To the editor:
Formation of superoxide anion (O2−) after ionizing radiation is a major determinant of the lethality of whole-body radiation exposure.1,2 Irradiated tissues release O2− for days to months after radiation exposure.3 Extracellular superoxide dismutase (ECSOD) is a potent antioxidant enzyme catalyzing the dismutation of O2−. ECSOD has been used in gene therapy of diseases involving oxidative stress.4 Mesenchymal stem cells (MSCs) are multipotent adult stem cells from bone marrow. These cells have advantages over other stem cells in that they can be easily isolated from patients or donors, readily expanded ex vivo, and efficiently gene engineered. Therefore, MSCs hold promise as vehicles for adult stem cell–based gene therapy.5
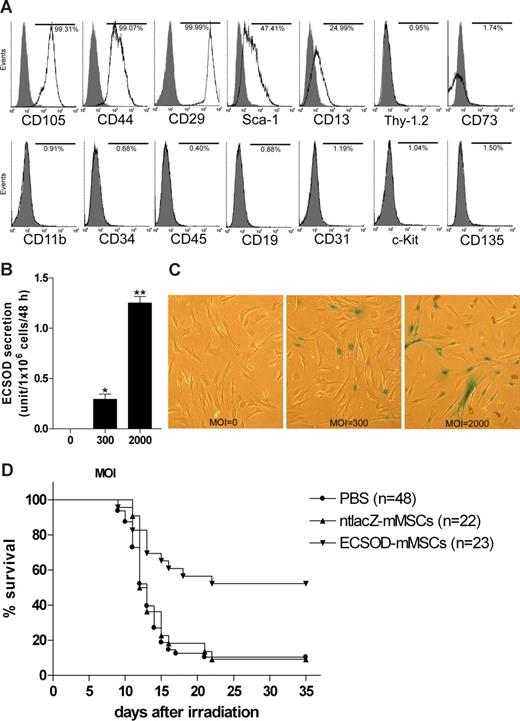
To test the hypothesis that MSCs genetically modified with ECSOD have a radioprotective effect, mouse MSCs (mMSCs) were isolated by their adherence to tissue-culture plastic from 6-week-old female BALB/c mice and ex vivo expanded as previously described.6,7 The cells were differentiated into osteoblasts and adipocytes in vitro, and cell phenotype was analyzed by flow cytometry. Figure 1A shows that the cells express CD105, CD44, CD29, stem cell antigen-1 (Sca-1), and CD13. The cells do not express CD11b, CD34, CD45, CD19, CD31, CD117 (c-Kit), CD135, CD90 (Thy-1.2), or CD73. Therefore, these cells are typical MSCs.
Radioprotective effect of mesenchymal stem cells genetically modified with extracellular superoxide dismutase. (A) Phenotype of mMSCs. Flow cytometric analysis was conducted on ex vivo–expanded mMSCs to determine the expression of CD11b, CD13, CD19, CD29, CD31, CD34, CD44, CD45, CD73, CD90 (Thy-1.2), CD105, CD117 (c-Kit), CD135, and Sca-1. Histograms show the relative intensity of mMSCs for various cell-surface antigens. Numbers indicate the percentage of cells in the population whose staining intensity with the specific antibody (white) was greater than that with the respective isotype control (gray). (B) Secretion of biologically active ECSOD by Ad5CMVECSOD-transduced mMSCs. mMSCs were transduced with Ad5CMVECSOD at multiplicity of infections (MOI, defined as plaque-forming units/cell) of 0, 300, or 2000 for 48 hours, the virus-containing culture medium was removed, and the cells were washed 3 times with PBS and further incubated in fresh culture medium for 48 hours. The culture supernatant was collected and analyzed for SOD activity using a SOD activity assay kit (Cayman Chemical Company, Ann Arbor, MI). Data were expressed as mean plus or minus SEM (n = 3) and analyzed statistically using a one-way analysis of variance (ANOVA) followed by post-hoc analysis with Tukey test. *P < .05 versus MOI 0; **P < .05 versus MOI 0 or 300. (C) Photomicrographs showing expression of nuclear-targeted β-galactosidase by Ad5CMVntlacZ-transduced mMSCs. mMSCs were transduced with Ad5CMVntlacZ at MOI 0, 300, or 2000 for 48 hours. The cells were X-gal stained for β-galactosidase activity and the blue nuclear-targeted β-galactosidase–positive Ad5CMVntlacZ-transduced mMSCs were identified. Original magnification ×40. (D) Intravenous administration of ECSOD gene-modified mMSCs improves survival of irradiated mice. Five-week-old female BALB/c mice were given 9 Gy total body γ irradiation from a 137Cs source (Gammacell 1000; MDS Nordion, Ottawa, ON) at a dose rate of 1.23 Gy/min. Twenty-four hours later, the animals were given a tail vein injection of 200 μL PBS, 0.5 × 106 ntlacZ gene-modified mMSCs (ntlacZ-mMSCs, MOI = 2000) in 200 μL PBS, or 0.5 × 106 ECSOD gene-modified mMSCs (ECSOD-mMSCs, MOI = 2000) in 200 μL PBS. Mouse survival was then monitored for 35 days. Kaplan-Meier survival curve was used for data analysis, and statistical significance was determined using log-rank test and one-way ANOVA followed by post-hoc analysis with Tukey test. P < .05 was considered statistically significant. The difference between the 3 groups was statistically significant by log-rank test (P = .002) and ANOVA (P < .001). Furthermore, P < .001 for ECSOD-mMSCs versus PBS, P < .001 for ECSOD-mMSCs versus ntlacZ-mMSCs, and P > .05 for ntlacZ-mMSCs versus PBS by Tukey test. In this study, 4 separate experiments were conducted and the result of each experiment was similar.
Radioprotective effect of mesenchymal stem cells genetically modified with extracellular superoxide dismutase. (A) Phenotype of mMSCs. Flow cytometric analysis was conducted on ex vivo–expanded mMSCs to determine the expression of CD11b, CD13, CD19, CD29, CD31, CD34, CD44, CD45, CD73, CD90 (Thy-1.2), CD105, CD117 (c-Kit), CD135, and Sca-1. Histograms show the relative intensity of mMSCs for various cell-surface antigens. Numbers indicate the percentage of cells in the population whose staining intensity with the specific antibody (white) was greater than that with the respective isotype control (gray). (B) Secretion of biologically active ECSOD by Ad5CMVECSOD-transduced mMSCs. mMSCs were transduced with Ad5CMVECSOD at multiplicity of infections (MOI, defined as plaque-forming units/cell) of 0, 300, or 2000 for 48 hours, the virus-containing culture medium was removed, and the cells were washed 3 times with PBS and further incubated in fresh culture medium for 48 hours. The culture supernatant was collected and analyzed for SOD activity using a SOD activity assay kit (Cayman Chemical Company, Ann Arbor, MI). Data were expressed as mean plus or minus SEM (n = 3) and analyzed statistically using a one-way analysis of variance (ANOVA) followed by post-hoc analysis with Tukey test. *P < .05 versus MOI 0; **P < .05 versus MOI 0 or 300. (C) Photomicrographs showing expression of nuclear-targeted β-galactosidase by Ad5CMVntlacZ-transduced mMSCs. mMSCs were transduced with Ad5CMVntlacZ at MOI 0, 300, or 2000 for 48 hours. The cells were X-gal stained for β-galactosidase activity and the blue nuclear-targeted β-galactosidase–positive Ad5CMVntlacZ-transduced mMSCs were identified. Original magnification ×40. (D) Intravenous administration of ECSOD gene-modified mMSCs improves survival of irradiated mice. Five-week-old female BALB/c mice were given 9 Gy total body γ irradiation from a 137Cs source (Gammacell 1000; MDS Nordion, Ottawa, ON) at a dose rate of 1.23 Gy/min. Twenty-four hours later, the animals were given a tail vein injection of 200 μL PBS, 0.5 × 106 ntlacZ gene-modified mMSCs (ntlacZ-mMSCs, MOI = 2000) in 200 μL PBS, or 0.5 × 106 ECSOD gene-modified mMSCs (ECSOD-mMSCs, MOI = 2000) in 200 μL PBS. Mouse survival was then monitored for 35 days. Kaplan-Meier survival curve was used for data analysis, and statistical significance was determined using log-rank test and one-way ANOVA followed by post-hoc analysis with Tukey test. P < .05 was considered statistically significant. The difference between the 3 groups was statistically significant by log-rank test (P = .002) and ANOVA (P < .001). Furthermore, P < .001 for ECSOD-mMSCs versus PBS, P < .001 for ECSOD-mMSCs versus ntlacZ-mMSCs, and P > .05 for ntlacZ-mMSCs versus PBS by Tukey test. In this study, 4 separate experiments were conducted and the result of each experiment was similar.
mMSCs were then transduced with Ad5CMVECSOD, an adenovirus carrying human ECSOD gene under the control of cytomegalovirus (CMV) promoter,8 and culture supernatant was analyzed for superoxide dismutase (SOD) activity. Figure 1B demonstrates a dose-dependent secretion of biologically active ECSOD by Ad5CMVECSOD-transduced mMSCs. mMSCs were further transduced with Ad5CMVntlacZ, an adenovirus carrying nuclear-targeted β-galactosidase gene ntlacZ under the control of CMV promoter,8 and analyzed by X-gal staining. As shown in Figure 1C, transduction efficiency is dose-dependent.
To determine whether intravenous administration of mMSCs genetically modified with ECSOD has a therapeutic effect for radiation damage, 5-week-old female BALB/c mice were given 9 Gy total body γ irradiation from a 137Cs source. Twenty-four hours later, the animals were given a tail vein injection of phosphate-buffered saline (PBS), Ad5CMVntlacZ-transduced mMSCs, or Ad5CMVECSOD-transduced mMSCs. As shown in Figure 1D, 52% of animals in the ECSOD gene-modified mMSC treatment group survived for 35 days, whereas only 9% of animals in the ntlacZ gene-modified mMSC treatment group and 10% of animals in the PBS treatment group survived for 35 days. Furthermore, all mice that survived for 35 days also survived for 5 months. These findings demonstrate for the first time that intravenous administration of MSCs genetically modified with ECSOD improves survival in irradiated mice, highlighting its clinical potential for the treatment of radiation damage resulting from a radiation accident, nuclear terrorism, and other radiologic emergencies.
Mice given 9 to 10 Gy total body irradiation die a hematologic death 10 to 14 days after exposure.9 It has been found that MSCs migrate to radiation-injured tissues such as bone marrow and gut after intravenous administration.10 Therefore, the improvement in survival of irradiated mice might result from the scavenger of O2− in the irradiated tissues such as bone marrow and gastrointestinal tract by ECSOD secreted from Ad5CMVECSOD-transduced MSCs.
Acknowledgments
We thank Richard West for assistance with flow cytometric assay and Lisa DeCamp, Dawna Dylewski, and Elissa Boguslawski for assistance with animal care and tail vein injection. This work was supported by research funding from Spectrum Health Foundation (Grand Rapids, MI) and Jay and Betty Van Andel Foundation (Grand Rapids, MI).
Authorship
Contribution: A.S.A.-M., A.J.S., D.W.P., and R.H.C. designed the research; T.A.G. and R.V.H. helped perform experiments; and W.D. designed the research and performed experiments. All authors analyzed results and wrote the paper.
Conflict-of-interest disclosure: A patent application related to the methodology described in the present work has been filed by W.D., A.S.A.-M., D.W.P., R.H.C., and A.J.S., all of whom are employees of Spectrum Health; the patent belongs to Spectrum Health. The authors declare no other competing financial interests.
Correspondence: Weiwen Deng, MD, PhD, Pediatric Blood and Bone Marrow Transplantation Program, MC185, Helen DeVos Children's Hospital, Spectrum Health, 100 Michigan Street NE, Grand Rapids, MI 49503; e-mail: weiwen.deng@devoschildrens.org.
References
Author notes
*A.S.A.-M. and A.J.S. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal