ligand-receptor interactions. The biologic significance of MVs has been largely overlooked for many years, and MVs were regarded just as simple cellular debris/fragments or part of apoptotic bodies. However, evidence is accumulating that these tiny membrane fragments orchestrate several biologic responses. MVs contain numerous bioactive proteins and lipids similar to those present in the membranes of the cells from which they originate. Furthermore, since they engulf some cytoplasm during membrane blebbing, they may also contain proteins and mRNA.2 Thus, MVs may stimulate target cells directly by surface-expressed ligands acting as a kind of “signaling complex.” In addition, they may transfer surface receptors from one cell to another and deliver proteins, mRNA, bioactive lipids, and even whole organelles (eg, mitochondria) into target cells. Finally, they may also serve as a vehicle to transfer infectious particles between cells, such as prions or HIV (“Trojan horse” mechanism of infection).
It is widely accepted that MVs circulating in peripheral blood originate from activated blood platelets, endothelial cells, and leukocytes. Platelet-derived microvesicles (PMVs) are released both from the platelet surface or from the endosomal compartments (exosomes) and, as shown in the figure, may (1) directly stimulate other cells (eg, hematopoietic cells, lymphocytes and endothelium),3 (2) transfer platelet expressed receptors (eg, CD41 or CXCR4)4,5 to the surface of other cells, and, in some situations, or (3) transfer mRNA, proteins, and even infectious particles (eg, prions, HIV) to the target cells. Interestingly, in healthy donors, the majority of circulating CD41+ MVs do not express surface activation marker CD62P, suggesting that they do not originate from activated platelets.
MVs are shed from both megakaryocytes (MegMVs) and platelets (PMVs). Evidence suggests that MVs may (1) stimulate target cells directly by surface-expressed ligands acting as a kind of “signaling complex,” (2) transfer surface receptors from one cell to another (eg, CD41, CXCR4), or (3) deliver proteins, mRNA, bioactive lipids, and even serve as a vehicle (“Trojan horse” mechanism) to transfer infectious particles between cells (eg, prions).
MVs are shed from both megakaryocytes (MegMVs) and platelets (PMVs). Evidence suggests that MVs may (1) stimulate target cells directly by surface-expressed ligands acting as a kind of “signaling complex,” (2) transfer surface receptors from one cell to another (eg, CD41, CXCR4), or (3) deliver proteins, mRNA, bioactive lipids, and even serve as a vehicle (“Trojan horse” mechanism) to transfer infectious particles between cells (eg, prions).
Here, Flaumenhaft et al report that a significant number of circulating CD41+ MVs in healthy individuals are derived directly from megakaryocytes (Megs). In a sequence of very elegant studies, the authors first demonstrate this via electron microscopy of spontaneous formation of Meg-derived MVs (MegMVs) from cultured murine Megs. Next, studies with cytoskeleton inhibitors reveal that the mechanism of MegMV formation is an active process different from platelet formation. Furthermore, both CD41 positive MegMVs and PMVs could be distinguished according to unique phenotype of surface markers. Accordingly, MegMVs are CD62− LAMP-1− and express full-length filamin in contrast to PMVs that are CD62+ LAMP-1+ and express little, if any, full-length filamin. Finally, direct studies on human Megs expanded from umbilical cord–blood CD34+ cells confirm that human Megs can also secrete MVs and suggest that CD41+ MVs circulating in healthy donors are similarly, as in mice, Meg-derived.
In conclusion, this article adds a complexity to our overall view on the population of MVs circulating in peripheral blood. Since new markers have been established to distinguish and potentially separate a population of CD41+ PMVs from CD41+ MegMVs, further work is needed to look for functional differences between PMVs and MegMVs. This opens a new area for further investigations. For example, in addition to differences in expression of surface makers, both types of MVs may differ in (1) mRNA content, (2) ability to stimulate target cells (eg, endothelium), (3) the role they play in supporting inflammatory reactions, and (4) complement activation. Finally, we can also envision that measuring both types of MVs in peripheral blood could have novel diagnostic and prognostic applications for several cardiovascular disorders, illnesses in which a fraction of PMVs released from activated platelets increases, changing the overall value of the MegMVs/PMVs ratio.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal