Abstract
The recessive dystrophic form of epidermolysis bullosa (RDEB) is a disorder of incurable skin fragility and blistering caused by mutations in the type VII collagen gene (Col7a1). The absence of type VII collagen production leads to the loss of adhesion at the basement membrane zone due to the absence of anchoring fibrils, which are composed of type VII collagen. We report that wild-type, congenic bone marrow cells homed to damaged skin, produced type VII collagen protein and anchoring fibrils, ameliorated skin fragility, and reduced lethality in the murine model of RDEB generated by targeted Col7a1 disruption. These data provide the first evidence that a population of marrow cells can correct the basement membrane zone defect found in mice with RDEB and offer a potentially valuable approach for treatment of human RDEB and other extracellular matrix disorders.
Introduction
Epidermolysis bullosa represents a family of severe, life-threatening skin disorders resulting from mutations in genes encoding protein components of the cutaneous basement membrane zone. Although some forms, such as the junctional type, are lethal in the neonatal period, others, such as the dystrophic forms, lead to years of painful skin blistering and mutilating scarring. The most severe form of dystrophic epidermolysis bullosa (the Hallopeau-Siemens type) is caused by recessive mutations in the type VII collagen gene (Col7A1).1
The recessive dystrophic form of epidermolysis bullosa (RDEB) is characterized by severely diminished type VII collagen (col7) production.2 The homotrimeric col7 protein is synthesized by fibroblasts and keratinocytes and represents the key component of anchoring fibrils that connect cutaneous basement membrane to the dermal matrix.3 Severe attenuation of anchoring fibrils in RDEB results in impaired dermal-epidermal cohesion and diminished adhesion of gastrointestinal mucosa at the basement membrane zone. Compromised integrity of the stratifying squamous epithelia leads to increased cutaneous and mucosal sensitivity to mechanical stress and stigmatizing, and to an eventually lethal, clinical phenotype. Children with RDEB develop painful skin and mucosal blistering, mutilating scarring, alopecia, corneal erosions, tooth decay, esophageal strictures, anemia, joint contractures, small epidermal inclusion cysts (milia), nail dystrophy, and fusion of fingers and toes (pseudosyndactyly or “mitten” deformity) by the age of 6 to 8 years. As a result of extreme skin fragility, aberrant tissue repair, and chronic inflammation, RDEB patients develop squamous cell carcinomas in the third decade of life.4
At this time, there is no therapeutic intervention with proven curative benefit. Palliative measures include complex bandaging of most of the body surface (to protect the skin from the slightest friction, and to prevent infection and excessive loss of body fluid), surgical debridement and analgesia, and nutritional support (using liquid or pureed food by mouth or via percutaneous gastric feeding tube) and analgesia. Faced with the limited impact and ultimate futility of the supportive measures, multiple attempts at designing more effective measures for local control of skin manifestations have been made. These included direct injection of col7 protein or cells, from either allogeneic wild-type source or gene-modified cells. Transgenic cells have been created using retroviral, lentiviral, transposon, ΦC31-based integrase vectors, or mini-genes.5-13 The lesions in RDEB, however, are distributed over multiple and large areas of the body, both on external (skin) and internal (gastrointestinal) sites, which makes local treatments impractical and unable to target the global pathology associated with RDEB. Thus, as these measures provide only local and temporal relief from the RDEB pathology, we wanted to design a strategy with the potential for systemic and durable correction of col7 deficiency.
To address the limitations of the current approaches in view of therapeutic considerations, we considered several lines of evidence. First, skin contains a population of easily accessible somatic stem cells similar to embryonic stem cells.14 Such epidermal stem cells and transit-amplifying cells contribute to all 3 germ layers of the embryo after injection into a blastocyst, and—critically—may express homing signals required for skin engraftment after systemic delivery. Second, recent advances in stem cell biology have shown the capacity of bone marrow (BM) to differentiate into cells and tissues of the 3 somatic lineages, leading to clinical trials for treating nonhematopoietic diseases.15 Marrow-derived stem cell populations, including mesenchymal stem cells16 and multipotent adult progenitor cells,17 have been shown to migrate to sites of injury and contribute to tissue repair.18 For example, the propensity of stromal cell populations to promote tissue healing has been shown in the settings of lung, brain, heart, and kidney injury.19-22 It has also been shown that cells in the marrow are capable of differentiation into the epithelial lineage23,24 and that after hematopoietic cell transplantation these cells are able to engraft in the skin of the recipient, especially in sites affected by graft-versus-host disease.25 Finally, directly or systemically administered BM cells have been shown to promote healing of skin wounds,26,27 which are the hallmark of RDEB.
Therefore, we hypothesized that stem cell populations from adult marrow may be capable of migrating to the integument and the internal mucosal sites, where they can modulate pathology through col7 protein production. If biochemical and phenotypic correction could be demonstrated in a relevant animal model, this would support the development of a novel therapeutic approach for children and adults with RDEB.
To test this hypothesis we used murine RDEB (Col7a1−/− mice) generated by targeted Col7a1 disruption,28 which results in widespread blistering beginning at birth due to an inability to form the anchoring fibrils needed for attachment of epidermis, mucosal layer, and basement membrane to underlying structures.28 Affected mice die within 2 weeks of birth.
Here we show that infusion of BM cells from a wild-type (Col7a1+/+) donor results in production of col7 protein, formation of anchoring fibrils, and enhanced survival in murine RDEB recipients. Our studies provide the first evidence that a systemic cellular therapy may effectively treat RDEB, and may offer a unique opportunity for treatment of other extracellular matrix disorders.
Methods
Mice
C57BL/6 (B6) mice (Col7a1−/−) were obtained from Dr Jouni Uitto. B6 green fluorescent protein (GFP) transgenic mice were obtained from Dr Jonathan Serody (University of North Carolina, Chapel Hill). Offspring of heterozygous Col7a1+/− mice were genotyped by polymerase chain reaction (PCR).28 All animal studies were approved by the University of Minnesota Institutional Animal Care and Use Committee.
Cell isolation
B6 multipotent adult progenitor cells, mesenchymal stem cells, epidermal stem cells, epidermal transit amplifying cells, and total BM were isolated using standard procedures.14,16,17,29,30 For CD150+48− enriched bone marrow cells,31 a negative selection was performed using fluorescein isothiocyanate (FITC)–conjugated anti–mouse CD48 antibody (clone HM 48-1, 0.25 μg/107 cells; eBioscience, San Diego, CA) with anti-FITC microbeads (10 μL/107 cells; Miltenyi Biotec, Auburn, CA), followed by a positive selection using FITC-conjugated anti–mouse CD150 antibody (clone 9D1, 1 μg/107 cells; eBioscience) with anti-FITC microbeads (10 μL/107 cells; Miltenyi Biotec). For flow cytometry, peripheral blood cells (obtained by a retro-orbital eye bleed and Ficoll isolation; Isolymph; Gallard-Schlesinger Industries, Plainview, NY) and BM cells (obtained by femur and tibia aspirations) were analyzed on FACScalibur (Becton Dickinson, Franklin Lakes, NJ). Cells were resuspended in 10 μL PBS and injected via facial vein.
PCR analysis
First-strand cDNA was synthesized from total RNA using oligo (dT) primer and SuperScript III RT (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Real-time PCR (RT-PCR) was performed on an ABI 7300 machine and analyzed with ABI Relative Quantification Study software (Applied Biosystems, Foster City, CA). For quantitative real-time reverse transcription–polymerase chain reaction experiments, a TaqMan gene expression assay for col7 was obtained from Applied Biosystems (probe sequence 6FAM-TCCTGAGGGGCCACCAGGACCCACT) using beta-2-microglobulin or glyceraldehyde-3-phosphate dehydrogenase as control genes. The 2−ΔΔCT method was used to generate RQ for quantifying col7 expression in samples in comparison with whole bone marrow. The formula used was RQ = {9i} (where BM is whole bone marrow).
Histology
Skin was cryopreserved in optimal cutting temperature medium (Sakura Finetek USA, Torrance, CA) at −80°C. Frozen sections (6 μm thick) were mounted on glass slides and fixed in acetone for 5 minutes at room temperature. Skin was stained for immunofluorescence using an anti-col7 antibody (1:2000; generously provided by Drs David Woodley and Mei Chen) and anti-GFP, FITC-conjugated antibody (1:400; Rockland, Gilbertsville, PA). Donkey antirabbit cy3 (1:400; Jackson Immunoresearch, West Grove, PA) was used as secondary antibody. Tissue sections were incubated with primary antibody overnight at 4°C and with secondary antibody for 1 hour at room temperature. Slides were coverslipped with nuclear stain 4′,6′diamidino-2-phenylindole (Molecular Probes, Eugene, OR) and examined by confocal microscopy. The following parameters were used: microscope: Olympus BX61 (Olympus Optical, Tokyo, Japan); objective lenses: 20×/0.7 and 40×/0.9; room temperature; imaging medium: oil-immersion; fluorochromes: GFP, Cy3; filters: FITC no. UM41001, Cy3 no. 41002; lasers: argon, green HeNe, red HeNe, DAPI blue diode; camera: RT Spot no. 2.3.1 (Diagnostic Instruments, Sterling Heights, MI); acquisition software: Flowview 500 (Olympus America, Lombard, IL).
Ultrastructural skin examination
For conventional transmission electron microscopy, small pieces of skin samples were fixed in half-strength Karnofsky fixative, followed by further fixation in 1% osmium tetroxide in distilled water. After en bloc staining with uranyl acetate, specimens were dehydrated in ethanol and embedded in Epon812 (Taab, Berkshire, United Kingdom). Ultrathin sections were stained with uranyl acetate and lead citrate.28
Data analysis
Group comparisons for survival data were made by log-rank test statistics. Differences between measurements were assessed using Student t test, with a P value less than .05 considered significant in all tests.
Results
Nonhematopoietic stem cells do not ameliorate murine RDEB
As previously reported, we noted a failure to thrive and death within approximately 2 weeks in untreated Col7a1−/− mouse pups28 (n = 18; Table 1; Figure 1A), necessitating curative attempts by early intervention. In nonconditioned, young mice (postnatal days 0-4), we evaluated multiple nonhematopoietic stem cell and progenitor cell BM populations that might be expected to contribute to col7 production.
Outcome of cell transfers into newborn Col7a1−/− mice
| Cell type . | Cell dose, 1 × 106 . | No. alive on day 21/no. injected (% alive) . |
|---|---|---|
| None | NA | 0/18 |
| MSC | 1 | 0/6 |
| MAPC | 0.5-1 | 0/48 |
| EpiSC | 1-5 | 0/20 |
| TAC | 5 | 0/8 |
| Nonenriched BM | 10 | 0/24 |
| BM CD150+48− | 8-10 | 3/20 (15%) |
| 1-5 | 0/34 | |
| BM CD150− | 8 | 0/7 |
| Cell type . | Cell dose, 1 × 106 . | No. alive on day 21/no. injected (% alive) . |
|---|---|---|
| None | NA | 0/18 |
| MSC | 1 | 0/6 |
| MAPC | 0.5-1 | 0/48 |
| EpiSC | 1-5 | 0/20 |
| TAC | 5 | 0/8 |
| Nonenriched BM | 10 | 0/24 |
| BM CD150+48− | 8-10 | 3/20 (15%) |
| 1-5 | 0/34 | |
| BM CD150− | 8 | 0/7 |
The indicated cell population and dose was given to Col7a1−/− mice intravenously between postnatal days 0 to 4.
MSC indicates mesenchymal stem cell; MAPC, multipotent adult progenitor cell; EpiSC, epidermal stem cell; TAC, transient amplifying cell; BM, bone marrow; and NA, not applicable.
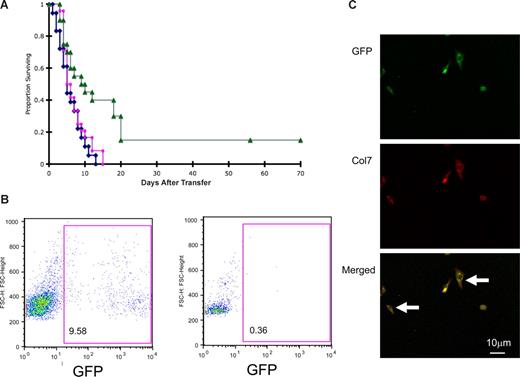
The effect of cellular infusions on survival and donor engraftment of Col7a1−/− (RDEB) mice. (A) Blue line indicates nontreated RDEB mice (n = 18); red line: RDEB mice given nonenriched BM (n = 24); and green line: RDEB mice treated with CD150+48− BM (n = 20; P = .005 and P = .005 versus nontreated or nonenriched BM-treated RDEB mice, respectively). (B) Histograms of peripheral blood of the animal with the highest donor engraftment (9.58%) 10 weeks after CD150+48− BM infusion (left panel) and unmanipulated control (right panel). (C) BM cells explanted from the surviving recipients express col7 protein. Representative example is shown. (Top panel) GFP-expressing donor cells (green); (middle panel) col7-expressing donor cells (red); (bottom panel) merged image; arrows point to donor cells coexpressing GFP and col7 protein.
The effect of cellular infusions on survival and donor engraftment of Col7a1−/− (RDEB) mice. (A) Blue line indicates nontreated RDEB mice (n = 18); red line: RDEB mice given nonenriched BM (n = 24); and green line: RDEB mice treated with CD150+48− BM (n = 20; P = .005 and P = .005 versus nontreated or nonenriched BM-treated RDEB mice, respectively). (B) Histograms of peripheral blood of the animal with the highest donor engraftment (9.58%) 10 weeks after CD150+48− BM infusion (left panel) and unmanipulated control (right panel). (C) BM cells explanted from the surviving recipients express col7 protein. Representative example is shown. (Top panel) GFP-expressing donor cells (green); (middle panel) col7-expressing donor cells (red); (bottom panel) merged image; arrows point to donor cells coexpressing GFP and col7 protein.
Despite abundant col7 mRNA (Table 2), neither the infusion of mesenchymal stem cells (106; n = 6 mice) nor multipotent adult progenitor cells (0.5-1 × 106; n = 48 mice) extended the life span of Col7a1−/− mice (Table 1). Similarly, neither col7 mRNA-expressing skin epidermal stem cells (1-5 × 106; n = 20 mice) nor skin transient amplifying cells (5 × 106; n = 8 mice) rescued Col7a1−/− mice from uniform lethality (Table 1). Due to the size of these populations, further cell dose escalation experiments could not be performed without substantial infusional toxicity. We concluded that the nonhematopoietic stem and progenitor cells given under these conditions did not appear to alter the course of the disease in this murine model of RDEB.
Relative expression of murine col7 RNA as assessed by quantitative RT-PCR
| Sample . | Cell type . | Col7 expression . |
|---|---|---|
| 1 | Wild-type whole BM | 1.00 |
| 2 | Wild-type EpiSC | 4034.01 (± 2.0) |
| 3 | Wild-type TAC | 911.44 (± 1.26) |
| 4 | Wild-type skin | 502.86 (± 0.8) |
| 5 | Wild-type MAPC | 275.71 (± 0.02) |
| 6 | Wild-type MSC | 15.68 (± 0.1) |
| 7 | Col7a1−/− MSC | 0 |
| 8 | Wild-type BM CD150+48− | 0.92 (± 0.18) |
| 9 | Wild-type BM Lin+ | 0 |
| 10 | Wild-type BM Lin− | 1.37 (± 0.38) |
| 11 | Wild-type BM CD150− | 0 |
| Sample . | Cell type . | Col7 expression . |
|---|---|---|
| 1 | Wild-type whole BM | 1.00 |
| 2 | Wild-type EpiSC | 4034.01 (± 2.0) |
| 3 | Wild-type TAC | 911.44 (± 1.26) |
| 4 | Wild-type skin | 502.86 (± 0.8) |
| 5 | Wild-type MAPC | 275.71 (± 0.02) |
| 6 | Wild-type MSC | 15.68 (± 0.1) |
| 7 | Col7a1−/− MSC | 0 |
| 8 | Wild-type BM CD150+48− | 0.92 (± 0.18) |
| 9 | Wild-type BM Lin+ | 0 |
| 10 | Wild-type BM Lin− | 1.37 (± 0.38) |
| 11 | Wild-type BM CD150− | 0 |
All assays were performed in duplicate and normalized to wild-type murine BM using the 2−ΔΔCT method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression served as the control gene.
BM indicates bone marrow; EpiSC, epidermal stem cell; TAC, transient amplifying cell; MAPC, multipotent adult progenitor cell; and MSC, mesenchymal stem cell.
Hematopoietic stem cell–enriched bone marrow cells correct basement membrane zone defect in murine RDEB
Purified hematopoietic stem cells (HSCs) transferred into lethally irradiated mice have been shown to differentiate into epithelial cells.32 Because rapid donor cell engraftment is required for survival in the Col7a1−/− murine model, studies were performed in newborn mice using high BM cell doses on a body-weight basis. BM was obtained from green fluorescent protein transgenic (GFP+) mice to enable tracking of hematopoietic cells to the skin lesion sites. Similar to nonhematopoietic stem and progenitor cells, the transfer of nonenriched GFP+ but otherwise congenic BM cells (10 × 106; n = 24 mice) was ineffective in reducing lethality or healing blisters in Col7a1−/− mice (Figure 1A).
To optimize cell dose delivery given the limitations of delivery volume and cell number that can be safely injected into newborn mice, BM cells first were processed to enrich for hematopoietic and progenitor cells in an attempt to increase the frequency of precursor cells with epithelial cell differentiation capacity. Because CD150+CD48− cells have been shown to contain rapidly engrafting cells for marrow repopulation and to increase stem cell purity,31,33 Col7a1−/− mice were given a comparable total number of CD150+CD48− GFP+ BM cells (8 × 106; n = 20) as nonenriched BM cells.
In contrast to all other marrow subpopulations, CD150+CD48− BM cells extended the median survival time compared with untreated or nonenriched BM-treated recipients (10.0 vs 5.6 vs 6.0 days, respectively; Table 1; Figure 1A). Moreover, the actuarial survival rates were significantly increased in Col7a1−/− mice treated with CD150+CD48− BM compared with either of the other groups (P = .005 vs nontreated, n = 18, or nonenriched BM-treated mice, n = 24; Table 1; Figure 1A).
Remarkably, 3 (15%) of 20 of mice were able to survive long term (Table 1; Figure 1A), indicating that a proportion of mice had sufficient repair of RDEB to prevent death from the diffuse epithelial cell injury characteristic of the disease. Neither the infusion of CD150− BM cells (8 × 106; n = 7) nor lower doses of CD150+48− BM cells (1-5 × 106; n = 34 mice) significantly prolonged survival (Table 1). These data suggest a threshold effect for col7-producing cells derived from the CD150+48− BM fraction that may be needed for timely repair of RDEB lesions and rescue from lethality. Although the apparent beneficial effect of CD150+CD48− cells over other BM-derived populations could be due to multiple factors, enhanced col7 mRNA expression was not seen in studies comparing expression levels between total BM and CD150+CD48− cells (Table 2).
At 7 weeks of age, the surviving mice (1 male and 2 females) weighed less than their normal heterozygous and wild-type littermates (n = 10; 5 females and 5 males): CD150+CD48− BM recipients had a mean weight (± SD) of 11.7 plus or minus 2 g versus normal littermates 20.6 plus or minus 2.2 g (P < .05).
One recipient was electively killed at week 8, and 2 were electively killed at week 10, for tissue analysis. Chimerism studies demonstrated 8.1% and 9.6% donor-derived GFP+ cells in the peripheral blood, and 3.6% and 4.4% in the BM (representative example is shown in Figure 1B).
At 10 weeks of age, the level of col7 RNA in the blistered footpads of CD150+CD48− BM-treated RDEB mice was more than 15-fold higher than in the blistered footpads of control RDEB animals (P < .05). Col7 RNA mRNA levels in the esophagus or in nonblistered skin on torso or ear (which are typically less exposed to trauma and develop fewer blisters) of treated versus untreated RDEB mice were not significantly different (data not shown).
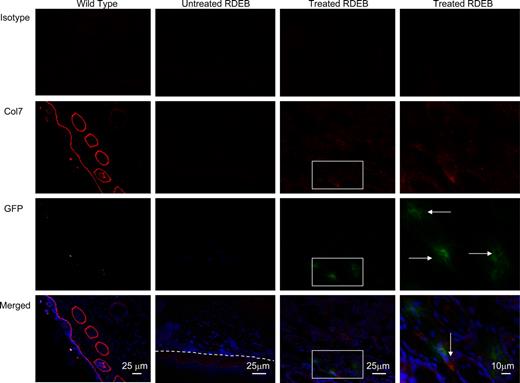
Furthermore, col7-producing cells were present in the skin of treated animals, clearly indicating that CD150+CD48− BM cells can home to injured skin of the RDEB animals (Figure 2). Consistent with this, BM cells explanted from the surviving recipients contained GFP+ cells that coexpressed col7 protein (Figure 1C). At 10 weeks of age, areas exposed to trauma (such as footpads due to walking, and oral cavity due to eating; Figures 2,Figure 3–4) showed numerous GFP+ donor cells spread widely in the dermis (not localized to hair follicles; Figure 4). Less frequently, dual GFP+ Col7+ cells or GFP+ donor cells were found next to the cells with col7 expression in the extracellular matrix (Figures 2,Figure 3–4).
Col7 protein is expressed in the skin of treated Col7a1−/− animals. Representative images of footpad skin of wild-type control (first column), untreated RDEB (second column; dashed line indicates dermal-epidermal junction), and CD150+CD48− BM-treated RDEB (third column and fourth—magnified view—column) are shown. Isotype control (viewed in red fluorescence channel; top row); anti-col7 antibody (red, second row, tight linear band at the skin basement membrane zone in the wild type in the leftmost column, and positive col7a staining in the treated RDEB in the rightmost column); anti-GFP antibody (green, third row, GFP-positive cells are indicated with horizontal arrows); and merged images with blue DAPI nuclear stain (bottom row, col7 indicated with vertical arrows). Merged images demonstrate the colocalization of GFP+ donor cells and collagen type VII in the skin 10 weeks after CD150+CD48− BM infusion.
Col7 protein is expressed in the skin of treated Col7a1−/− animals. Representative images of footpad skin of wild-type control (first column), untreated RDEB (second column; dashed line indicates dermal-epidermal junction), and CD150+CD48− BM-treated RDEB (third column and fourth—magnified view—column) are shown. Isotype control (viewed in red fluorescence channel; top row); anti-col7 antibody (red, second row, tight linear band at the skin basement membrane zone in the wild type in the leftmost column, and positive col7a staining in the treated RDEB in the rightmost column); anti-GFP antibody (green, third row, GFP-positive cells are indicated with horizontal arrows); and merged images with blue DAPI nuclear stain (bottom row, col7 indicated with vertical arrows). Merged images demonstrate the colocalization of GFP+ donor cells and collagen type VII in the skin 10 weeks after CD150+CD48− BM infusion.
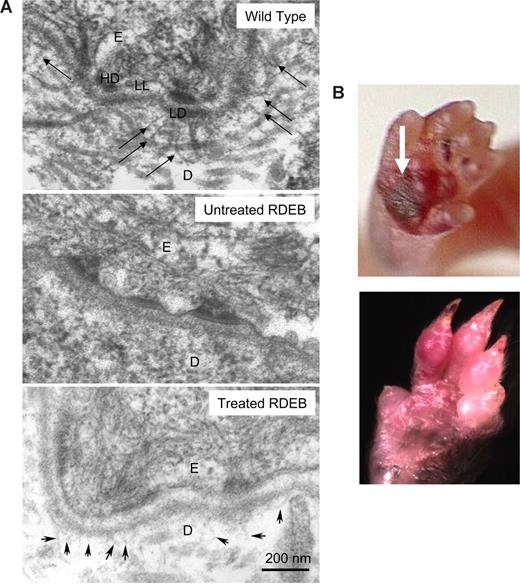
High-dose CD150+CD48− BM from Col7a1 wild-type congenic donors corrects basement membrane zone defect. (A) Anchoring fibrils are fan-shaped structures in the skin that are composed of col7 protein. Transmission electron microscopy demonstrates that anchoring fibrils emanate from the papillary dermis into the lamina densa (top panel, arrows) in the wild-type skin (as expected), and in the skin of treated RDEB animals (bottom panel, arrowheads). No anchoring fibrils are deposited at the skin basement membrane zone in the RDEB skin (middle panel). D indicates dermis; E, epidermis; LL, lamina lucida; LD, lamina densa; and HD, hemidesmosome. (B) Skin blisters characteristic of RDEB healed in all 3 long-term surviving Col7a1−/− recipients of CD150+CD48− BM (top panel, arrow points to a skin blister at 3 days of age; bottom panel, 10 weeks of age). Representative examples are shown. Camera: Coolpix 4300 (Nikon, Tokyo, Japan).
High-dose CD150+CD48− BM from Col7a1 wild-type congenic donors corrects basement membrane zone defect. (A) Anchoring fibrils are fan-shaped structures in the skin that are composed of col7 protein. Transmission electron microscopy demonstrates that anchoring fibrils emanate from the papillary dermis into the lamina densa (top panel, arrows) in the wild-type skin (as expected), and in the skin of treated RDEB animals (bottom panel, arrowheads). No anchoring fibrils are deposited at the skin basement membrane zone in the RDEB skin (middle panel). D indicates dermis; E, epidermis; LL, lamina lucida; LD, lamina densa; and HD, hemidesmosome. (B) Skin blisters characteristic of RDEB healed in all 3 long-term surviving Col7a1−/− recipients of CD150+CD48− BM (top panel, arrow points to a skin blister at 3 days of age; bottom panel, 10 weeks of age). Representative examples are shown. Camera: Coolpix 4300 (Nikon, Tokyo, Japan).
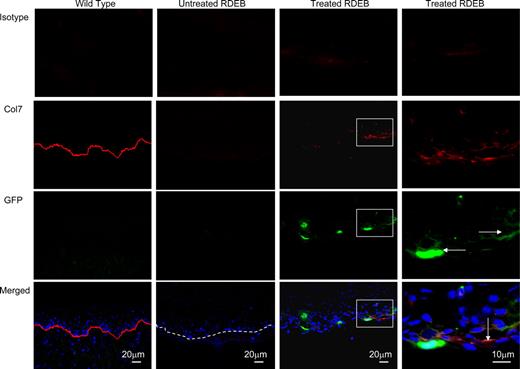
Col7 protein is expressed in the oral cavity of treated Col7a1−/− animals. Representative images of tongue sections of wild-type control (first column), untreated RDEB (second column; dashed line indicates dermal-epidermal junction), and CD150+CD48− BM-treated RDEB (third column and fourth—magnified view—column) are shown. Isotype control (viewed in red fluorescence channel; top row); anti-col7 antibody (red, second row, tight linear band at the skin basement membrane zone in the wild type in the leftmost column, and discontinuous, yet linear, signal in the treated RDEB in the rightmost column); anti-GFP antibody (green, third row, GFP-positive cells are indicated with horizontal arrows); and merged images with blue DAPI nuclear stain (bottom row, col7 indicated with the vertical arrow). Merged images demonstrate the colocalization of GFP+ donor cells and collagen type VII in the tongue 10 weeks after CD150+CD48− BM infusion.
Col7 protein is expressed in the oral cavity of treated Col7a1−/− animals. Representative images of tongue sections of wild-type control (first column), untreated RDEB (second column; dashed line indicates dermal-epidermal junction), and CD150+CD48− BM-treated RDEB (third column and fourth—magnified view—column) are shown. Isotype control (viewed in red fluorescence channel; top row); anti-col7 antibody (red, second row, tight linear band at the skin basement membrane zone in the wild type in the leftmost column, and discontinuous, yet linear, signal in the treated RDEB in the rightmost column); anti-GFP antibody (green, third row, GFP-positive cells are indicated with horizontal arrows); and merged images with blue DAPI nuclear stain (bottom row, col7 indicated with the vertical arrow). Merged images demonstrate the colocalization of GFP+ donor cells and collagen type VII in the tongue 10 weeks after CD150+CD48− BM infusion.
Critically, anchoring fibrils (wheat stack–shaped structures composed of collagen type VII emanating from the papillary dermis into the lamina densa of the basement membrane zone) were identified using transmission electron microscopy in surviving treated Col7a1−/− animals (aged 10 weeks; Figure 5A). These anchoring fibrils are smaller and less developed than the ones observed in the wild-type animals, yet the difference compared with untreated mutant murine RDEB skin (where they are absent entirely; Figure 5A) is significant. In addition, each survivor demonstrated marked amelioration of new blister formation (blisters develop consistently in the areas of trauma, including footpads due to walking), with evidence of old blisters healing (Figure 5B).
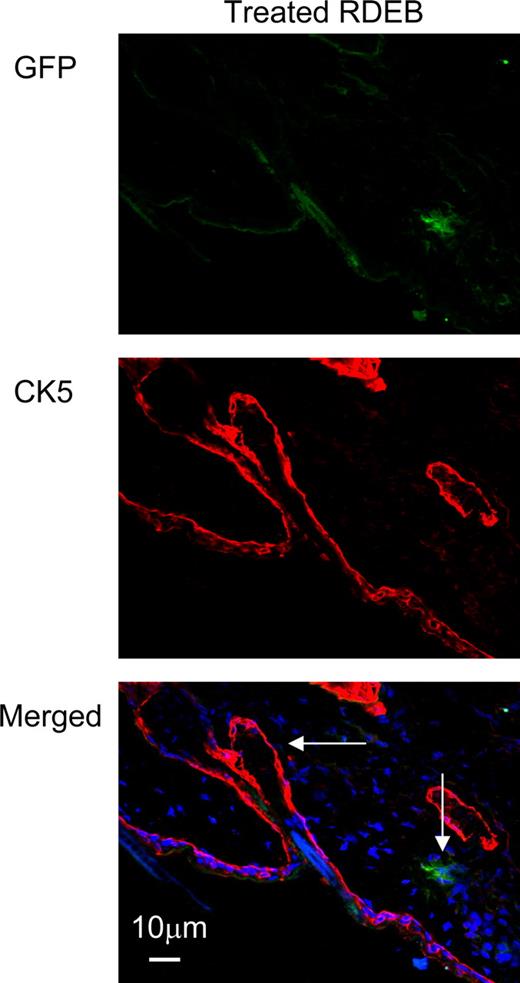
Donor GFP+ cells are not localized in the hair follicles of the skin. Immunofluorescence staining of the skin of the animals treated with GFP+CD150+CD48− BM infusion shows that GFP+ donor cells have localized outside of the hair follicles. Donor cells were visualized using anti-GFP antibody (green, top panel), and hair follicles were visualized using an anti–cytokeratin 5 antibody (red, middle panel). Donor cells and hair follicles are shown (vertical and horizontal arrows, respectively) on the image merged with blue DAPI nuclear stain (bottom panel).
Donor GFP+ cells are not localized in the hair follicles of the skin. Immunofluorescence staining of the skin of the animals treated with GFP+CD150+CD48− BM infusion shows that GFP+ donor cells have localized outside of the hair follicles. Donor cells were visualized using anti-GFP antibody (green, top panel), and hair follicles were visualized using an anti–cytokeratin 5 antibody (red, middle panel). Donor cells and hair follicles are shown (vertical and horizontal arrows, respectively) on the image merged with blue DAPI nuclear stain (bottom panel).
These data unequivocally demonstrate the ability of BM-derived cell populations to biochemically and phenotypically correct RDEB.
Discussion
The main finding of this study is that wild-type marrow-derived cells can migrate to the skin lesions seen in RDEB, produce col7 protein and anchoring fibrils, prevent blister formation, and extend survival in a murine model of RDEB. Thus, “proof of principle” for the use of marrow-derived cells in the treatment of an extracellular matrix disorder has been achieved.
Intuitively, this is unexpected, as RDEB is a deficiency of a structural protein, and the capacity of cross-correction of protein deficiency has been traditionally associated only with enzyme defects such as those observed in some congenital lysosomal storage disorders. In these latter disorders, hematopoietic cell transplantation is a treatment of choice due to the ability of engrafting cells to provide a life-long source of the deficient enzyme that can be taken up by recipient's cells.34 On closer inspection, however, col7 is not a “true” structural protein as it does not contribute to the cellular structure. Instead, it is a cell-excreted extracellular matrix protein polymerized into large suprastructures (200-700 nm–long anchoring fibrils) deposited at the basement membrane zone. Therefore, there appear to be no conceptual hurdles for phenotypic correction of RDEB by cellular transfer, provided that donor wild-type cells transit or home to the skin and produce col7 in sufficient quantity to form anchoring fibrils.
None of the 82 recipients of nonhematopoietic stem or progenitor cell populations survived longer than untreated Col7a1−/− controls (Table 1). Although we concluded that nonhematopoietic stem cells did not correct murine RDEB, we cannot exclude the possibility that nonhematopoietic stem cells (such as epidermal stem and progenitor cells) could be beneficial if given at different doses, infused into myeloablated or otherwise immune-suppressed recipients, or modified to be more effective at in vivo skin homing or persistence.
In contrast, hematopoietic cells, enriched for the signaling lymphocytic activation molecule (SLAM) family markers, ameliorated the manifestations of murine RDEB. Although the cells isolated by this process clearly include true hematopoietic stem cells, other cells with various degree of “stemness” and capacity to give rise to hematopoietic or nonhematopoietic progeny are likely carried along during the isolation of the SLAM cells. This process is inevitably influenced by starting cell numbers, characteristics of donors, time on the column, and the choice of the CD48 and CD150 antibodies.31,33,35-37 In this context, it is relevant that our data have not shown that CD150+CD48− cells alone were responsible for biochemical amelioration of col7 deficiency. Our observations are equally consistent with a possibility that cells copurified along with CD150+CD48− cells were, at least in part, responsible for the development of anchoring fibrils. We fully appreciate that our data do not allow us to conclude that CD150+CD48− cells are unique in their capacity to correct skin defect in RDEB. Yet, although the full understanding of the origin of the precursor cell responsible for improvement of skin blistering and shearing in Col7a1−/− mice will require future studies, we can conclude that the cells capable of cross-correction of the col7 defect are (1) intrinsic to BM and (2) transferable from wild-type donors to mutant immunocompetent recipients.
Thus, both cutaneous cells (keratinocytes and fibroblasts) and extracutaneous cells (BM cells) can express col7. In regard to the former, Woodley et al showed that systemically administered human RDEB fibroblasts corrected with lentiviral-mediated gene replacement of Col7a1 promoted skin wound healing in athymic nude mice.38 Although hematopoietic cell transplantation is not without its own complications, treatment with wild-type BM cells is potentially more advantageous than other possible forms of RDEB therapy in several respects. For example, normal donor hematopoietic cells have a potential to provide a continuous source of wild-type cells (responding to migration signals from injured skin) that can home to skin wounds, in contrast to fibroblasts that would presumably have a more limited life span. By using normal donor hematopoietic cells, gene replacement of autologous cells is not needed, avoiding risks associated with genetic modification39 or transient gene expression. In addition, the use of myeloablative therapy along with donor hematopoietic cell rescue would foster a state of immunologic tolerance to the wild-type protein rather than engender a foreign antigen immune response, analogous to the immune reactivity seen in the acquired autoimmune form of epidermolysis bullosa (epidermolysis bullosa acquisita).40
The cause of death for untreated Col7a1−/− mice is likely mucosal disruption with consequent weight loss and malnutrition. In support of this hypothesis, the clusters of donor GFP+ cells were seen in the submucosal layer of the tongue and the esophagus (Figure 3). These data suggest that the failure of HSC-enriched BM cells to uniformly protect RDEB mice from lethality may be due to the rapidity and frequency of donor GFP+ cells to localize to the gastrointestinal epithelial cell layers. It is striking, and consistent with our observations of partial correction of the col7 deficiency, that growth retardation observed in the hypomorphic mouse RDEB model41 was similar to that observed in the surviving genetically fully penetrant enriched BM-treated RDEB mice. The hypomorphic RDEB mice have approximately 10% of normal col7 expression and survive into adult life if fed a liquid diet. However, the fully penetrant forms in both murine and human RDEB are those that have a high mortality rate. Therefore, the strategy of BM infusions (even if it leads to only a fraction of wild-type col7 production) may have the potential to stabilize the skin and attenuate the lethal forms of human RDEB, resulting in a less severe phenotype.
Furthermore, it is conceivable that the strategy we used, systemic infusion of wild-type BM cells, could provide benefit to other human disorders of extracellular matrix disorders. Efforts are under way to identify the requirements of BM-derived cells capable of efficiently homing to wounded skin and producing an array of extracellular matrix proteins such as col7a protein.
As the principal advantage of systemic therapy is its potential to target not only the skin, the largest organ of the body, but also the mucosa of the mouth and gastrointestinal tract, the clinical testing of efficacy of human BM for treatment of human RDEB is under way to determine whether it is of more substantial benefit than local protein, gene, or cellular therapy as investigated by others at the current time.9,42-44
In summary, despite multiple attempts to provide life-saving interventions, no definitive therapy exists for this painful, mutilating, cancer-causing disease—which is ultimately lethal. We show that infusion of wild-type BM cells results in expression of col7, formation of anchoring fibrils, and healing of the blisters. To our knowledge, this is the first report of BM engraftment in the appropriate skin niche that results in sufficient numbers to provide cross-correction of the col7 deficiency. Thus, our data demonstrate “proof-of-principle” of BM transfer for amelioration of the basement membrane zone defect in RDEB and provide a rationale for a clinical trial to assess the safety and efficacy of treatment of human RDEB by hematopoietic cell transplantation.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Hisham Bazzi, Christopher Lees, Amanda Kobs, Justin White, Jose Roig-Lopez, and Katherine Fantauzzo for their excellent technical help.
This work was supported in part by grants from the EB Fund (Minneapolis, MN), National Institutes of Health (NIH, Bethesda, MD; 5RO1-HL049997), NYSTAR, Minnesota Medical Foundation, and Children's Cancer Research Fund (Minneapolis, MN).
National Institutes of Health
Authorship
Contribution: J.T., A.I-Y., M.R., R.T.M., L.X., and C.S. performed experimental work and collected data; M.O., T.L., and J.U. interpreted and reviewed the paper; and J.T., A.M.C., J.E.W., and B.R.B. designed the study, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jakub Tolar, Division of Pediatric Hematology, Oncology, Blood and Marrow Transplantation, University of Minnesota, MMC 366, 420 Delaware Street SE, Minneapolis, MN 55455; e-mail: tolar003@umn.edu.
References
Author notes
*A.M.C., J.E.W., and B.R.B. contributed equally to this work.