Abstract
Pulmonary arterial hypertension (PAH) is emerging as a major complication and independent risk factor for death among adults with sickle cell disease (SCD). Using surface-enhanced laser desorption/ionization time of flight mass spectrometry (SELDI-TOF MS), we searched for biomarkers of PAH in plasma specimens from 27 homozygous sickle cell anemia (HbSS) patients with PAH and 28 without PAH. In PAH patients, analysis consistently showed lower abundance of a 28.1-kDa peak (P < .001), identified by high-resolution mass spectrometry as the oxidant-scavenging protein apolipoprotein A-I (apoA-I), which correlated with clinical assays of apoA-I (r = .58, P < .001) and high-density lipoprotein (HDL) levels (r = .50, P = .001). Consistent with endothelial dysfunction that may mediate this effect in PAH, HbSS patients with lower apoA-I levels also displayed impaired vasodilatory responses to acetylcholine (mean ± SEM, 189% ± 34% [n = 13] vs 339% ± 51% [n = 13], P < .001). As a group, patients with SCD demonstrated significantly lower apoA-I levels than African-American control subjects. The PAH cohort was further characterized by high levels of apolipoproteins A-II and B and serum amyloid A, and low levels of haptoglobin dimers and plasminogen. These results imply a relationship of apolipoproteins to the development of PAH vasculopathy in SCD, potentially involving an unexpected mechanistic parallel to atherosclerosis, another proliferative vasculopathy.
Introduction
Pulmonary arterial hypertension (PAH) has emerged as a major complication and independent risk factor for sudden death among adults with sickle cell disease (SCD). A prospective study of 195 sickle cell patients revealed a PAH prevalence of 32% with a relative risk of mortality of 10.1 with tricuspid regurgitant jet velocity (TRV) higher than 2.5 m/s on transthoracic Doppler echocardiography, an indirect indicator of elevated pulmonary artery pressure.1 This high prevalence and mortality rate are confirmed in other retrospective and prospective studies of PAH in SCD.2-7 Markers that correlate significantly with PAH in SCD include increasing age, hemolysis, iron overload, renal dysfunction, and cholestatic liver dysfunction.1,6-9 Patients with SCD exhibit worse levels of exercise performance, maximal oxygen consumption, and mortality rates at milder elevations of pulmonary arterial pressures than do patients with idiopathic PAH.2,10 These data indicate that PAH in SCD is associated with impaired cardiopulmonary function and early mortality
Recent epidemiologic, pathophysiological, and biochemical findings have linked PAH in SCD to a hemolysis-linked reduced bioavailability of nitric oxide (NO), a potent vasodilatory, antioxidant, and anti-inflammatory molecule produced endogenously in endothelium. PAH in these patients is further characterized by hemolysis-associated activation of platelets,11 and variable activation of hemostatic proteins.12 The only identified plasma marker of PAH in patients with SCD has been amino-terminal brain-type natriuretic peptide.13 However, there have been no published exploratory studies to date of proteomic biomarkers of PAH in patients with SCD. Identification of new disease markers might provide new mechanistic insights, prospective risk assessment, and potential risk reduction interventions.
We have undertaken an exploratory screen for markers indicative of new mechanisms contributing to the development of PAH in patients with sickle cell disease. We have screened patient plasma specimens using surface-enhanced laser desorption/ionization–time-of-flight mass spectrometry (SELDI-TOF MS), which allows high-throughput analysis of complex protein mixtures, effective in resolving proteins with extremes in molecular weights, hydrophobicity, and isoelectric points.14 SELDI-TOF has limitations in resolution, but this is counterbalanced by its robust high-throughput capability.15 Candidate biomarkers then were identified by high-resolution mass spectrometry, followed by unambiguous immunoassay validation. We present data here from an exploratory plasma proteomics screen unexpectedly implicating the apolipoprotein pathway in SCD PAH, corroborated by association of endothelial dysfunction with low levels of apolipoprotein A-I.
Methods
Patient selection
A bank of 247 frozen EDTA plasma samples from patients with homozygous sickle cell anemia (HbSS), collected with patients' informed consent in accordance with the Declaration of Helsinki between February 2001 and January 2005 on a protocol approved by the National Institutes of Health (NIH) Institutional Review Board, was at our disposal for proteomic study (ClinicalTrials.gov identifier NCT00011648). Patients harboring a heterozygous hemoglobin S and C, D, or β+-thalassemia phenotype (SC, SD, or Sβ+thalassemia, respectively) by high-performance liquid chromatography analysis of hemoglobin were eliminated, as were patients with serum creatinine 221 μM (2.5 mg/dL) or higher. Of the 190 eligible SCD patients, the 27 study subjects with the highest resting TRV were selected to comprise the PAH-positive group. A control PAH-negative HbSS group was chosen from the lowest TRV quartile to provide a similar distribution of age, sex, and renal function to the PAH group. Table 1 describes the clinical characteristics of the cohorts, including very high plasma levels of N-terminal pro–brain type natriuretic peptide (NT-proBNP) in the PAH group, a well-described marker of high pulmonary arterial pressures.13 The statistically significant differences seen between the 2 groups are consistent with those previously reported. The characteristics of the patients in the forearm blood flow study have been previously reported.16 The 280 patients in the convenience sample were recruited during ambulatory visits in steady state. β-Globin genotypes have been determined for 140 of the patients by gene sequencing. These fell into the following categories: HbSS, 86%; HbSC, 8%; Sβ+ thalassemia, 4%; and Sβ0, 2%. Of the homozygous SCD patients, 55 also had been subjects in the proteomics screen, and 18 had been in the forearm blood flow study.
Patient characteristics
| Variable . | No PAH . | PAH . | P . |
|---|---|---|---|
| No. | 28 | 27 | |
| Sex, fraction female | 0.61 | 0.56 | NS |
| Age, y | 34 (8) | 42 (11) | < .01 |
| TRV, m/s | 1.4 (0.5) | 3.1 (0.4) | < .001 |
| Leukocytes, ×109/L | 10.6 (3.4) | 11.2 (4.1) | NS |
| Hemoglobin, g/L | 93 (13) | 84 (14) | < .05 |
| Platelets, ×109/L | 415 (149) | 384 (133) | NS |
| Reticulocytes, ×109/L | 314 (314) | 266 (142) | NS |
| Lactate dehydrogenase, IU/L | 334 (115) | 492 (204) | < .001 |
| Alkaline phosphatase, IU/L | 114 (91) | 159 (90) | < .05 |
| Aspartate aminotransferase, IU/L | 41 (21) | 52 (27) | NS |
| Alanine aminotransferase, IU/L | 28 (17) | 29 (14) | NS |
| Bilirubin, total, μM | 46 (22) | 56 (36) | NS |
| Bilirubin, direct, μM | 7 (3) | 12 (12) | < .01 |
| Creatinine, μM | 53 (15) | 61 (23) | < .05 |
| Ferritin, pM | 1723 (2647) | 2916 (3737) | < .05 |
| NT-proBNP, pg/mL | 75 (71) | 1069 (3222) | < .001 |
| Variable . | No PAH . | PAH . | P . |
|---|---|---|---|
| No. | 28 | 27 | |
| Sex, fraction female | 0.61 | 0.56 | NS |
| Age, y | 34 (8) | 42 (11) | < .01 |
| TRV, m/s | 1.4 (0.5) | 3.1 (0.4) | < .001 |
| Leukocytes, ×109/L | 10.6 (3.4) | 11.2 (4.1) | NS |
| Hemoglobin, g/L | 93 (13) | 84 (14) | < .05 |
| Platelets, ×109/L | 415 (149) | 384 (133) | NS |
| Reticulocytes, ×109/L | 314 (314) | 266 (142) | NS |
| Lactate dehydrogenase, IU/L | 334 (115) | 492 (204) | < .001 |
| Alkaline phosphatase, IU/L | 114 (91) | 159 (90) | < .05 |
| Aspartate aminotransferase, IU/L | 41 (21) | 52 (27) | NS |
| Alanine aminotransferase, IU/L | 28 (17) | 29 (14) | NS |
| Bilirubin, total, μM | 46 (22) | 56 (36) | NS |
| Bilirubin, direct, μM | 7 (3) | 12 (12) | < .01 |
| Creatinine, μM | 53 (15) | 61 (23) | < .05 |
| Ferritin, pM | 1723 (2647) | 2916 (3737) | < .05 |
| NT-proBNP, pg/mL | 75 (71) | 1069 (3222) | < .001 |
Values for all continuous variables represent means, with standard deviations indicated in parentheses. Significance was determined by Wilcoxon-Mann-Whitney test, except for sex (χ2 test).
PAH indicates pulmonary arterial hypertension; TRV, tricuspid regurgitant jet velocity; and NT-proBNP, N-terminal pro–brain-type natriuretic hormone.
Summary of m/z species of highest significance in sickle cell patients with pulmonary hypertension
| m/z by SELDI-TOF MS, Da . | Multivariate analysis of significance . | Identity . | Identification method . | Level in PAH . | P . |
|---|---|---|---|---|---|
| 28 101 | RF, SLR | Apolipoprotein A-I | LC-MS/MS MALDI-TOF | Low | .001 |
| 8900 | SLR | Apolipoprotein A-II | LC-MS/MS | High | .03 |
| 13 455 | RF | Serum amyloid A-4 | LC-MS/MS | High | .001 |
| 89 443 | RF | Plasminogen | LC-MS/MS | Low | .004 |
| 75 188 | RF | Haptoglobin dimer | LC-MS/MS MALDI-TOF | Low | .004 |
| 18 475 | RF | Galectin-1 (tentative) | Literature search | Low | .001 |
| m/z by SELDI-TOF MS, Da . | Multivariate analysis of significance . | Identity . | Identification method . | Level in PAH . | P . |
|---|---|---|---|---|---|
| 28 101 | RF, SLR | Apolipoprotein A-I | LC-MS/MS MALDI-TOF | Low | .001 |
| 8900 | SLR | Apolipoprotein A-II | LC-MS/MS | High | .03 |
| 13 455 | RF | Serum amyloid A-4 | LC-MS/MS | High | .001 |
| 89 443 | RF | Plasminogen | LC-MS/MS | Low | .004 |
| 75 188 | RF | Haptoglobin dimer | LC-MS/MS MALDI-TOF | Low | .004 |
| 18 475 | RF | Galectin-1 (tentative) | Literature search | Low | .001 |
m/z indicates mass-to-charge ratio; SELDI-TOF MS, surface-enhanced laser desorption/ionization–time-of-flight mass spectrometry; Da, Daltons; RF, Random Forest; SLR, stepwise logistical regression; LC-MS/MS, liquid chromatography tandem mass spectrometry; MALDI-TOF, matrix assisted laser desorption-ionization time-of-flight mass spectrometry; and P, significance by 2-tailed unpaired t test.
Exploratory proteomic screening and mass spectrometry
Detailed methods are provided in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Briefly, plasma samples were fractionated by ion exchange chromatography with step pH gradient elution. The resulting fractions were bound to specific affinity matrix surfaces, and unbound proteins were washed from the surfaces. Optimization experiments indicated the minimum number of surfaces and fractions for resolution of the maximum number of nonredundant peaks from each patient. CiphergenExpress 3.0 (current version known as ProteinChip Data Manager software; Bio-Rad Laboratories, Hercules, CA) was used to process and detect peaks in the resulting spectra. For each of the experimental data sets and pooled reference data sets, the baseline was adjusted and spectra were normalized to the total ion current. Univariate analysis was performed through P value and receiver operating curve area calculation of individual peaks by CiphergenExpress 3.0 software. Multivariate analysis was performed by logistic regression (JMP 5.1; SAS, Cary, NC) and by Random Forest (Salford Systems, San Diego, CA) classification/regression. Ten independently significant peaks of interest were selected from the collective results of the Random Forest and stepwise logistical regression models for identification.
To identify the proteins corresponding to the SELDI-TOF peaks of interest, plasma samples from representative patients were fractionated by denaturing polyacrylamide gel electrophoresis, and a range of bands were excised of molecular weight bracketing the estimated molecular weight of the peaks of interest from the SELDI-TOF screen. Proteins were eluted from the gel slices, and the gel slice selected that matched the mass to charge ratio of the peaks of interest. The identities of these proteins were determined by high-resolution mass spectrometry (matrix-assisted laser desorption/ionization time-of-flight/time-of-flight [MALDI-TOF-TOF] or liquid chromatography/mass spectrometry/mass spectrometry [LC-MS/MS]).
Apolipoprotein assays
ApoA-I and apoB levels were measured in unfractionated plasma from 3 groups of patients: the 56 patients in the SELDI-TOF screening cohort (“Exploratory proteomic screening and mass spectrometry”); 26 homozygous sickle cell patients from the forearm blood flow study (“Forearm blood flow studies”); and a convenience sample of 83 patients of all genotypes of SCD, sampled during steady state. ApoA-I was measured on a standard nephelometric clinical analyzer, which uses a polyclonal antibody that reacts to apoA-I after a denaturation treatment step with a detergent. Because apoA-I is relatively stable to proteolysis and a polyclonal antibody was used, it is stable to numerous freeze-thaw cycles. The clinical laboratory that performed the apoA-I assay for the study routinely freezes all clinical samples before analysis and has not observed any change from fresh samples. Furthermore, apoA-I has been routinely measured on frozen samples, using the same or very similar nephelometric methods, in many previous studies on the link between high-density lipoprotein (HDL) and atherosclerosis.
Forearm blood flow studies
Archived plasma samples were retrieved from frozen storage at − 80°C (ClinicalTrials.gov identifier NCT00542230). These correspond to previously published forearm blood flow measurements induced by test doses of acetylcholine and sodium nitroprusside.16 Apolipoprotein levels were measured; and 2-way ANOVA analysis was performed using Prism 4.0 (GraphPad Software, San Diego, CA).
Results
Mass spectrometry screening and protein identification
An optimized protocol was developed to derive a maximal number of mass spectrometry peaks with minimal redundancy, using fractions of plasma eluted at different pH step gradients or with organic solvent (Document S1). A group of 28 patients, the most significant PAH patients, were selected from the NIH sickle cell disease cohort, with a control group of 27 patients without PAH, chosen to provide a similar age and sex distribution (Table 1). The comparisons reflected the same relationships previously seen by us and others. For each experimental subject, SELDI-TOF MS analysis on the plasma fractions on the various matrix types resolved approximately 955 peaks per patient. The peak intensities were compared in the data sets from the PAH patients with the corresponding data sets from the non-PAH patients.
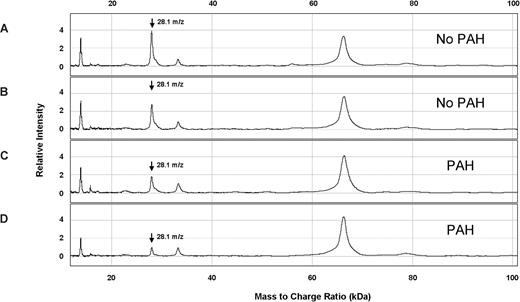
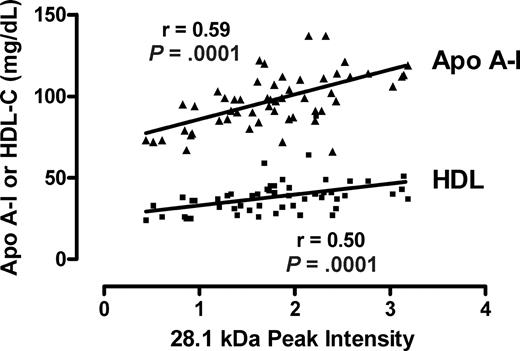
Several peaks of interest were identified by ranking highly on one or more statistical methods of analysis (Table 2). The Random Forest algorithm and stepwise logistical regression modeling of these peaks consistently showed lower abundance of a 28 100-m/z peak in PAH patients (Figure 1; P < .001). Upon further purification of this peak, MALDI-TOF MS and LC-MS/MS identified it as the oxidant scavenging protein apolipoprotein A-I (apoA-I; Figure 2; Document S1). The 28 100-m/z peak intensity values correlated with standard clinical laboratory assays of apoA-I performed on unfractionated plasma from the same subjects (r = .58, P < .001, Spearman correlation) and with HDL levels (r = .50, P = .001), supporting the mass spectrometry identification (Figure 3).
Representative SELDI-TOF MS spectra from plasma from patients with SCD with and without PAH. The vertical axes represent intensity of peaks in arbitrary units, and the horizontal axes represent mass to charge ratio (m/z). These 4 spectra were obtained from plasma eluted from ion anion exchange resin, bound to IMAC30-Cu++ matrix, and ionized for SELDI-TOF MS. Two specimens are from SCD patients without PAH (A,B) and 2 are from SCD patients with PAH (C,D). A peak at m/z 28.1 kDa was observed at lower average intensity in SCD patients with PAH ( ).
).
Representative SELDI-TOF MS spectra from plasma from patients with SCD with and without PAH. The vertical axes represent intensity of peaks in arbitrary units, and the horizontal axes represent mass to charge ratio (m/z). These 4 spectra were obtained from plasma eluted from ion anion exchange resin, bound to IMAC30-Cu++ matrix, and ionized for SELDI-TOF MS. Two specimens are from SCD patients without PAH (A,B) and 2 are from SCD patients with PAH (C,D). A peak at m/z 28.1 kDa was observed at lower average intensity in SCD patients with PAH ( ).
).
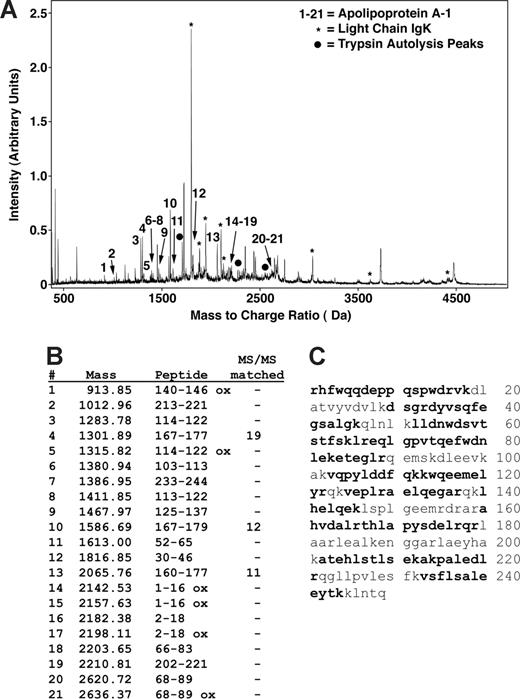
Peptide mapping of apoA-I by MALDI mass spectrometry. (A) MS and MS/MS analysis of a trypsin-digested gel splice resulted in matching of most of the major peaks as either apoA-I (peaks indicated by nos. 1-21), immunoglobulin kappa light chain (IgK, *), or trypsin autolysis products (•). No other proteins were identified as significant matches. (B) A total of 21 different peaks matched to apoA-I with MS/MS sequencing of 3 of these matching 19, 12, and 11 fragment ion peaks. (C) Matching peptides indicated in bold represent 66% of the sequence and are distributed throughout the sequence.
Peptide mapping of apoA-I by MALDI mass spectrometry. (A) MS and MS/MS analysis of a trypsin-digested gel splice resulted in matching of most of the major peaks as either apoA-I (peaks indicated by nos. 1-21), immunoglobulin kappa light chain (IgK, *), or trypsin autolysis products (•). No other proteins were identified as significant matches. (B) A total of 21 different peaks matched to apoA-I with MS/MS sequencing of 3 of these matching 19, 12, and 11 fragment ion peaks. (C) Matching peptides indicated in bold represent 66% of the sequence and are distributed throughout the sequence.
Correlation of the m/z 28 101 peak with apoA-I and HDL-C assays. The intensities of the m/z 28 101 from all patients with or without PAH peak are plotted against each clinical laboratory measurement of apolipoprotein A-I (apoA-I) and high-density lipoprotein cholesterol (HDL-C) from the same specimens. The Spearman correlation coefficients and P values are indicated.
Correlation of the m/z 28 101 peak with apoA-I and HDL-C assays. The intensities of the m/z 28 101 from all patients with or without PAH peak are plotted against each clinical laboratory measurement of apolipoprotein A-I (apoA-I) and high-density lipoprotein cholesterol (HDL-C) from the same specimens. The Spearman correlation coefficients and P values are indicated.
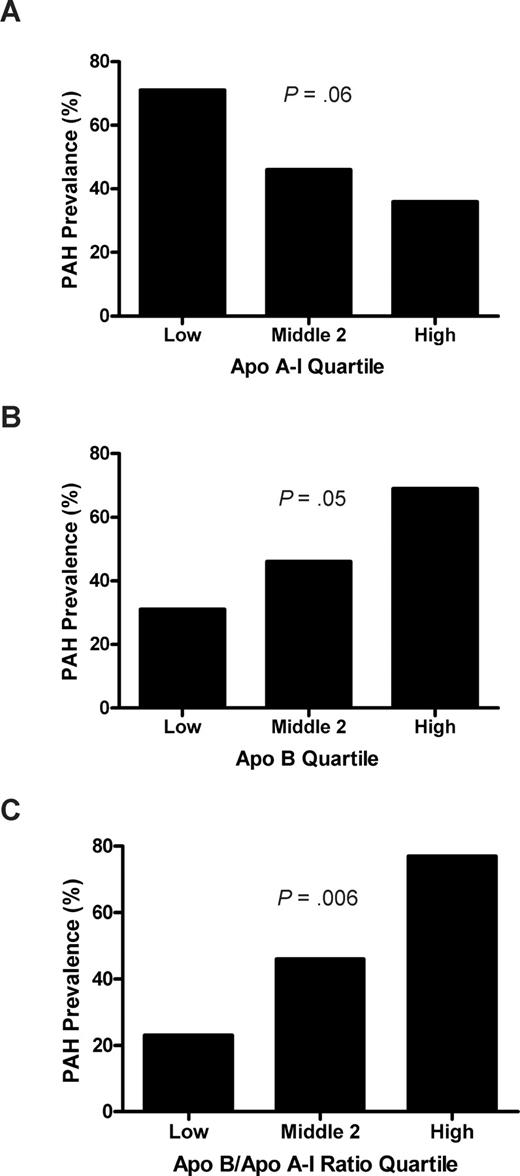
The prevalence of PAH was associated with plasma levels of apolipoproteins. In those patients whose apoA-I levels fell in the lowest quartile (< .87 g/L [87 mg/dL]), the prevalence of PAH was 71%, compared with a prevalence of 36% in the highest apoA-I quartile (> 1.11 g/L [111 mg/dL]; odds ratio [OR], 2.0), with an intermediate prevalence in the combined middle 2 quartiles (.87-1.11 g/L [87-111 mg/dL]; P = .06, log rank test for trend; Figure 4). Although apolipoprotein B (apoB) was not detected on SELDI-TOF, likely a consequence of the fractionation approach, these levels also were assayed by nephelometry in a linked assay with apoA-I. The PAH prevalence in the upper quartile of apoB levels (> .73 g/L [73 mg/dL]) was 69%, compared with 31% in the lowest quartile (< .49 g/L [49 mg/dL];OR, 2.3; P = .05). The top quartile of the ratio of apoB/apoA-I (> .72) was even more strongly associated with PAH prevalence compared with the lowest quartile (< .51) (77% vs 23%; OR, 3.3; P = .006). The receiver operating characteristic curve area of the apoB/apoA-I ratio as an independent risk marker of PAH was calculated as 0.67 (P = .04). The higher risk of PAH with low apoA-I and relatively high apoB levels is reminiscent of the same pattern in another proliferative vasculopathy, atherosclerosis, which is linked to endothelial dysfunction.
Prevalence of pulmonary arterial hypertension (PAH) by apolipoprotein quartiles. (A) Low apoA-I levels trend with high prevalence of PAH. The apoA-I levels from Figure 1 were grouped into the lowest, highest, and middle 2 quartiles, and the percentage of patients with TRV 2.5 m/s or higher was calculated. There is a strong trend toward higher prevalence of PAH in the lower apoA-I group in this analysis (P = .06, χ2 test for trend). (B) A similar analysis for apoB shows the highest prevalence of PAH in the highest quartile of apoB levels (P = .05) and (C) even more strikingly, with high apoB/apoA-I ratio (P = .006).
Prevalence of pulmonary arterial hypertension (PAH) by apolipoprotein quartiles. (A) Low apoA-I levels trend with high prevalence of PAH. The apoA-I levels from Figure 1 were grouped into the lowest, highest, and middle 2 quartiles, and the percentage of patients with TRV 2.5 m/s or higher was calculated. There is a strong trend toward higher prevalence of PAH in the lower apoA-I group in this analysis (P = .06, χ2 test for trend). (B) A similar analysis for apoB shows the highest prevalence of PAH in the highest quartile of apoB levels (P = .05) and (C) even more strikingly, with high apoB/apoA-I ratio (P = .006).
Endothelial dysfunction and apoA-I level
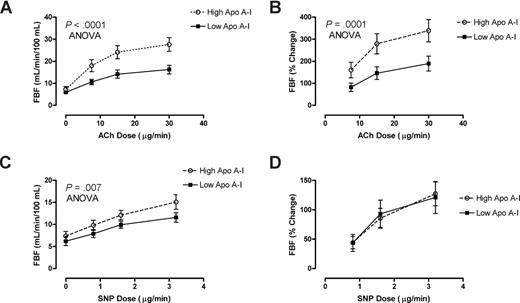
Further biologic validation of a link between low apoA-I levels and endothelial dysfunction was obtained in patients with HbSS who underwent forearm blood flow physiological studies. Patients with HbSS with lower than median levels of apoA-I (60-88 mg/dL, n = 13) demonstrated remarkably blunted mean responses to the endothelium-dependent vasodilator acetylcholine (ACh), but those with higher levels of apoA-I (.89-1.49 g/L [89-149 mg/dL], n = 13) had significantly better vasodilatory responses (percentage increase in blood flow, P < .001, ANOVA; Figure 5A,B). In sharp contrast, the lower apoA-I group showed no difference in the rate of blood flow increase in response to the endothelium-independent vasodilator sodium nitroprusside (SNP; Figure 5D), although the low apoA-I group showed globally slightly lower absolute blood flow at baseline and all doses of SNP (Figure 5C). This distinctive pattern of diminished endothelial-dependent response with unaltered endothelial-independent response in HbSS patients with lower apoA-I is once again parallel to that seen in other disorders of endothelial dysfunction, including atherosclerosis.17
Low apoA-I level is a marker for endothelial dysfunction. Forearm blood flow was measured in 26 patients with SCD with venous occlusion plethysmography following test doses of acetylcholine (ACh) and sodium nitroprusside (SNP) infused into the brachial artery. Patients with higher than median levels of apoA-I ( ) demonstrated dose-dependent vasodilation to ACh close to previously published normal values. In sharp contrast, those with lower than median levels of apoA-I had markedly blunted responses measured as (A) absolute blood flow (P < .001, 2-way ANOVA) or as (B) percentage of increase from baseline (P = .001). Although the (C) absolute blood flow was lower at baseline and all doses of SNP in low apoA-I patients (P = .007), the (D) percentage increase from baseline did not differ by apoA-I status.
) demonstrated dose-dependent vasodilation to ACh close to previously published normal values. In sharp contrast, those with lower than median levels of apoA-I had markedly blunted responses measured as (A) absolute blood flow (P < .001, 2-way ANOVA) or as (B) percentage of increase from baseline (P = .001). Although the (C) absolute blood flow was lower at baseline and all doses of SNP in low apoA-I patients (P = .007), the (D) percentage increase from baseline did not differ by apoA-I status.
Low apoA-I level is a marker for endothelial dysfunction. Forearm blood flow was measured in 26 patients with SCD with venous occlusion plethysmography following test doses of acetylcholine (ACh) and sodium nitroprusside (SNP) infused into the brachial artery. Patients with higher than median levels of apoA-I ( ) demonstrated dose-dependent vasodilation to ACh close to previously published normal values. In sharp contrast, those with lower than median levels of apoA-I had markedly blunted responses measured as (A) absolute blood flow (P < .001, 2-way ANOVA) or as (B) percentage of increase from baseline (P = .001). Although the (C) absolute blood flow was lower at baseline and all doses of SNP in low apoA-I patients (P = .007), the (D) percentage increase from baseline did not differ by apoA-I status.
) demonstrated dose-dependent vasodilation to ACh close to previously published normal values. In sharp contrast, those with lower than median levels of apoA-I had markedly blunted responses measured as (A) absolute blood flow (P < .001, 2-way ANOVA) or as (B) percentage of increase from baseline (P = .001). Although the (C) absolute blood flow was lower at baseline and all doses of SNP in low apoA-I patients (P = .007), the (D) percentage increase from baseline did not differ by apoA-I status.
Characterization of apoA-I and B levels in SCD
To characterize the levels of apolipoproteins in patients with SCD, serum apolipoprotein levels were measured in a convenience sample of 280 adults with SCD at steady state and 50 healthy African-American control subjects. SCD males had the lowest levels of apoA-I and apoB, significantly lower than SCD females and male African-American controls (Table 3). Likewise, SCD females had significantly lower apoA-I and apoB levels than female African-American controls (Table 3). The ratio of apoB to apoA-I was not significantly different between the groups, suggesting that patients with high ratios constitute distinct subgroups within each subgroup, rather than being an intrinsic feature of SCD.
Apolipoprotein levels in patients with sickle cell disease and healthy African-American control subjects
| Sex/ group . | n . | apoA-I . | apoB . | apoB/apoA-I ratio . |
|---|---|---|---|---|
| Males | ||||
| SCD | 127 | .98 (.90, 1.15)*† | .58 (.47, .72)‡§ | 0.57 (0.47, 0.78) |
| Control | 15 | 1.33 (1.15, 1.50) | .89 (.72, 1.05) | 0.70 (0.56, 0.73) |
| Females | ||||
| SCD | 153 | 1.13 (.98, 1.32)* | .65 (.52, .80)‡ | 0.58 (0.47, 0.72) |
| Control | 35 | 1.43 (1.30, 1.56) | .79 (.65, .90) | 0.53 (0.44, 0.71) |
| Sex/ group . | n . | apoA-I . | apoB . | apoB/apoA-I ratio . |
|---|---|---|---|---|
| Males | ||||
| SCD | 127 | .98 (.90, 1.15)*† | .58 (.47, .72)‡§ | 0.57 (0.47, 0.78) |
| Control | 15 | 1.33 (1.15, 1.50) | .89 (.72, 1.05) | 0.70 (0.56, 0.73) |
| Females | ||||
| SCD | 153 | 1.13 (.98, 1.32)* | .65 (.52, .80)‡ | 0.58 (0.47, 0.72) |
| Control | 35 | 1.43 (1.30, 1.56) | .79 (.65, .90) | 0.53 (0.44, 0.71) |
Apolipoprotein levels are expressed in g/L.
P < .001 compared with same-sex controls;
P < .001 compared with SCD females;
P = .001, compared with same sex controls;
P = .01, compared with SCD females; Mann-Whitney test.
Identification of additional dysregulated proteins
Five additional peaks showed differential intensities in HbSS patients with PAH, 4 of which were identified by high-resolution mass spectrometry (Table 2). Two additional apolipoproteins emerged due to their significantly higher expression in PAH. An 8900-m/z peak, significant in combination with the 28-100 m/z apoA-I peak in an exploratory stepwise logistical regression model (P = .007; ROC area = .88) was identified by LC-MS/MS as apolipoprotein A-II (Table 3). In addition, a 13 400-m/z peak, also important by the Random Forest algorithm and t test (P = .001), was identified by LC-MS/MS as the inflammatory response apolipoprotein serum amyloid A (SAA) (Table 3).
The 3 remaining peaks of interest were found at low levels in HbSS patients with PAH, both emerging as significant by the Random Forest algorithm. A SELDI-TOF peak of m/z 89 443 Da (P = .004) was determined by LC-MS/MS to be plasminogen, the precursor form of the fibrinolytic enzyme plasmin. Another peak of m/z 75 188 Da (P = .004) by SELDI-TOF proved to be the dimeric form of haptoglobin, the hemoglobin scavenger protein. A final peak of approximately m/z 18 475 Da by SELDI-TOF remains to be definitively identified, although its molecular weight resembles that of a previously reported marker, galectin-1.18
Discussion
We have used high-throughput SELDI-TOF MS as a screening tool in our investigation of pulmonary hypertension in patients with sickle cell disease to identify peaks of interest for further characterization by high-resolution mass spectrometry and immunoassay for actual identification of biomarkers. By using the SELDI-TOF MS spectra only for initial high-throughput screening for potential markers, we overcame its limitations of lower resolution and precision. Our investigation did not yield a diagnostic marker of PAH, but instead has provided initial clues to an unexpected pathway that might contribute to risk of PAH. Our results demonstrate a preliminary epidemiologic association of the development of PAH in patients with SCD with altered expression of apolipoprotein A-I, apoA-II, apoB, and SAA. All of these proteins are known to be risk indicators of vascular disease in patients without SCD.19,20 Recent studies have shown that the ratio of apoB to apoA-I has a higher predictive value than total cholesterol for risk of coronary artery disease, myocardial infarction, and stroke.21
Our results were unexpected, because patients with SCD have long been known to have very low rates of atheroma formation. Our study and several others have found levels of total cholesterol, HDL, low-density lipoprotein (LDL), apoA-I, and apoB to be significantly lower in children and adults with SCD compared with healthy controls, and this may contribute to the low atheroma rate.22-24 Low cholesterol levels were proposed by Sasaki et al to be largely a consequence of increased cholesterol consumption by membrane synthesis during increased erythrocyte production.23 ApoA-I was found not only to be low at baseline in sickle cell patients compared with normal healthy controls, but to drop even further during vaso-occlusive crisis.25 The concept of SCD vasculopathy first proposed by Hebbel, Osarogiagbon, and Kaul in 2004 highlighted the interaction of sickle erythrocytes with vascular endothelium and the role of inflammatory pathways, including IL-1β.26 Their groundbreaking model is confirmed and extended by our direct observations in humans and demonstrated preliminary evidence of a role of apolipoprotein dysregulation.
Although atherosclerosis is very rare in SCD, it is intriguing that several features are shared between the vasculopathy of sickle cell PAH and that of atherosclerotic vascular disease in those without SCD. Sickle vasculopathy is known to demonstrate vascular smooth muscle proliferation leading to histologic luminal narrowing, impaired NO vasodilation, endothelial damage with risk of in situ thrombosis, inflammation, and oxidant stress, elements also common to atherosclerotic vascular disease.26-29 The impaired response to acetylcholine in forearm blood flow studies indicative of endothelial dysfunction in patients with atherosclerosis17 we now also find in patients with SCD who have lower levels of apoA-I. The evidence presented in this study for similar dysregulation of the apolipoprotein pathway with linked endothelial dysfunction in sickle cell patients with PAH introduces yet another factor common to both atherosclerosis and sickle vasculopathy.
There are several candidate mechanisms by which apolipoprotein dysregulation might affect sickle vasculopathy. In addition to SCD, low levels of apoA-I and high SAA have been demonstrated in inflammatory disease,30-34 contributing to a proinflammatory transformation of HDL.35-37 The latter suggests that these are not only markers, but perhaps mediators of the effect of inflammation on vasculopathy, and this pathway might account for the activation of monocytes and macrophages considered a pivotal event in the vascular inflammatory process in SCD.38 ApoA-I is also linked to antioxidant function that is proposed to be involved in its vasculoprotective activity, apparently by complexing with paraoxonase-1.39,40 Oxidative stress is a prominent feature of SCD,41-45 and antioxidant activity linked to apoA-I might mediate part of its apparent protection against sickle vasculopathy and pulmonary hypertension. Finally, apoA-I plays a prominent role in the activity of scavenger receptor B-I (SRBI)–NO, an endothelial apoA-I receptor that is involved not only in reverse cholesterol transport, but also in production of NO by endothelial NO synthase (eNOS).46 Mechanistically, a defect in eNOS activation due to low apoA-I binding to SRBI would be consistent with our observed blunting of acetylcholine-induced blood flow in SCD patients with low apoA-I levels. In support of this concept, the apoA-I mimetic L-4F was found to dramatically reverse a severe defect in acetylcholine-induced blood flow in the sickle cell mouse.47 Combined with our results associating endothelial dysfunction in SCD patients with low apoA-I, the animal model data suggest a potential role for apoA-I in endothelium-regulated vasodilation in SCD.
There are several limitations to note in our present study. The SELDI-TOF platform provides a lower resolution than high-resolution mass spectrometry, and some previous publications by other groups may have overrepresented the diagnostic value of its profiles. However, its lower resolution is compensated by its strength in rapid fractionation and high throughput, providing an increase in statistical power in population studies like ours. The 2-step fractionation procedure of plasma, first by ion-exchange chromatography and second by the affinity surface binding, reduced sample complexity significantly, improving resolution of discrete peaks, but may have contributed somewhat to variability of peak intensity, as seen by the less than perfect correlation of the 28.1-kDa peak to the values obtained on unfractionated plasma in the standard apoA-I clinical laboratory assay. Here again, the very highly significant P value indicates the high confidence of the assignment of the 28.1-kDa peak to apoA-I. Most importantly, the biologic implication of the association of low apoA-I levels with pulmonary hypertension is corroborated by the link of apoA-I levels with the gold standard blood flow assay of endothelial dysfunction. Our results suggest that apoA-I levels are significantly related to PAH, but not sensitive or specific enough to be a diagnostic test. Additional testing is needed to verify whether it may be a prospective risk factor for PAH, as it is for atherosclerosis. The principal value of our observation is to identify an unexpected pathway that may be contributing to the multifactorial pathophysiology of PAH in SCD.
This apoA-I–related defect in endothelium-dependent blood flow in SCD appears to be distinct from the defect we previously reported in endothelium-independent blood flow due to hemolysis-associated scavenging of NO.16,48 We propose that these 2 defects in blood flow in patients with SCD may accelerate the development of a proliferative vasculopathy, manifested as PAH. Since our recent work has also linked PAH to priapism and cutaneous leg ulceration,1,49 it would be intriguing to investigate whether relative deficiency of apoA-I might also predispose patients to these other 2 vascular complications. The results in this paper suggest that the pathophysiology of sickle vasculopathy may overlap partially with that of atherosclerosis, but without cholesterol deposition and in a different anatomic distribution. Prospective evaluation in larger patient cohorts and in laboratory models will be required to further investigate these concepts.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the protocol coordination by Mary Hall, specimen procurement by clinical research nurses James Nichols and Lori Hunter, and clinical database management by Wynona Coles.
This research was supported by intramural research funds from the National Institutes of Health (NIH). S.Y. and A.T. were supported by the Clinical Research Training Program (CRTP), a public-private partnership supported jointly by the NIH and a grant to the Foundation for the NIH from Pfizer Pharmaceuticals Group (New York, NY).
National Institutes of Health
Authorship
Contribution: S.Y. and G.T.H. performed SELDI-TOF screening and data analysis; A.T. and A.H.C. collected and analyzed clinical data; G.W. performed LC-MS/MS analysis; X.X. and S.Y. performed the bioinformatics analyses; A.T.R. supervised confirmatory apolipoprotein assays; P.J.M. supervised bioinformatics analyses; R.F.S. supervised LC-MS/MS analysis; S.K.D. performed MALDI-TOF analysis; A.F.S. and G.J.K. supervised SELDI-TOF screening and data analysis; A.F.S. and G.J.K. designed the study; and S.Y. and G.J.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gregory J. Kato, National Institutes of Health, 10 Center Drive, MSC 1476, Building 10 CRC, Room 5-5140, Bethesda, MD 20892-1476; e-mail: gkato@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal