Abstract
Fcγ receptors (FcγRs) on mononuclear phagocytes trigger autoantibody and immune complex–induced diseases through coupling the self-reactive immunoglobulin G (IgG) response to innate effector pathways, such as phagocytosis, and the recruitment of inflammatory cells. FcRγ-based activation is critical in the pathogenesis of these diseases, although the contribution of FcγR-mediated calcium signaling in autoimmune injury is unclear. Here we show that macrophages lacking the endoplasmic reticulum–resident calcium sensor, STIM1, cannot activate FcγR-induced Ca2+ entry and phagocytosis. As a direct consequence, STIM1 deficiency results in resistance to experimental immune thrombocytopenia and anaphylaxis, autoimmune hemolytic anemia, and acute pneumonitis. These results establish STIM1 as a novel and essential component of FcγR activation and also indicate that inhibition of STIM1-dependent signaling might become a new strategy to prevent or treat IgG-dependent immunologic diseases.
Introduction
Pathogenic self-reactive antibody either in the form of soluble immune complexes or as a cellular-bound cytotoxic antibody produces fatal inflammatory responses in human autoimmune disease, such as systemic lupus erythematosus (SLE), rheumatoid arthritis, autoimmune hemolytic anemia (AIHA), immune thrombocytopenia (ITP), and hypersensitivity pneumonitis. Previous studies of immune complex–induced tissue injury in rodents have identified the roles of Fc receptors (FcRs) for immunoglobulin G (IgG; FcγRs) and the complement anaphylatoxins (C3a and C5a) as well as their receptors (C3aR and C5aR).1-3 By simultaneous triggering of activating and inhibitory signaling pathways, FcγRs control a wide array of cellular responses, including phagocytosis, antibody-dependent cell-mediated cytotoxicity, and release of inflammatory mediators, which ultimately can lead to cellular destruc-tion and the amplification of normal and pathologic immune reactions in vivo.4,5
In the mouse, there are 3 classes of activating FcγRs: the high- affinity receptor FcγRI, the low-affinity receptor FcγRIII, and the recently described FcγRIV.3,6 All these FcγRs form hetero-oligomeric complexes with the same FcR γ-chain (FcRγ), which contains an immunoreceptor tyrosine-based activation motif (ITAM) sequence that is required for cell activation.1 IgG ligand cross-linking of FcRγ-associated FcγRs on innate immune cells, such as macrophages, mast cells, natural killer cells, and neutrophils, results in tyrosine phosphorylation of the ITAM with subsequent recruitment of SH2-containing molecules and adaptor proteins that regulate the activation of effector enzymes, including phospholipase (PL) Cγ leading to production of diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3) followed by influx of extracellular Ca2+ into the cytosol.3,7-10
In immune cells, the predominant pathway of Ca2+ entry is thought to involve IP3-receptor mediated Ca2+ release from the endoplasmic reticulum (ER) Ca2+ store, which in turn induces the opening of plasma membrane–expressed store-operated Ca2+ (SOC) channels, also known as calcium release–activated Ca2+ (CRAC) channels by a mechanism known as store-operated Ca2+ entry (SOCE).11,12 In addition, DAG and some of its metabolites have been shown to induce non–store-operated Ca2+ entry (non-SOCE).13
Although SOCE has long been recognized as a major pathway of Ca2+ signaling, the principal proteins mediating this process have been discovered only recently. Stromal interaction molecule 1 (STIM1) is an ER-resident Ca2+ sensor that connects ER store depletion to the activation of SOC/CRAC channels.14-17 In its N terminus, STIM1 contains an “EF hand” that is located in the ER lumen and binds Ca2+ with low affinity. After store depletion, STIM1 accumulates in regions of ER-plasma membrane appositions (puncta), which appear to be the sites of Ca2+ entry. There it colocalizes with the 4-transmembrane domain protein Orai1 (also called CRACM1),15,18-21 which has been identified as the predominant CRAC channel in human T cells22 and mast cells.23 In addition, STIM1 has also been reported to interact with other SOC channel candidates such as transient receptor potential channels (TRPCs) 1, 2, and 4.24 Although SOCE is thought to be a major pathway of Ca2+ entry in virtually all nonexcitable cells, this has only been directly shown for T cells15,16,25 and IgE-dependent mast cell activation.26
In contrast, while CRAC activity has been detected in macrophages in one study27 and suggested to be required for chemokine receptor or Toll-like receptor (TLR)–mediated activation and expression of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α),28-30 the role of (store-operated) Ca2+ entry in FcγR-mediated phagocytosis has been controversial,31-33 and its significance for FcγR-dependent macrophage activation and autoimmune disease has remained elusive. In the current study, we analyzed Stim1−/− mice and found severely defective FcγR-mediated Ca2+ entry and phagocytosis and partially impaired induction of inflammatory cytokines in macrophages. As a consequence, Stim1−/− mice are protected from IgG-induced autoimmune inflammation.
Methods
Mice
The generation of Stim1−/− mice has been described previously.34 For the generation of bone marrow chimeras, 5- to 6-week-old C57Bl/6 female mice were lethally irradiated with a single dose of 10 Gy, and bone marrow cells from 6-week-old wild-type or Stim1−/− mice derived from the same litters were injected intravenously into the irradiated mice (4 × 106 cells/mouse). Eight weeks after transplantation, peritoneal macrophages were isolated, and STIM1 deficiency was confirmed by Western blot analysis using anti-STIM1 monoclonal antibodies from BD Transduction (GOK/Stim1, clone number 44; BD Biosciences, Franklin Lakes, NJ) and Abnova (clone 5A2, H00006786-M01; Heidelberg, Germany). The relative STIML signal for each sample was determined densitometrically and normalized to the β-tubulin signal of the same sample. All recipient animals received acidified water containing 2 g/L neomycin sulfate for 6 weeks after transplantation. FcRγ−/− B6 mice were purchased from Taconic Farms (Germantown, NY) and used at 8 to 16 weeks of age. Experiments were conducted in accordance with the regulations of the local authorities and were approved by the institutional review boards of all participating institutions.
Antibodies and fluorescence-activated cell sorting analysis
The following antibodies were used: anti-FcγRI (clone 290322) and anti-FcγRIII (clone 275003; both R&D Systems, Wiesbaden, Germany), anti-FcγRIV (clone 9E9),6 and anti-Ly17.2 (clone K9.361).35 Expression of FcγRI, FcγRII, FcγRIII, and FcγRIV on F4/80-positive peritoneal macrophage (PM) cells (2 × 104) were analyzed with Alexa 647–conjugated 290322, K9.361, 275003, and 9E9 by flow cytometry, using a FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany). The specificity of 290322 and 275003 was confirmed on PM from the FcγRI−/− and FcγRIII−/− mice, respectively, where specific staining was abrogated (see Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Intracellular calcium measurements
PM cells in Tyrode buffer without calcium were loaded with fura-2/AM (5 μM) in the presence of Pluronic F-127 (0.2 μg/mL; Molecular Probes/Invitrogen, Karlsruhe, Germany) for 25 minutes at 37°C. After labeling, the cells were washed once and activated with 5 μM thapsigargin or 10 μg/mL 2.4G2 (anti-FcγRII/IIIa; BD Transduction) followed by 20 μg/mL rabbit anti–rat IgG antibodies (DAKO, Glostrup, Denmark). The analyses were performed in Tyrode buffer containing 1 mM Ca2+ or 0.5 mM ethyleneglycoltetraacetic acid (EGTA), and fluorescence was measured with an LS 55 fluorimeter (PerkinElmer, Waltham, MA). Excitation was alternated between 340 and 380 nm, and emission was measured at 509 nm. Each measurement was calibrated using Triton X-100 and EGTA.
Rosetting and phagocytosis of IgG-opsonized red blood cells by peritoneal macrophages in vitro
Resident PM cells were flushed out of the peritoneal cavity of Stim1+/+ and Stim1−/− bone marrow transplanted mice or FcRγ3+/− and the respective wild-type mice, washed twice with phosphate-buffered saline (PBS) and suspended in RPMI 1640 medium and 10% fetal calf serum (FCS). The PM cells were allowed to adhere for 4 hours on chamber slides (Nunc, Roskilde, Denmark) at a density of 3 × 105 cells per well, followed by the removal of nonadherent cells and overnight incubation in RPMI 1640 and 1% FCS medium. Freshly isolated red blood cells (RBCs) from B6 mice were washed 2 times with ice-cold PBS by centrifugation at 1600 rpm and processed for opsonization. Hereby, 10 μL pelleted RBCs were incubated at 4°C for 1 hour with 10 μL mouse anti-RBC IgG2a and IgG2b at concentrations of 50 μg/mL. For rosetting, 250 μL 2% opsonized RBC suspension were added to each well, incubated at 4°C for 4 hours, and washed 2 times with PBS to remove unbound RBCs. To study phagocytosis, incubations were performed at 37°C for 4 hours, followed by hypotonic lysis of noningested extracellular RBCs and 2 washes with PBS. Supernatants were saved and assayed for the presence of monocyte chemotactic protein-1 (MCP-1) by enzyme-linked immunosorbent assay (ELISA; R&D Systems). PM cells were fixed with 4% paraformaldehyde, conventionally stained with Unna-Pappenheim stain, and rosette formation and phagocytosis were determined by light microscopy as described.36
Experimental AIHA
Mouse anti–mouse RBC IgG2a monoclonal autoantibody 34-3C37 was purified from tissue-culture supernatant by protein G affinity chromatography. Mice were injected intraperitoneally with 150 to 300 μg of the pathogenic monoclonal antibody (mAb), survival was monitored, and daily hematocrits (Ht) were determined with heparinized microhematocrit capillary tubes in a microfuge (4 minutes at 11 000 rpm) using blood samples obtained from the retro orbital plexus. Hematoxylin and eosin (H&E)–stained formalin-fixed sections of liver were prepared from mice killed at days 0 and 2 after 34-3C treatment and examined for histopathologic changes.
Transwell migration assay of neutrophils in vitro
Stim1−/− or wild-type bone marrow (0.75 × 106 cells in 100 μL RPMI 1640 medium, 0.5% bovine serum albumin [BSA]) was placed into the insert of a Transwell chemotaxis chamber (Costar, Cambridge, MA) and incubated for 2 hours with either RPMI/0.5% BSA supplemented with 50 ng/mL C5a or 250 ng/mL macrophage-inflammatory protein-2 (MIP-2). The neutrophils that transmigrated into the lower chamber were vigorously suspended and counted with a FACSCalibur for 1 minute at 12 μL/minute with gating on forward and side scatter. Migration of polymorphonuclear cells (PMN) from the insert to the bottom well was quantitated as the percentage of total PMN loaded into the upper chamber.
Experimental hypersensitivity pneumonitis
Mice were anesthetized with ketamine and xylazine, the trachea was cannulated, and 150 μg protein G–purified rabbit anti–ovalbumin (OVA) IgG Ab was applied. Immediately thereafter, 20 mg/kg OVA Ag was given intravenously. Mice were killed at 5 hours after initiation of pulmonary immune complex (IC) inflammation. Bronchoalveolar lavage (BAL) was performed 5 times with 1 mL PBS at 4°C. The total cell count of the BAL fluid (BALF) was assessed with a hemocytometer (Neubauer Zählkammer, Gehrden, Germany). The amount of erythrocytes represented the degree of hemorrhage. For quantitation of alveolar PMN accumulation, differential cell counts were performed on cytospins (10 minutes at 55g) stained with May-Grünwald-Giemsa using 300 μL BALF. The concentrations of TNF-α and MIP-2 in BALF were assayed with ELISA kits. The presence of bioactive C5a in BALF was determined by Transwell chemotaxis assays, using C5aR+/+ and C5aR−/− bone marrow cells. The difference in the percentage migration of C5aR+/+ and C5aR−/− PMN was quantitated.38 Myeloperoxidase (MPO) activity of lavaged lung tissue was assayed as described.39 In brief, homogenized tissue was suspended in 50 mM potassium phosphate buffer, pH 6.0, and 0.5% hexadecyltrimethyl ammonium bromide, subsequently exposed to 3 freeze-thaw cycles, and finally sonicated. A total of 0.167 mg/mL o-dianisidine dihydrochloride and 0.0005% hydrogen peroxide were added to the supernatant. The change in optical density (OD) at λ equals 450 nm was recorded. A serial dilution of MPO from human PMNs (Calbiochem-Novabiochem, Bad Soden, Germany) served as a standard.
Experimental thrombocytopenia and anaphylaxis
Ether-anesthetized mice received the indicated amounts of anti-GPIIb/IIIa antibodies40 intravenously, and platelet counts were determined using an automated cell counter (Sysmex) and confirmed by flow cytometry. For hypothermia measurement, body temperature was measured at the indicated times with a rectal probe.
Statistical analysis
Statistical analysis was performed using the SPSS version 9.0 statistical package (SPSS, Chicago, IL). To analyze differences in mean values between groups, a 2-sided unpaired Student t test was used.
Results
To address the function of STIM1 in FcγR activation and autoimmune inflammation, we analyzed Stim1−/− mice. As recently reported, STIM1 deficiency is associated with approximately 70% perinatal lethality, probably related to a cardiopulmonary defect. The surviving animals display pronounced growth retardation and a maximal life span of 4 to 6 weeks.26,34 Therefore, and to restrict STIM1 deficiency to the hematopoietic system, we transplanted lethally irradiated wild-type mice with bone marrow from Stim1−/− or wild-type control mice and analyzed them after 8 to 10 weeks. Blood cell counts in Stim1−/− chimeric mice were similar to the controls, although higher numbers of monocytes were noted (Figure 1A). To study the effect of STIM1 deficiency on macrophage functions, we isolated PMs and found increased numbers in Stim1−/− chimeras compared with the control (Figure 1B). Western blot analysis confirmed the absence of STIM1 in PM preparations from the mutant chimeras, whereas the protein was strongly expressed in control PM (Figure 1C). STIM1 was also absent in PM directly isolated from Stim1−/− mice, which served as a control. PM were microscopically indistinguishable from wild-type controls (not shown). Together, this demonstrates that STIM1 is not required for monocyte/macrophage development and transmigration into the peritoneum.
Defective SOCE in Stim1−/− macrophages. (A) Mean blood monocyte counts (± standard deviation [SD]) in wild-type and Stim1−/− chimeras (n = 5). (B) Mean PM numbers (± SD) isolated from wild-type and Stim1−/− chimeras (n = 5, *P < .05). (C) Western blot analysis of STIM1 expression in wild-type and Stim1−/− PM; β-tubulin served as a loading control. Top panel: densitometric analysis of 4 individual blots. The results shown are the mean signal intensities (± SD) for STIM1, each normalized to the signal intensity of the β-tubulin signal of the same sample (arbitrarily defined as 1.0). Bottom panel: representative blot. (D) Fura-2–loaded PM were stimulated with 5 μM TG for 10 minutes followed by addition of extracellular Ca2+ and monitoring of [Ca2+]i. Representative measurements (left) and maximal (Δ[Ca 2+]i ± SD) (n = 5 per group) before and after addition of 1 mM Ca2+ (right) are shown (***P < .001).
Defective SOCE in Stim1−/− macrophages. (A) Mean blood monocyte counts (± standard deviation [SD]) in wild-type and Stim1−/− chimeras (n = 5). (B) Mean PM numbers (± SD) isolated from wild-type and Stim1−/− chimeras (n = 5, *P < .05). (C) Western blot analysis of STIM1 expression in wild-type and Stim1−/− PM; β-tubulin served as a loading control. Top panel: densitometric analysis of 4 individual blots. The results shown are the mean signal intensities (± SD) for STIM1, each normalized to the signal intensity of the β-tubulin signal of the same sample (arbitrarily defined as 1.0). Bottom panel: representative blot. (D) Fura-2–loaded PM were stimulated with 5 μM TG for 10 minutes followed by addition of extracellular Ca2+ and monitoring of [Ca2+]i. Representative measurements (left) and maximal (Δ[Ca 2+]i ± SD) (n = 5 per group) before and after addition of 1 mM Ca2+ (right) are shown (***P < .001).
Defective SOCE and FcγR-dependent calcium signaling and phagocytosis in Stim1−/− macrophages
To assess the role of STIM1 for SOCE in macrophages, we induced SOC influx in wild-type and Stim1−/− PM with the sarcoplasmic/endoplasmatic reticulum Ca2+ ATPase (SERCA) pump inhibitor thapsigargin (TG). TG-induced Ca2+ store release was reduced approximately 50% in Stim1−/− PM compared with wild-type controls (Figure 1D). Furthermore, subsequent TG-dependent SOC influx was almost completely absent in Stim1−/− PM, demonstrating that STIM1 is essential for SOCE in macrophages and indicating that is also required for proper control of store content in those cells.
Flow cytometric analysis revealed that Stim1−/− PM express significantly reduced (approximately 45%) levels of FcγRIII, whereas FcγRIV levels were increased by approximately 58% compared with the wild type. In contrast, no differences were found in the levels of FcγRI (Figure 2A) and FcγRII (anti-Ly17.2; mean fluorescence intensity [MFI]: 157.3 ± 32.2, n = 3 vs 170.5 ± 24.9, n = 4, P = .566). Staining of the cells with the 2.4G2 antibody, which binds to FcγRII, III, and probably also IV41 was also not significantly different between wild-type and mutant PM (MFI: 343.5 ± 56 vs 294.0 ± 60, n = 4, P = .13). To determine the significance of defective SOCE for FcγR-dependent macrophage activation, we assessed changes in [Ca2+]i in response to direct FcγR activation by the 2.4G2 antibody cross-linked by a secondary antibody (Figure 2B). While a pronounced increase in [Ca2+]i was observed in wild-type PM upon addition of the cross-linking antibody, this response was dramatically reduced in Stim1−/− PM. This demonstrated for the first time that STIM1-dependent SOCE is a central mechanism of Ca2+ entry in macrophages in response to FcγR activation.
Essential role for STIM1 in macrophage FcγR-dependent calcium signaling and phagocytosis, but not MCP-1 production. (A) PM cells of Stim1+/+ and Stim−/− chimeric mice were stained with Alexa 647–conjugated FcγRI, FcγRIII, and FcγRIV-specific mAbs in combination with PE-F4/80 and analyzed by fluorescence-activated cell sorting (FACS). Data shown are MFI (± SD; n = 4, **P < .01). (B) Fura-2–loaded PM were incubated with 10 μg/mL 2.4G2 for 4 minutes followed by addition of 20 μg/mL cross-linking anti–rat IgG antibodies and monitoring of [Ca2+]i. Representative measurements (left) and maximal (Δ[Ca2+]i ± SD, n = 5 per group) are shown. (C-E) PM cells were incubated with uncoated (control) or IgG2-coated MRBC. (C) To study binding, incubations were performed for 4 hours at 4°C. To study phagocytosis (D) and MCP-1 release (E), incubations were performed for 4 hours at 37°C followed by the lysis of extracellular MRBC. (C,D) The percentage of positive cells that formed rosettes with more than 3 erythrocytes or ingested more than 1 erythrocyte was assessed microscopically. The results shown are mean (± standard error of the mean [SEM]) of 2 independent experiments performed in duplicate (**P < .001).
Essential role for STIM1 in macrophage FcγR-dependent calcium signaling and phagocytosis, but not MCP-1 production. (A) PM cells of Stim1+/+ and Stim−/− chimeric mice were stained with Alexa 647–conjugated FcγRI, FcγRIII, and FcγRIV-specific mAbs in combination with PE-F4/80 and analyzed by fluorescence-activated cell sorting (FACS). Data shown are MFI (± SD; n = 4, **P < .01). (B) Fura-2–loaded PM were incubated with 10 μg/mL 2.4G2 for 4 minutes followed by addition of 20 μg/mL cross-linking anti–rat IgG antibodies and monitoring of [Ca2+]i. Representative measurements (left) and maximal (Δ[Ca2+]i ± SD, n = 5 per group) are shown. (C-E) PM cells were incubated with uncoated (control) or IgG2-coated MRBC. (C) To study binding, incubations were performed for 4 hours at 4°C. To study phagocytosis (D) and MCP-1 release (E), incubations were performed for 4 hours at 37°C followed by the lysis of extracellular MRBC. (C,D) The percentage of positive cells that formed rosettes with more than 3 erythrocytes or ingested more than 1 erythrocyte was assessed microscopically. The results shown are mean (± standard error of the mean [SEM]) of 2 independent experiments performed in duplicate (**P < .001).
We then investigated the effect of STIM1 deficiency on FcγR-dependent phagocytosis. PM cells were cultured overnight, incubated for 4 hours with mouse red blood cells (MRBCs) opsonized with anti-MRBC IgG and measured for binding (at 4°C) and phagocytic ingestion (at 37°C) of MRBCs by light microscopy as described.36 Despite their intact functionality to bind IgG-coated MRBCs (Figure 2C), macrophages from Stim1−/− chimeras showed strongly diminished IgG2a- and IgG2b-mediated phagocytosis (Figure 2D), providing direct evidence that STIM1 is essential for FcγR-mediated phagocytosis. We also examined FcγR-induced MCP-1 production. Strikingly, similar levels of MCP-1 were found in the cell-culture supernatant of wild-type and Stim1−/− PM stimulated by IgG-bound MRBCs (Figure 2E). To test whether the reduced expression of FcγRIII in Stim1−/− PM could explain the phagocytosis defect, we analyzed PM from FcRγ+/− mice. While FcγRIII expression levels were reduced by approximately 50% in those cells compared with the wild-type, binding and phagocytosis of IgG2a-opsonized RBCs was unaltered (Figure S2), clearly showing that the reduced expression of FcγRIII is not sufficient to significantly inhibit phagocytosis. Together, these results demonstrated that FcγR-induced phagocytosis, but not its associated cytokine induction is dependent on STIM1.
STIM1 in AIHA
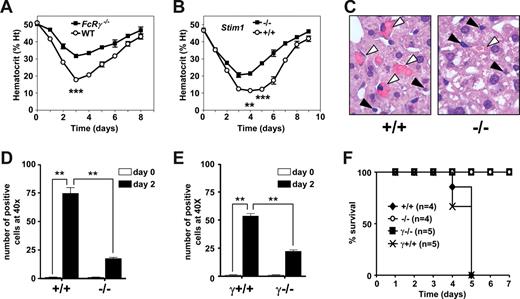
The above results demonstrated that STIM1 is essential for FcγR-mediated phagocytosis, but also that other FcγR-mediated effector functions, such as MCP-1 production, in response to FcγR activation are preserved in the absence of the calcium sensor. To test the significance of this selective defect for IgG-triggered pathologies, we subjected wild-type and Stim1−/− mice to a model of AIHA, where the disease is induced by a single intraperitoneal injection of the anti-MRBC 34-3C mAb.36,37,42,43 The resultant anemia, as evidenced by a reduction in Ht, is to a great extent mediated through FcRγ-chain–dependent erythrophagocytosis by Kupffer cells (Figure 3A). Four days after the treatment with 150 μg of the pathogenic anti-MRBC mAb, wild-type chimeras showed a significantly stronger reduction in Ht levels than Stim1−/− chimeras (wild-type Ht, 11.4% ± 1.7% vs Stim1−/− Ht, 21.4% ± 2.1%, P < .001) (Figure 3B). Qualitative and quantitative histologic examinations of the liver showed that Kupffer cells in wild-type chimeras contained phagocytosed RBCs. In contrast, only a minor population of the Kupffer cells in Stim1−/− chimeras showed phagocytosis of RBCs (Figure 3C). Moreover, the cell count of phagocytic liver cells per microscopic field (original magnification, ×40) was markedly reduced in the Stim1−/− chimeric animals (Figure 3D) down to levels as observed in FcRγ-chain–deficient mice (Figure 3E). To further substantiate the observation that STIM1 deficiency is protective against IgG-induced anemia, we also determined the cytotoxic effects of higher doses of the 34-3C mAb. An amount of 300 μg of this antibody was sufficient to induce lethal AIHA in wild-type chimeras, whereas all Stim1−/− chimeras survived. As a control, FcRγ-chain–deficient mice were analyzed and all of them survived (Figure 3F).
Experimental AIHA in FcRγ and STIM1 wild-type and knockout mice. Transient anemia was induced in FcRγ+/+ and FcRγ−/− B6 mice (A,E) and in Stim1+/+ (+/+) and Stim1−/− (−/−) chimeras (B-D) by injection of 150 μg of the pathogenic 34-3C IgG2a mAb, and daily hematocrit was assessed (A,B). Liver H&E sections (original magnification, ×40) from 2 days anemic mice were evaluated both qualitatively (C) and quantitatively (D,E) for the occurrence of erythrophagocytosis. (F) Mice also received a lethal dose of anti-RBC 34-3C (300 μg), and survival was monitored. Results were obtained from 4 to 5 mice in each group (*P < .05, **P < .001).
Experimental AIHA in FcRγ and STIM1 wild-type and knockout mice. Transient anemia was induced in FcRγ+/+ and FcRγ−/− B6 mice (A,E) and in Stim1+/+ (+/+) and Stim1−/− (−/−) chimeras (B-D) by injection of 150 μg of the pathogenic 34-3C IgG2a mAb, and daily hematocrit was assessed (A,B). Liver H&E sections (original magnification, ×40) from 2 days anemic mice were evaluated both qualitatively (C) and quantitatively (D,E) for the occurrence of erythrophagocytosis. (F) Mice also received a lethal dose of anti-RBC 34-3C (300 μg), and survival was monitored. Results were obtained from 4 to 5 mice in each group (*P < .05, **P < .001).
STIM1 in immune complex-mediated pneumonitis
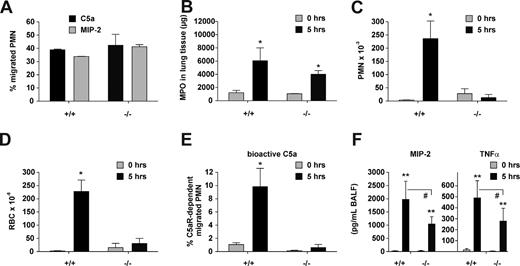
To further assess the function of STIM1 in inflammatory reactions involving tissue-resident macrophages, we then used the in vivo IgG IC-mediated model of hypersensitivity pneumonitis/alveolitis.44 We injected OVA-specific IgG into the trachea of wild-type and Stim1−/− chimeras followed by intravenous injection of the OVA antigen to induce the acute reaction. In this type of lung injury, the activation of alveolar macrophages (AM) causes neutrophil influx into the lung and their subsequent transmigration into the alveolar compartment by a mechanism that depends on the cooperation of FcγRIII and the C5a anaphylatoxin receptor, C5aR.38,39,45,46 Neutrophil counts in blood from wild-type and Stim1−/− chimeras were comparable (800 ± 200 vs 1000 ± 100, n = 5, P > .05). IC-treated mice were killed at 5 hours, and their lung tissues and bronchoalveolar lavage (BAL) fluids were analyzed for hemorrhage, neutrophil infiltration, and the presence of PMN recruitment factors as described.44 Despite their capacity to normally respond to C5a and MIP-2 stimuli in vitro (Figure 4A) and to infiltrate the lung interstitium upon IgG-IC challenge in vivo (Figure 4B), neutrophils in Stim1−/− chimeras were unable to migrate into alveoli (Figure 4C). This defect appeared to be based on impaired generation of vasoactive C5a, as neither enhanced vascular permeability, as evaluated by hemorrhage (Figure 4D), nor the appearance of bioactive C5a (Figure 4E) was observed in Stim1−/− chimeras. In contrast, both MIP-2 and TNF-α were substantially produced in the mutant animals, although at somewhat lower levels than in control mice (Figure 4F). Taken together, these results suggest that STIM1 is required for the FcγR-induced generation of C5a, and, consistent with previous reports,38,46 indicate that this process is essential to induce neutrophil accumulation at extravascular sites in pneumonitis.
Experimental hypersensitivity pneumonitis in Stim1−/− chimeras. (A) Polymorphonuclear cell (PMN) migration in response to C5a and MIP-2 as measured in vitro by Transwell chemotaxis assays using PMN from bone marrow of the indicated mice. (B-F) IgG immune complex–induced lung injury was initiated in Stim1+/+ (+/+) and Stim1−/− (−/−) chimeric mice, and interstitial neutrophil influx in lung tissue was assessed by measuring MPO activity (B). Content of PMN (C), RBC (D), C5a-dependent bioactivity (E), and contents of MIP-2 and TNF-α (F) in BAL fluids were determined in samples obtained 5 hours after immune complex–induced pneumonitis. *P < .05 and **P < .001 compared with 0-hour controls. #P < .05 wild-type compared with Stim1−/− 5-hour response. For each bar, n = 4 to 5 mice.
Experimental hypersensitivity pneumonitis in Stim1−/− chimeras. (A) Polymorphonuclear cell (PMN) migration in response to C5a and MIP-2 as measured in vitro by Transwell chemotaxis assays using PMN from bone marrow of the indicated mice. (B-F) IgG immune complex–induced lung injury was initiated in Stim1+/+ (+/+) and Stim1−/− (−/−) chimeric mice, and interstitial neutrophil influx in lung tissue was assessed by measuring MPO activity (B). Content of PMN (C), RBC (D), C5a-dependent bioactivity (E), and contents of MIP-2 and TNF-α (F) in BAL fluids were determined in samples obtained 5 hours after immune complex–induced pneumonitis. *P < .05 and **P < .001 compared with 0-hour controls. #P < .05 wild-type compared with Stim1−/− 5-hour response. For each bar, n = 4 to 5 mice.
STIM1 in IgG-mediated thrombocytopenia and anaphylaxis
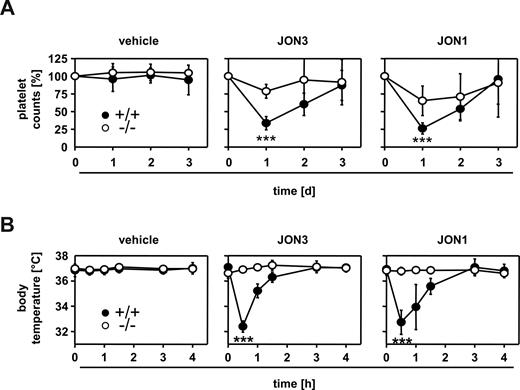
Next, we sought to determine the role of STIM1 in IgG-induced thrombocytopenia. In human ITP, platelet surface glycoprotein IIb/IIIa (GPIIb/IIIa, αIIbβ3 integrin) is the most common antigenic target with approximately 70% to 80% of patients displaying autoantibodies to this receptor complex.47-49 Therefore, we injected wild-type and Stim1−/− chimeras with 7 μg anti-GPIIb/IIIa (JON) antibodies and monitored platelet counts as described.50 We have previously shown that JON3 (rat IgG1)-induced pathologies are predominantly mediated through FcγRIII, whereas JON1 (rat IgG2b)–induced pathologies depend on FcγRIII and probably also FcγRIV.51 Both JON3 and JON1 induced profound thrombocytopenia in wild-type chimeras, with a maximal reduction of platelet counts of 66.3 (± 9.3) and 77.0% (± 5.4%) after 24 hours, respectively, (Figure 5A) confirming previous results.50 In contrast, thrombocytopenia was significantly ameliorated in Stim1−/− chimeras with a maximal reduction in platelet counts of 21.3% (± 9.7%) for JON3 and 30.0 (± 16.0) for JON1, respectively. These results demonstrate that STIM1-dependent processes are crucial for the destruction of IgG-opsonized platelets.
Stim1−/− mice are protected from antiplatelet IgG-induced thrombocytopenia and anaphylaxis. (A) Wild-type or Stim1−/− chimeras received 7 μg JON3 (rat IgG1) or JON1 (rat IgG2b; both directed against GPIIb/IIIa) or vehicle IV and platelet counts were monitored. The results shown are mean (± SD) of 6 mice per group. (B) Wild-type or Stim1−/− chimeras received vehicle or 100 μg JON3 orJON1 and body temperature was monitored with a rectal probe. The results shown are mean (± SD) of 5 mice per group (***P < .001).
Stim1−/− mice are protected from antiplatelet IgG-induced thrombocytopenia and anaphylaxis. (A) Wild-type or Stim1−/− chimeras received 7 μg JON3 (rat IgG1) or JON1 (rat IgG2b; both directed against GPIIb/IIIa) or vehicle IV and platelet counts were monitored. The results shown are mean (± SD) of 6 mice per group. (B) Wild-type or Stim1−/− chimeras received vehicle or 100 μg JON3 orJON1 and body temperature was monitored with a rectal probe. The results shown are mean (± SD) of 5 mice per group (***P < .001).
We have previously shown that anti-GPIIb/IIIa antibodies are unique among antiplatelet antibodies in that a bolus injection of a high dose triggers, in addition to thrombocytopenia, an (eventually lethal) acute systemic reaction by FcγR-dependent mechanisms.40,51,52 To determine the relative contribution of STIM1 in this part of the pathology, we injected wild-type and Stim1−/− chimeras with 100 μg JON3 or JON1 and monitored body temperature. In agreement with the previous observations, wild-type chimeras developed anaphylactic shock and severe hypothermia within minutes upon intravenous injection. In contrast, Stim1−/− chimeras were completely protected from anti-GPIIb/IIIa induced anaphylaxis (Figure 5B).
Discussion
The identification of STIM1 as the “missing link” between intracellular Ca2+ store depletion and the activation of plasma membrane CRAC channels has enabled gain-of-function 53 and loss-of-function 25,26,34 studies to examine the significance of SOCE for mammalian physiology and disease processes. Here we have used STIM1-deficient mice to demonstrate that STIM1 is an essential component of distinct FcγR-induced signaling events in macrophages and a central mediator of IgG-triggered autoimmune inflammation.
The analysis of mice with genetic ablation of Stim1 gene expression has recently demonstrated that STIM1 is a critical mediator of SOCE downstream of T-cell receptor (TCR) stimulation25 and mast cell activation through FcϵR.26 These studies confirmed the proposed central role of SOCE in these 2 cell types, which have been the best characterized cell systems to study CRAC activity.11,12,54 In contrast, less was known about the mechanisms of FcγR-induced Ca2+ entry in immune cells and its contribution to cellular activation and subsequent effector functions. We found severely defective TG-induced SOCE and FcγR-triggered Ca2+ responses in Stim1−/− macrophages (Figures 1E, 2A) demonstrating for the first time that SOCE is the predominant mechanism of Ca2+ entry downstream of FcγR activation and that STIM1 is a critical mediator of this process. The residual FcγR-induced Ca2+ influx detected in Stim1−/− PM might be explained by STIM2-regulated SOCE55 or the existence of a non-SOCE pathway in those cells, possibly induced by DAG or one of its metabolites.13
In addition to defective Ca2+ entry, we also observed reduced TG-induced Ca2+ release from intracellular stores indicating a lower filling state of the ER (Figure 1E). This has also been reported for Stim1−/− mast cells26 suggesting a function of STIM1 in the filling of intracellular Ca2+ stores. Because normal store content has been detected in mast cells lacking the CRAC channel Orai1 (CRACM1),23 this may occur through interactions of STIM1 with the IP3 receptors or SERCA pumps in the ER rather than CRAC channels in the plasma membrane.
FcγR-dependent phagocytosis was almost completely abrogated in PM from Stim1−/− chimeras, demonstrating that SOCE is essential for this response to occur. This finding provides a definitive answer to the long-standing question on the role of calcium signals in the process of FcγR-dependent phagocytosis in macrophages.12,31-33 Although significant alterations in the expression levels of FcγRIII and IV were noted (Figure 2A), these do not account for the described phenotype of Stim1−/− mice for the following reasons. First, while phagocytosis of opsonized RBCs is inhibited in Stim1−/− marcrophages, MCP-1 production is normal, demonstrating that signal transduction through the receptors is not generally altered in the mutant cells. Second, we show that PM from FcRγ+/− mice display unaltered phagocytosis of IgG2a-opsonized RBCs despite an approximately 50% reduction in FcγRIII expression (Figure S2). Third, the inhibition of phagocytosis is seen with both IgG2a as well as IgG2b, which trigger predominantly FcγRIII, or FcγRIII and IV, respectively. Because FcγRIV levels are increased in the mutant cells, this clearly shows that the lack of phagocytosis is a functional defect downstream of the receptors rather than an effect of inappropriate receptor levels. These findings also demonstrate that FcγR-dependent phagocytosis can be mechanistically uncoupled from the associated cytokine induction in macrophages and thereby reveals that these processes are mediated by different signaling pathways. In vivo, pulmonary Stim1−/− macrophages produced substantial amounts of TNF-α and MIP-2 downstream of immune complex–induced FcγR activation, but the response was significantly reduced compared with the control. Thus, STIM1-dependent SOCE contributes to, but is not essential for FcγR-induced inflammatory cytokine production in macrophages. Similar observations have been reported in Stim1−/− mast cells26 and T cells,25 although the defect was somewhat more pronounced in those cells. While these results show that STIM1 is dispensable for ITAM-triggered cytokine expression in immune cells, they do not exclude a requirement for elevated [Ca2+]i.
Type II inflammation, which is characterized by autoantibody-mediated cellular destruction, contributes to many human autoimmune conditions and is specifically causal in AIHA56,57 and ITP.47-49,58 In line with the severe defect in FcγR-dependent phagocytosis, both FcRγ-deficient mice and Stim1−/− chimeras were significantly protected from IgG-induced elimination of red blood cells in anemia (Figure 3). Similar results were also obtained for thrombocytopenia (Figure 5A), confirming that FcγRs are key effectors in type II tissue injury and that STIM1 is a central mediator of pathology in these 2 examples of autoimmune disease.
HIV-1–related ITP in humans has been reported to involve formation of platelet-derived IgG immune complexes,59 and we previously observed similar microparticle-like structures in murine ITP induced by mAbs JON1/3, resulting in Fc-dependent acute anaphylaxis.40,51,52 We found an almost complete absence of this unique kind of type I inflammation in STIM1-deficient mice. This shows that, in addition to its indispensable role for IgE-dependent anaphylaxis by FcϵR-bearing mast cells,26 STIM1 is also of critical importance in certain systemic reactions that are IgG-dependent and mediated by FcγR. It is important to note, however, that it is at present not clear whether macrophages or other cell types, most notably mast cells, mediate this response.
In addition to type I/II inflammation, we also observed that Stim1−/− chimeras were resistant to the development of pneumonitis/alveolitis in the lung Arthus reaction, the classical animal model for type III IgG IC-triggered inflammatory disorders in humans. FcγR-induced C5a bioactivity was absent in alveoli of Stim1−/− mice, providing strong in vivo evidence for the essential role of STIM1 in the generation of C5a, which was suggested by previous studies to mainly depend on macrophage serine protease activity.60 TNF-α and MIP-2 secretion was inhibited but not abolished in Stim1−/− chimeras. Despite production of these 2 inflammatory mediators, Stim1−/− neutrophils did not transmigrate from the pulmonary vasculature into the alveolar compartment in the functional absence of C5a. This failure was not caused by an intrinsic defect of Stim1−/− PMN migration, as indicated by substantial accumulation of those cells in lung interstitium. Collectively, these data suggest for a critical and selective requirement of STIM1 in C5a, but not cytokine production, and confirm FcγR-dependent C5a as the recently proposed dominant initiator of the type III inflammatory cascade.38,46
In conclusion, we have shown that SOCE is a crucial component of FcγR-triggered signaling and autoimmune inflammation and that STIM1 is an essential mediator in this process. These findings extend the crucial role of SOCE as a central mechanism of immune cell activation beyond T cells and mast cells and may serve as basis for the development of novel pharmacologic agents to control or prevent such diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank L. Engling and S. Kumari for their help with the PMN migration assays and in vivo experiments.
This work was supported by the Rudolf Virchow Center, the Int. MD/PhD Program of Molecular Medicine at Hannover Medical School, and the Deutsche Forschungsgemeinschaft (DFG; SFB 688 and grant Ni556/7-1 to B.N.; GRK705, SFB587, and grant Ge892/10-1 to J.E.G.). D.S. was supported by a grant of the German Excellence Initiative to the Graduate School of Life Sciences, University of Würzburg.
Authorship
Contribution: A.B. generated the gene-disrupted mice, contributed to the ITP experiments, calcium measurements, and blood cell analyses; D.V.S. established the calcium measurements, characterized the bone marrow chimeras, and contributed to the in vivo experiments; S.N.S. did the phagocytosis and AIHA experiments; S.K. did the immune complex alveolitis experiments; D.S. and T.V. did the ITP experiments; R.E.S. contributed to the writing of the manuscript; and J.E.G. and B.N. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Nieswandt, Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University of Würzburg, Zinklesweg 10, 97078 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de; or J. Engelbert Gessner, Hannover Medical School, Molecular Immunology Research Unit, Clinic for Immunology and Rheumatology, Hannover, Germany; e-mail: gessner.johannes@mh-hannover.de.
References
Author notes
*A.B. and J.E.G. contributed equally to this work.

![Figure 1. Defective SOCE in Stim1−/− macrophages. (A) Mean blood monocyte counts (± standard deviation [SD]) in wild-type and Stim1−/− chimeras (n = 5). (B) Mean PM numbers (± SD) isolated from wild-type and Stim1−/− chimeras (n = 5, *P < .05). (C) Western blot analysis of STIM1 expression in wild-type and Stim1−/− PM; β-tubulin served as a loading control. Top panel: densitometric analysis of 4 individual blots. The results shown are the mean signal intensities (± SD) for STIM1, each normalized to the signal intensity of the β-tubulin signal of the same sample (arbitrarily defined as 1.0). Bottom panel: representative blot. (D) Fura-2–loaded PM were stimulated with 5 μM TG for 10 minutes followed by addition of extracellular Ca2+ and monitoring of [Ca2+]i. Representative measurements (left) and maximal (Δ[Ca 2+]i ± SD) (n = 5 per group) before and after addition of 1 mM Ca2+ (right) are shown (***P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/5/10.1182_blood-2008-05-158477/4/m_zh80020929130001.jpeg?Expires=1765925217&Signature=eX3TTWaxS24ifmnZBm8F-vuRYtZJPZ1PRQG1ZMmRsEC90ENOpzFAh6nWCltHzmUvfnn-VcTdbP-3rv4tBUP~bXNvLLtXYSnYfkN8YJNOasYqdD7XGHy0dtolIdghJt2479r~W6zHLu1t01apAynjXDObwLJz8pVZ8gXDjNnTBcHlO4VmLBdOnzxU-ZA0iRGrcU2l-pVZ87ehk4llcbHkL7kTsQTp9z-Psgjk65p0wSFawFkGxtklYIqTZ~4K094pNAYGNZRt2p9l82amE9RXHC83L2OqXVnUIkS61f503k~vGcRKC4izOTRFQByUd9MUZDoOkHGDgupVE8aZEFlCKA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Essential role for STIM1 in macrophage FcγR-dependent calcium signaling and phagocytosis, but not MCP-1 production. (A) PM cells of Stim1+/+ and Stim−/− chimeric mice were stained with Alexa 647–conjugated FcγRI, FcγRIII, and FcγRIV-specific mAbs in combination with PE-F4/80 and analyzed by fluorescence-activated cell sorting (FACS). Data shown are MFI (± SD; n = 4, **P < .01). (B) Fura-2–loaded PM were incubated with 10 μg/mL 2.4G2 for 4 minutes followed by addition of 20 μg/mL cross-linking anti–rat IgG antibodies and monitoring of [Ca2+]i. Representative measurements (left) and maximal (Δ[Ca2+]i ± SD, n = 5 per group) are shown. (C-E) PM cells were incubated with uncoated (control) or IgG2-coated MRBC. (C) To study binding, incubations were performed for 4 hours at 4°C. To study phagocytosis (D) and MCP-1 release (E), incubations were performed for 4 hours at 37°C followed by the lysis of extracellular MRBC. (C,D) The percentage of positive cells that formed rosettes with more than 3 erythrocytes or ingested more than 1 erythrocyte was assessed microscopically. The results shown are mean (± standard error of the mean [SEM]) of 2 independent experiments performed in duplicate (**P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/5/10.1182_blood-2008-05-158477/4/m_zh80020929130002.jpeg?Expires=1765925217&Signature=4~nkoe6sBt96Ea69IpavMsHBut7NZPvPdvviwaLuDGnp6ATKofgUsQ4lH5JBwiwnLSclQAYXJOk~y~SodCxdeQYYs111mbvb3wq8udFOkVFGuknziKUU1tAXeAgNJZ6t25uwljgIV~tMl6l7EYKvyakj2wD2oNYDcuW6hVsORFUVlQm8rdu3TypMbXoJ0SBjjIU-E8adPBuUSqDZw7~koSL1T0XQZNzKEDfXdjxVNb49l5oToCjTxFHWrIBKjNNEVI8oFkG4HODqBErMd81aefUboTrhik~KGt1cyCpQJ5z1dgeXadXJ5ey0HtKqwncK7TLY~XRAxl1vlNyw5oebVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal