Abstract
Follicular lymphoma (FL) is a morphologically and genetically well-characterized B-cell non-Hodgkin lymphoma that can show predominantly follicular, combined follicular and diffuse, or predominantly diffuse growth patterns. Although approximately 85% of FLs harbor the translocation t(14;18)(q32;q21) and consistently display a follicular growth pattern, predominantly diffuse FLs are less well characterized on the phenotypical, molecular, and clinical level. We studied 35 predominantly diffuse FL by immunohistochemistry, classical chromosome banding analysis, fluorescence in situ hybridization (FISH), and gene expression profiling. A total of 28 of 29 analyzable cases lacked t(14;18), and 27 of 29 cases revealed a unifying chromosomal aberration, a deletion in 1p36. Morphologically, 12 FLs were grade 1 and 23 were grade 2, and the immunophenotype with frequent expression of CD10, BCL6, and CD23 was in line with a germinal center B-cell phenotype. The gene expression profiles of 4 predominantly diffuse FLs fell into the spectrum of typical FL, with a unique enrichment of specific gene signatures. Remarkably, patients with diffuse FL frequently presented with low clinical stage and large but localized inguinal tumors. These results suggest that predominantly diffuse FL represent a distinct subtype of t(14;18)-negative nodal FL with a unifying genetic alteration (deletion of 1p36) and characteristic clinical features.
Introduction
The World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues1 distinguishes disease entities on the basis of their different morphologic and immunologic features, genetic constitution, and clinical behavior, thus attempting to define homogeneous biologic disease entities. In this respect, follicular lymphoma (FL) represents an archetype of a disease entity, being characterized by rather uniform morphologic features, a typical immunophenotype, and a recurring primary genetic aberration, the t(14;18)(q32;q21) chromosome translocation that can be found in the majority of cases. Despite these uniform features present in roughly 70% to 85% of FLs, some disease variants do constitute exceptions to the general rule. Their recognition and correct classification is important to understand the diagnostic spectrum of the disease. For example, FL grade 3B (FL3B) are composed of blasts exclusively, lack—as a rule—the t(14;18), may be CD10 negative, and do not express the BCL-2 protein in a considerable number of cases.2,3 FL3B in combination with a diffuse large B-cell component (FL3B+DLBCL) frequently harbor translocations involving the BCL6 gene in chromosomal band 3q27, thus exemplifying that variant histologies may predict for a distinct genetic constitution and, hence, biology.
In this article, we describe a hitherto unrecognized subtype of FL that is characterized by an unusual, predominantly diffuse growth pattern, a frequent presentation in the inguinal region, and the formation of large, localized tumors. These FLs show a particular cytogenetic constitution that lacks the t(14;18) and displays a characteristic uniform chromosomal aberration, a deletion in the terminal parts of the short arm of chromosome 1, del(1p36).
Methods
Tumor specimens
A total of 35 cases of nodal malignant B-cell non-Hodgkin lymphoma (B-NHL) that were referred to the German Reference Center for Lymph Node Pathology at the Department of Pathology, University of Würzburg, the Department of Pathology, Caritas-Krankenhaus Bad Mergentheim, or the Department of Clinical Pathology, Robert-Bosch-Krankenhaus, between 1986 and 2007 were investigated in the present study. The study was approved by the local ethics committees of all participating institutions, and informed consent was obtained in accordance with the Declaration of Helsinki. All cases were classified as FL with a predominantly diffuse growth pattern according to the criteria of the WHO classification system (< 25% follicularity).1 Slides were cut from formalin-fixed and paraffin-embedded tissues and stained with hematoxylin and eosin, Giemsa, and periodic acid-Schiff (PAS).
Immunohistochemistry
For diagnostic purposes, we performed immunostaining on paraffin sections using B-cell markers CD20 (Clone L26; DAKO, Glostrup, Denmark; 1:1000) and CD23 (Clone 1B12; Novocastra, Newcastle upon Tyne, United Kingdom; 1:80) and T-cell markers CD3 (Novocastra; 1:80) and CD5 (Clone 4C7; Novocastra; 1:40). Proliferation indices (PIs) were recorded after staining with the MIB1 antibody (MM1; Novocastra; 1:30), detecting the Ki67 antigen, in increments of 10%. We evaluated CD10, BCL-2, and BCL-6 expressions on paraffin sections using monoclonal antibodies to CD10 (NCL-CD10 270; Novocastra; 1:100), BCL-2 (Clone124; DAKO, Hamburg, Germany; 1:50), and BCL-6 (Clone PG-B6p; DAKO; 1:5), respectively. The presence of follicular dendritic cell (FDC) meshworks was ascertained either by staining for CD23 (Clone 1B12; Novocastra; 1:80) or using an antibody to CD21 (Clone 1F8; DAKO; 1:200). All immunohistochemical reactions were performed after antigen retrieval by pressure cooking using the peroxidase antiperoxidase (PAP) method.
Clinical data
Basic clinical data of all 35 patients with FL demonstrating a predominantly diffuse growth pattern were retrieved from treating physicians according to clinical records, and treatment information was obtained from 22 patients (Table 1). A total of 34 (97%) of 35 of the specimens were obtained before treatment and represented initial diagnostic biopsies. One patient had already been treated by the time the biopsy was performed.
Clinical data of 35 follicular lymphomas with a predominantly diffuse growth pattern investigated in the present study
| Clinical feature . | Value . | No. of cases with information available . |
|---|---|---|
| Mean age, y (range) | 57 (27-85) | 35 |
| Male sex, n (%) | 18 (51) | 35 |
| Ann Arbor stage I/II, n (%) | 15 (75) | 20 |
| Median/average diameter of tumor (range) | 4.00 cm/5.6 cm (1.8-18 cm) | 33 |
| Localization of lymph nodes, n (%) | 35 | |
| Inguinal | 29/35 (83) | |
| Cervical | 3/35 (9) | |
| Axillary | 3/35 (9) | |
| Bone marrow infiltration, n (%) | 1 (5) | 19 |
| Primary diagnostic material, n (%) | 34 (97) | 35 |
| Treatment | ||
| Median/average follow-up, mo | 29/52.6 (1-191) | 21 |
| Death, n (%) | 3 (15)* | 20 |
| No therapy, n (%) | 2 (11) | 19 |
| Chemotherapy (CHOP/CHOP-like), n (%) | 3 (16) | 19 |
| Radiotherapy, n (%) | 8 (42) | 19 |
| Combined radio- and chemotherapy, n (%) | 6 (32) | 19 |
| CR after initial therapy, n (%) | 15 (94) | 16 |
| Recurrence after initial therapy, n (%) | 2 (13) | 16 |
| Progression after initial therapy, n (%) | 1 (6) | 16 |
| Clinical feature . | Value . | No. of cases with information available . |
|---|---|---|
| Mean age, y (range) | 57 (27-85) | 35 |
| Male sex, n (%) | 18 (51) | 35 |
| Ann Arbor stage I/II, n (%) | 15 (75) | 20 |
| Median/average diameter of tumor (range) | 4.00 cm/5.6 cm (1.8-18 cm) | 33 |
| Localization of lymph nodes, n (%) | 35 | |
| Inguinal | 29/35 (83) | |
| Cervical | 3/35 (9) | |
| Axillary | 3/35 (9) | |
| Bone marrow infiltration, n (%) | 1 (5) | 19 |
| Primary diagnostic material, n (%) | 34 (97) | 35 |
| Treatment | ||
| Median/average follow-up, mo | 29/52.6 (1-191) | 21 |
| Death, n (%) | 3 (15)* | 20 |
| No therapy, n (%) | 2 (11) | 19 |
| Chemotherapy (CHOP/CHOP-like), n (%) | 3 (16) | 19 |
| Radiotherapy, n (%) | 8 (42) | 19 |
| Combined radio- and chemotherapy, n (%) | 6 (32) | 19 |
| CR after initial therapy, n (%) | 15 (94) | 16 |
| Recurrence after initial therapy, n (%) | 2 (13) | 16 |
| Progression after initial therapy, n (%) | 1 (6) | 16 |
CR indicates complete remission.
One patient died of lymphoma, and 2 of myocardial infarction and stroke, respectively.
Classical cytogenetic banding analysis
Classical cytogenetic studies were conducted by following established protocols.4 In brief, unstimulated and/or phorbol-12,13-dibutyrate (substance P)–stimulated5 10-mL cell cultures with 1 to 2 × 106 cells per milliliter RPMI 1640 medium were set up after mechanical disaggregation of the specimens using a 100-micron nylon gauze and directly processed or allowed to grow overnight (stimulated cell cultures).
For metaphase preparation, cultures were pulsed with colchicine for 30 minutes before harvesting and were then exposed to a hypotonic solution of 0.075 mol/L KCl, fixed in methanol/acetic glacial acid (3:1), and dropped onto glass slides. The slides were stored for several days at room temperature for maturation purposes. We stained the metaphases using a trypsin-Giemsa standard technique and evaluated them according to the guidelines of the International System for Human Cytogenetic Nomenclature (ISCN).6 A chromosomal aberration was regarded as clonal if 2 or more metaphases of one case harbored the same structural alteration or chromosomal gain or if a loss of a whole chromosome was found in at least 3 different metaphases. Images were captured with a Zeiss Axioskop2 microscope (Zeiss, Jena, Germany) and were evaluated with the IKAROS imaging system (MetaSystems, Altlussheim, Germany).
Bicolor fluorescence in situ hybridization analysis
Bicolor fluorescence in situ hybridization (FISH) was successfully performed on 29 (83%) of 35 tumor specimens. In 6 cases with either methanol-acetic acid fixed or frozen material available, 3:1 methanol-acetic acid–fixed cell suspensions from cytogenetic preparations or isolated from frozen blocks were dropped onto glass slides to obtain well-preserved and spread-out mononuclear cells.
In the remaining cases, only neutral-buffered, formalin-fixed, paraffin-embedded material was available. To prepare isolated nuclei, cells/nuclei were extracted from paraffin blocks according to previously described methods7-10 with minor modifications. In brief, depending on the size of the tissue block, 2 to 5 sections (35 μm thick) were cut and paraffin was dissolved by adding 8 mL of xylene at 37°C. The xylene was discarded after 30 minutes or 60 minutes, and the procedure was repeated 3 times. Thereafter, the tissue was rehydrated by incubation with 8 mL of 100%, 70%, and 50% ethanol for 30 minutes at 37°C. After twice adding 8 mL of distilled water for 30 minutes at 37°C, and incubating the mixture with 8 mL of 37°C prewarmed digestion buffer (3.4 mmol/L tri-sodium citrate, 0.1% NP-40, and 0.5 mmol/L TrisHCI, pH 7.6) for 30 minutes, the tissue was mechanically disaggregated with Medimachine System (DAKO, Glostrup, Denmark) to achieve a cell suspension. Enzymatic digestion was performed by replacing the solution with digestion-buffer containing 0.5% trypsin (Serva, Heidelberg, Germany) for 2 hours at 37°C. The isolated nuclei were harvested after centrifugation at 1000 rpm (78g) for 10 minutes and 3 additional washings with 10 mL of phosphate-buffered saline (PBS) solution. The remaining nuclei were fixed by resuspension and vortexing in 2 changes of freshly prepared, −20°C cold Carnoy‘s fixative (methanol:acetic glacial acid = 3:1) and stored at least for 24 hours at −70°C. Then the cell suspension was dropped onto 3-aminopropyltriethoxysilane (APES)-coated slides.
Locus-specific, directly labeled probes were applied to check for the presence of the translocation t(14;18)(q32;q21) leading to IGH/BCL2 gene rearrangement using the Vysis LSI IGH/BCL2 Dual Color, Dual Fusion Translocation Probe (Vysis/Abbott Molecular Diagnostics, Wiesbaden-Delkenheim, Germany) and for detection of the centromeric region of chromosome 1 (1p11.1-1q11.1) using the alpha satellite DNA probe CEP 1 (D1Z5) SpectrumGreen Probe (Vysis/Abbott Molecular Diagnostics), following the manufacturer's advice.
For the detection of chromosomal alterations involving chromosome band 1p36.3, we applied the locus-specific bacterial artificial chromosome (BAC) probe BAC RP4-755G511 and for chromosomal bands 1p22 and 1q32, we applied the yeast artificial chromosome (YAC) probes YAC 968g8 and YAC 958e1, respectively, as previously described.12,13
Signal visualization was accomplished with the use of a Zeiss Axioskop2 fluorescence microscope (Zeiss) and illustrations were made with the ISIS imaging system (MetaSystems, Altlussheim, Germany). Signals were evaluated in at least 200 intact nuclei. An aberrant clone was defined according to the cutoff level evaluated for each probe in control studies with 5 reactive neutral-buffered formalin-embedded lymph node specimens calculating the mean prevalence of a given signal in at least 200 cells plus 3 standard deviations (Table 2).
Panel of the locus-specific FISH probes and cutoff thresholds
| Locus . | Probe information . | Cutoff, % . |
|---|---|---|
| 1p36.3 | BAC RP4–755G5 | 5 |
| 1p22 | YAC 968g8 | 5 |
| 1p11.1-q11.1 | CEP 1 (D1Z5) | 5 |
| 1q32 | YAC 958e1 | 6 |
| t(14;18)(q32;q21) | LSI IGH/BCL2 | 5 |
| Locus . | Probe information . | Cutoff, % . |
|---|---|---|
| 1p36.3 | BAC RP4–755G5 | 5 |
| 1p22 | YAC 968g8 | 5 |
| 1p11.1-q11.1 | CEP 1 (D1Z5) | 5 |
| 1q32 | YAC 958e1 | 6 |
| t(14;18)(q32;q21) | LSI IGH/BCL2 | 5 |
FISH indicates fluorescence in situ hybridization.
Gene expression analysis and statistical evaluation
We performed gene expression analysis of 4 FLs with predominantly diffuse growth pattern, documented 1p36 deletion, and frozen material available by following the standard Affymetrix protocol for eukaryotic gene expression analysis (available at www.affymetrix.com), with use of the HG U133A chip from Affymetrix (Affymetrix, Santa Clara, CA), whereas gene expression data of 150 typical FL cases (grades 1 and 2) were available from the previous publication by Dave et al.14 A 2-sided t test, cluster analysis, and gene set enrichment analysis (GSEA; http://www.broad.mit.edu/gsea) were performed to compare the gene expression profiles of the 2 groups. GSEA was performed as described by Subramanian et al15 and Mootha et al.16 A total of 122 gene expression signatures17 from the signature database of the Staudt laboratory (http://lymphochip.nih.gov/signaturedb) were used as gene sets. Gene sets were assessed as significantly enriched in one of the phenotypes, if the nominal P value was less than .05 and the FDR-q value 0.25 or less. Because one of our datasets comprised fewer than 7 samples, “gene set” was chosen as permutation type in the settings of the GSEA software, as recommended by the GSEA team.
The results of the t test were sorted by P value and difference in mean expression of the 2 groups. For cluster analysis and visualization of the data, the Cluster and TreeView software programs from Michael Eisen (Lawrence Berkeley National Laboratory, Berkeley, CA) were used.
Results
Follicular lymphomas with a predominantly diffuse growth pattern at disease onset display particular morphologic and cytogenetic features
At the study onset, 5 FL samples with available cytogenetic data were classified as FL grade 1 (n = 2) or FL grade 2 (n = 3) with a predominantly diffuse growth pattern. They exhibited a follicular growth pattern with atypical follicles in less than 25% of the lymph node area infiltrated by the tumor (Figure 1A). Frequently, the follicular compartment consisted of some isolated or small groups of follicles only, whereas the majority of the tissue was diffusely infiltrated (> 90% of the area). All 5 FLs revealed the typical cytology of FL consisting of a mixture of—distinctly prevailing—centrocytes and only some intermingled centroblasts in the neoplastic follicles (Figure 1B), whereas the diffuse infiltrates were predominantly composed of small centrocyte-like or round cells (Figure 1C).
Morphologic and immunohistochemical features of FL with predominantly diffuse growth pattern. (A) A diffuse infiltration pattern prevails with only few intermingled atypical follicles. In the neoplastic follicles, centrocytic cells dominate (B), whereas in the diffuse areas tumor cells present with rounder nuclear contours (C). Tumor cells express CD20 (D), and reactive T cells (CD3) are prominent in diffuse areas (E). (F) The proliferative activity (Ki-67) is accentuated in the atypical follicular structures and decreased in the diffuse infiltrates. CD10 is expressed in atypical follicles and diffusely infiltrated areas in the majority of cases (G), as is CD23 (H). (I) BCL6 expression can be observed in all atypical follicles and in the nuclei of some interspersed B cells in the diffuse infiltrates. CD21 highlights FDCs in the atypical follicle. No CD21 staining is observed in the diffuse infiltrate (J). BCL2 expression is variable between cases but also within different areas of the same case (K,L). Magnification: panels A, D-G, I,J: ×200; panels B,C,H,I (inset), K,L: ×400.
Morphologic and immunohistochemical features of FL with predominantly diffuse growth pattern. (A) A diffuse infiltration pattern prevails with only few intermingled atypical follicles. In the neoplastic follicles, centrocytic cells dominate (B), whereas in the diffuse areas tumor cells present with rounder nuclear contours (C). Tumor cells express CD20 (D), and reactive T cells (CD3) are prominent in diffuse areas (E). (F) The proliferative activity (Ki-67) is accentuated in the atypical follicular structures and decreased in the diffuse infiltrates. CD10 is expressed in atypical follicles and diffusely infiltrated areas in the majority of cases (G), as is CD23 (H). (I) BCL6 expression can be observed in all atypical follicles and in the nuclei of some interspersed B cells in the diffuse infiltrates. CD21 highlights FDCs in the atypical follicle. No CD21 staining is observed in the diffuse infiltrate (J). BCL2 expression is variable between cases but also within different areas of the same case (K,L). Magnification: panels A, D-G, I,J: ×200; panels B,C,H,I (inset), K,L: ×400.
These 5 FL samples were part of a series of 6 cases of predominantly diffuse FL, in which cytogenetic analysis was attempted, and clonal karyotypic alterations were found in 5 tumors. The t(14;18)(q32;q21) was absent in all cases, and this finding was confirmed by FISH analysis conducted on metaphase spreads and/or nuclear preparations. A total of 4 of the 5 cases harbored, as recurring aberrations, deletions in the short arm of chromosome 1 (1p), with breakpoints spanning from bands 1p32 to 1p36 (cases 1-4, Table 3 and Figure 2). In all 4 cases, chromosomal material from band 1p36 was lost. We again confirmed this finding with FISH analysis using locus-specific BAC and YAC probes to 1p36, 1q32, and 1cen. In case 5, although clonal chromosomal aberrations were present, no unequivocal deletion in 1p could be ascertained by banding analysis. Interestingly enough, however, FISH analysis conducted from nuclear suspensions also revealed a clonal 1p36 deletion in this case (in 90 of 200 nuclei analyzed).
Karyotype information obtained by classical banding analysis in 5 index follicular lymphoma cases with predominantly diffuse growth pattern
| Case no. . | Karyotype . | Aberrant metaphases/total no. of metaphases analyzed . |
|---|---|---|
| 1 | 50∼56,XY,+X,del(1)(p35),−2,add(3)(p22),+3,+7,+22,+1∼3mar[4] | 4/4 |
| 2 | 50∼51,XX,+X,der(1)t(1;?4)(p36;?q21),+5,+7,+8,+11,−18[8] | 5/8 |
| 3 | 47∼48,XY,der(1)t(1;2)(?p32;?p14),del(7)(q22q32),+7,+12,−14,add(21)(q22) [5] | 2/5 |
| 4 | 45∼49,XY,add(1)(p36)[8],t(7;11)(q11;q21)[3],+11[2],+8[2],der(18) t(9;18)(q13;q23),[cp8] | 8/9 |
| 5 | 46∼47,XY,der(1)?t(1;3)(q44;q21)[2],del(7)(q22)[9],+8[7],+mar[2],[cp9] | 9/12 |
| Case no. . | Karyotype . | Aberrant metaphases/total no. of metaphases analyzed . |
|---|---|---|
| 1 | 50∼56,XY,+X,del(1)(p35),−2,add(3)(p22),+3,+7,+22,+1∼3mar[4] | 4/4 |
| 2 | 50∼51,XX,+X,der(1)t(1;?4)(p36;?q21),+5,+7,+8,+11,−18[8] | 5/8 |
| 3 | 47∼48,XY,der(1)t(1;2)(?p32;?p14),del(7)(q22q32),+7,+12,−14,add(21)(q22) [5] | 2/5 |
| 4 | 45∼49,XY,add(1)(p36)[8],t(7;11)(q11;q21)[3],+11[2],+8[2],der(18) t(9;18)(q13;q23),[cp8] | 8/9 |
| 5 | 46∼47,XY,der(1)?t(1;3)(q44;q21)[2],del(7)(q22)[9],+8[7],+mar[2],[cp9] | 9/12 |
Cytogenetic alterations affecting the chromosomal region 1p36 in FL with a predominantly diffuse growth pattern. G-banded pairs of chromosome 1 (cases 1 and 4, see Table 3) with structural alterations affecting the short arm (A). Representative FISH results on isolated nuclei from paraffin-embedded tumor tissue are shown for when the BAC probe RP4-755G5 for the chromosomal region 1p36 (red signal) and the YAC probe 968g8 for the region 1p22 (green signal) are used. Loss of genetic material in 1p36 is evident, whereas 2 copies of the region 1p22 are retained (B).
Cytogenetic alterations affecting the chromosomal region 1p36 in FL with a predominantly diffuse growth pattern. G-banded pairs of chromosome 1 (cases 1 and 4, see Table 3) with structural alterations affecting the short arm (A). Representative FISH results on isolated nuclei from paraffin-embedded tumor tissue are shown for when the BAC probe RP4-755G5 for the chromosomal region 1p36 (red signal) and the YAC probe 968g8 for the region 1p22 (green signal) are used. Loss of genetic material in 1p36 is evident, whereas 2 copies of the region 1p22 are retained (B).
FL cases with a predominantly diffuse growth pattern display characteristic clinical and immunophenotypic features
We next reviewed 183 primary nodal FL cases from the databases of the participating institutions that had been classified as predominantly diffuse FL and also reviewed the available clinical data. Lymphomas that were diagnosed on fine-needle biopsies or in which the review diagnosis was other than predominantly diffuse FL were excluded. Altogether, 29 additional tumors were identified with available paraffin blocks, in which the diagnosis of FL, predominantly diffuse, had been rendered on an excised lymph node and was confirmed according to the criteria of the WHO classification system of hematopoietic neoplasms (> 75% diffuse infiltration).1 In those samples, the number of paraffin blocks available for study varied considerably. Consultation cases usually consisted of 1 block only (mean, 1.2 blocks), whereas cases sent in directly for diagnostic purposes were extensively worked up, with one having up to 8 paraffin blocks (mean, 2.5 blocks) available for morphologic evaluation in a given case. The minimum diameter of the tissues that were able to be evaluated was 1 cm. According to clinical records available, all but 1 case represented initial diagnostic specimens. Twelve lymphomas (34%) were classified as FL grade 1, and 23 tumors (66%) as FL grade 2.
All 35 lymphomas classified as predominantly diffuse FL displayed similar clinical and morphologic features. The predominating clinical presentation was that of an isolated large tumor, frequently presenting in the inguinal region. The median size of the excised lymph nodes was 5 cm, with a range of 1.8 cm to 18 cm. On histologic examination, a diffuse or vaguely nodular infiltration pattern prevailed. In all cases, however, some atypical follicles were present with reactivity for CD10 and BCL-6. The total area of follicular infiltrates was less than 25% in all cases. In the neoplastic follicles, centrocytic cells were dominating, whereas only few centroblasts were intermingled. In the diffuse areas, the majority of the tumor cells seemed to present with rounder nuclear contours, and basophilic blasts were only occasionally identified (Figure 1A-C). On the basis of these cytologic features as well as the presence of atypical follicles, the diagnosis of FL was made.
Immunophenotyping was consistent with this diagnosis, albeit it showed some unique features. In all cases, the infiltrating neoplastic cells were CD20 positive. T cells (positive for CD3 and CD5) were densely intermingled, especially in the diffuse parts (Figure 1D,E). The follicles, as a rule, were positive for CD10 and BCL-6 (Figure 1G-I), whereas BCL-2 was expressed in the diffuse areas. In the neoplastic follicles, the expression of BCL-2 varied considerably between cases and occasionally also from follicle to follicle, ranging from entirely negative to moderately strong in most of the germinal center B cells (Figure 1K,L). In the diffusely infiltrated areas, CD10 was positive in 30 cases (85%; Figure 1G). BCL6, in these areas, was positive in the nuclei of some interspersed B cells (Figure 1I inset). Strikingly, in roughly two-thirds of cases, CD23 was coexpressed on the neoplastic B cells, predominantly in the diffuse areas (27 tumors; 77%), but also within germinal centers in 17 cases (49%; Figure 1H). CD21 reactivity was confined to FDCs within the neoplastic follicles (Figure 1J). Ki67 showed varying reactivity ranging from 15% to 45% on average, with a slight accentuation in the atypical follicles, in which the Ki67-positive cells were randomly distributed, failing to show a zonation phenomenon (Figure 1F).
Predominantly diffuse FL sample are negative for the t(14;18) chromosomal translocation and harbor deletions in 1p36 as a unifying chromosomal aberration
All 35 FL samples with a predominantly diffuse growth pattern were subjected to FISH analysis, and 6 typical FLs from the inguinal region were added as control samples. FISH signals could be evaluated in 29 of 35 tumors and in all 6 control samples, whereas analyzable signals were not obtained from 6 (17%) lymphomas. Of 6 typical FL samples, 5 (83%) showed a signal distribution indicative of a t(14;18), whereas 28 (97%) of 29 diffuse FL samples were negative for the translocation (Figure 3). No signal distributions were noted, indicating amplification of BCL2. Remarkably, all but 2 (27/29) diffuse FL samples that were analyzed harbored deletions in the terminal part of the short arm of chromosome 1 as detected by the signal constellation of the FISH probe RP4-755G5 located at 1p36.3, showing only one signal in 15% to 70% of cells (cutoff value 5%). In contrast, the chromosomal band 1p22 represented by YAC 968g8 was conserved in all but 1 case tested (case 23 in Figure 3), and this case failed to harbor a 1p36 deletion. The single t(14;18)-positive tumor (case 27) also harbored a 1p36 deletion. The centromeric region of chromosome 1 (CEP 1 D1Z5) was conserved in all cases tested. The presence of DNA sequences in the long arm of chromosome 1 was tested in 7 cases. Using YAC 958e1, we discovered that all 7 cases were disomic for the DNA probe located at 1q32, thus excluding losses of 1q sequences in those cases. All 6 typical FL samples tested were negative for deletions at 1p22 or 1p36.
FISH in 29 FLs with predominantly diffuse growth pattern. Detailed FISH results are provided for the chromosomal loci 1p36.3, 1p22, the centromeric region of chromosome 1 (1cen), and for the status of the translocation t(14;18).
FISH in 29 FLs with predominantly diffuse growth pattern. Detailed FISH results are provided for the chromosomal loci 1p36.3, 1p22, the centromeric region of chromosome 1 (1cen), and for the status of the translocation t(14;18).
GSEA, t test, and cluster analysis
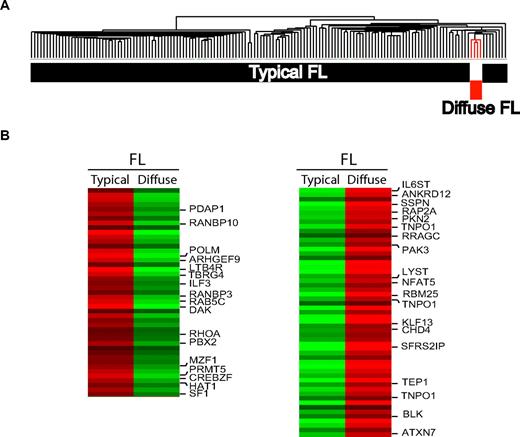
Cluster analysis of the gene expression profiles of 4 predominantly diffuse FL cases and 150 previously published, typical FL samples showed that FL samples with a striking, predominantly diffuse growth pattern belong to the gene expression spectrum of FL but nevertheless represent a distinct subgroup (Figure 4A). A 2-sided t test revealed a significant difference in gene expression patterns, with more than 3000 probe sets being differentially expressed between these groups (P < .001; Figure 4B). Comparing the gene expression data of diffuse and typical FL samples with GSEA, we found a significant enrichment of GCB cell, proliferation, cell- cycle, and B-cell signatures in the typical FL cases. In predominantly diffuse FL cases, we found an enrichment of several T-cell, natural killer (NK)–cell, and 2 DC subset signatures (BDCA1-positive DCs derived from blood and CD123-positive DCs derived from tonsils18 ).
Gene expression profiling of FL with predominantly diffuse growth pattern. (A) Hierarchical clustering demonstrates that gene expression profiles of diffuse FL fall into the spectrum of typical FL but nevertheless show a distinct gene expression signature. (B) Top 100 probe sets that are differentially expressed between typical FL and diffuse FL (according to the results of a t test between the groups).
Gene expression profiling of FL with predominantly diffuse growth pattern. (A) Hierarchical clustering demonstrates that gene expression profiles of diffuse FL fall into the spectrum of typical FL but nevertheless show a distinct gene expression signature. (B) Top 100 probe sets that are differentially expressed between typical FL and diffuse FL (according to the results of a t test between the groups).
Clinical and survival data
Most frequently, diffuse FL samples involved inguinal lymph nodes. Specifically, 29 (83%) of the 35 diagnostic specimens represented inguinal lymph nodes; 3 tumors were from cervical and 3 from axillary locations (9% each). According to the clinical records, the most frequent clinical presentation was an isolated, painless swelling in the groin with a median tumor size of 5 cm (range, 1-18 cm). Of 20 patients with staging data available, 8 had stage I, 7 stage II, and only 5 (25%) had stage III or IV. In all 15 patients with stage I or II disease, the inguinal region was affected, and additional sites affected in the 7 stage II patients were the contralateral inguinal, iliacal, and para-aortal lymph nodes.
Data on therapy were available from 19 FL patients. Of those, 8 patients with stages I and II disease were treated with radiotherapy alone, 6 patients received chemotherapy and radiotherapy, and 3 patients underwent chemotherapy alone. In 2 patients, no therapy was given. Follow-up data were available from 16 patients. Median follow-up time was 29 months (range, 1-191 months). Complete remission was achieved in 15 patients, and 1 patient presented with progressive disease. This latter patient was the only one who succumbed to his disease after 14 months. Death occurring during the follow-up period in 2 more patients was unrelated to lymphoma (cerebral stroke and myocardial infarction). Details of clinical data are given in Table 1.
Discussion
FL is the most frequent form of NHL in the Western world, comprising roughly 25% of all newly diagnosed lymphoid tumors. In its classical form, FL grades 1 and 2, it displays a predominantly follicular pattern of growth, the atypical follicles being positive for CD10 and BCL-2. Predominantly diffuse FLs have been accepted as an entity in the WHO classification of lymphoid tumors1 and are characterized by a strikingly diffuse growth pattern of centrocytes and centroblasts with less than 25% follicularity. It has been stressed, however, that this diagnosis be made with caution. Specifically, mantle cell lymphoma (MCL) and marginal zone B-cell lymphoma (MZBCL) have to be ruled out, and evidence of germinal center differentiation is required, for example, by demonstrating reactivity of the neoplastic cells for CD10 and/or BCL-6 or presence of the t(14;18) chromosome translocation. In the literature, predominantly diffuse FLs are rarely described, and their prognosis has been termed intermediate.19,20 Most of these tumors, especially the t(14;18)-positive diffuse FL, however, may have been progressed from typical follicular FL or may have been diagnosed in small biopsies that lacked a clear follicular infiltration pattern. Therefore, it has been doubted whether entirely diffuse FL exists as an entity.
In the present work, we describe 35 cases of a malignant lymphoid tumor with characteristic and unique morphologic, clinical, and genetic features, thus fulfilling the criteria for a biologic entity beyond typical FL. These tumors are characterized by a predominantly diffuse growth pattern with only few or rare neoplastic follicles, consistent with the diagnosis of predominantly diffuse FL. The immunohistochemical features of these tumors are well in line with FL, being negative for CD5 in all cases, and displaying reactivity for CD10 and BCL-6 in the neoplastic follicles. Of importance, there also is reactivity for CD23, especially in the diffuse parts of the infiltrate, combined with coreactivity of CD10 in a substantial number of cases, thus showing an immunophenotype that is consistent with a derivation from a germinal center B cell. In support of this view, gene expression profiling of diffuse FL revealed a phenotype that falls into the spectrum of typical FL but nevertheless shows distinct features (Figure 4). In particular, GSEA unraveled an enrichment of GCB-cell, proliferation, B-cell, and cell-cycle signatures in typical FL cases. These findings are consistent with the observation that the proliferative activity as measured by Ki-67 labeling is relatively low within the diffuse areas, that BCL-6 expression is down-regulated, and that CD10 expression is strongly reduced in some diffuse FL cases. In contrast, diffuse FL cases were found to be enriched in T-cell, NK-cell, and DC signatures, pointing to an increased admixture of nonmalignant bystander cells, which is also supported by morphologic and immunohistochemical observations. Whether the increased number of bystander cells in diffuse FL cases creates a growth supportive microenvironment that is provided by the germinal center micromilieu in typical FL is presently unclear. It is noteworthy that the gene expression signatures of 2 particular DC subsets, as defined in a recent study,18 rather than markers of follicular DC such as CD21, CD23, or CD35, were enriched in diffuse FL cases. Specifically, signatures for blood-derived BDCA1-positive DC and tonsillar CD123-positive DC were found to be enriched.18 However, the enrichment of both DC signatures in diffuse FL was predominantly based on genes (eg IL13RA1, PTCRA, IL27RA and IL3RA) that are also widely expressed by other cells of the immune system, such as T and NK cells21-24 and it is therefore unclear, if increased numbers of specific DC subsets account for the enrichment of these 2 gene expression signatures.
In contrast to typical FL, diffuse FL cases do present with characteristic clinical features. They frequently arise in and are confined to the inguinal region with a propensity to form large localized tumors, and patients follow a particularly indolent clinical course. In this context, 2 aspects deserve a brief discussion. First, we have recently described that partial lymph node infiltration, with partial colonization of reactive germinal centers, is a frequent feature in typical FLs of low clinical stage (stages I and II) and that the inguinal region is a recurring primary site of origin in these tumors.25 These FLs, however, were typical FL cases in the sense that all but one case tested were positive for the t(14;18), as evidenced by FISH analysis. Second, in a recent article examining CD23 expression in FL, Thorns et al26 described—apart from an unexpectedly high overall frequency of CD23 positivity in FL—an unusually high coincidence of CD23 staining in FL arising in inguinal lymph nodes (38%).26 The large majority of these cases, however, were described as typical, predominantly follicular FLs.
One of the most surprising findings in our study was that, despite the presence of atypical follicles, only 1 of 33 diffuse FL tested turned out to harbor the t(14;18) chromosome translocation that is considered the cytogenetic hallmark of grade 1 and 2 FL.27,28 For the sake of scientific accuracy, however, it has to be stated that no variant BCL2 translocations, eg, to the loci of the immunoglobulin light chains, would have been recognized by the FISH strategy used. However, t(14;18) negativity does not by itself exclude a diagnosis of FL. Roughly 15% to 25% of otherwise-typical FL grades 1 and 2 are negative for the BCL2 rearrangement inferred by the t(14;18), and these tumors may present with particular phenotypic and genetic features.29,30 However, we and others have shown that certain subtypes of FL grade 3, the purely follicular FL3B and those with an additional DLBCL component, also, as a rule, are BCL2/t(14;18) negative.2,3,31,32 The site of origin itself may also predict for the t(14;18) status in FL: Although primary duodenal and intestinal FL, for example, are t(14;18) positive in the most cases, primary cutaneous FL are BCL2 rearranged in less than 50% of cases.33,34
Ideally, malignant tumors with similar morphologic and immunophenotypic features and comparable clinical presentations also display similar genetic features. This is the case for the so-called primary translocations in certain NHL subtypes thus defining biologic entities, such as the t(14;18) in FL, the t(11;14) in MCL, or the t(3q27)/BCL6 rearrangement in DLBCL. However, recurring gains or deletions of chromosomal regions also have been shown to be characteristic of certain tumor entities. For example, gains in the chromosomal band 9q34 have been identified as a characteristic aberration in intestinal T-cell lymphoma.35 Likewise, the formation of an isochromosome 7q is a characteristic finding in hepatosplenic T-cell lymphoma. In B-cell malignancies, however, numerical imbalances that can serve as characteristic and tumor-specific alterations usually are not encountered, with the possible exception of certain chromosomal imbalances in MZBCL recently defined by array or conventional CGH,34,36 such as the 7q32 deletion encountered in some cases of splenic MZBCL.37
In this study, we have documented that deletions in chromosomal band 1p36 are a recurring genetic aberration in predominantly diffuse FL. In our series, this aberration was found in 28 (97%) of 29 cases, suggesting that the del(1p36) may constitute a primary aberration in this particular tumor type. Rearrangements of band 1p36 are recurring aberrations encountered by cytogenetics in 12% to 20% of NHL, and the majority of these are associated with the t(14;18) chromosome translocation.38,39 A thorough investigation by Lestou et al40 with a multicolor chromosomal banding technique revealed that chromosomal aberrations in 1p lead to deletions of 1p36 in roughly 60% of cases. These aberrations, frequently in the form of unbalanced translocations, obviously involve several partner chromosomes, although chromosome 8 has been identified as the most frequent partner involved. When more refined molecular techniques are used, deletions of chromosomal band 1p36 are encountered even more frequently. Rajgopal and colleagues11 analyzed the occurrence of microdeletions in 1p36 in 20 NHL cases previously characterized by chromosomal banding and judged to be nonaltered in this region and found microdeletions at 1p36.33 in 5 (25%), including 2 of 8 FL. This finding is in line with the observation in one of our cases, in which the deletion was only recognizable after FISH analysis as well. In a recent study, using SNP arrays41 on 58 FACS-sorted FL samples, the authors revealed loss of heterozygosity (LOH) and, especially, copy-neutral LOH (uniparental disomy) in this region in 50% of samples, making the 1p36 chromosome region the second most frequent region altered in FL.
No tumor suppressor gene of potential importance for the pathogenesis of FL has been identified in 1p36. Several genes, with putative tumor suppressor activity, have been mapped to this chromosomal region, including TP73,42 RUNX3,43 TNFR2,44 ID3,45 PAX7,46 DAN,47 and CDC2L1.48
It is interesting to note that, in our cases, in contrast to the majority of FL, only one case exhibited a del(1p36) in association with the t(14;18), whereas the majority of deletions in this region are associated with a BCL2 rearrangement and occur in typical follicular FL. This finding possibly points to a different target gene in typical follicular FL and diffuse FL or, alternatively, to the overwhelming biologic importance of the t(14;18) that, in case of its presence, leads to the phenotype of classical (predominantly follicular) FL irrespective of an additional deletion in 1p36.
In summary, we describe here a new subtype of FL with a predominantly diffuse infiltration pattern, frequent clinical presentation with large, localized tumors in the inguinal region, and characteristic genetic features with lack of the t(14;18) and a unifying chromosomal deletion in 1p36. Future studies will have to address the clinical behavior of these tumors in the context of previous or ongoing prospective clinical trials in FL as well as the identification of the molecular event in 1p36 that may be crucial in the pathogenesis of predominantly diffuse FL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Irina Eichelbrönner and Heike Brückner for their technical assistance, as well as the Robert-Bosch-Stiftung, Stuttgart, Germany, and the Interdisciplinary Center for Clinical Research (IZKF), University of Würzburg, Würzburg, Germany, for their financial support.
G.O. and H.S. are supported by the Robert-Bosch-Stiftung. A.R., E.H., and E.L. are supported by the Interdisciplinary Center for Clinical Research (IZKF), University of Würzburg, Würzburg, Germany.
Authorship
Contribution: T.K. and E.L. performed research, analyzed and interpreted the data, and helped to draft the manuscript; J.K., S.B., and E.H. analyzed and interpreted the data and performed research; H.S., S.W., HK.M-H., and M.O. analyzed and interpreted the data; and G.O. and A.R. designed research, analyzed and interpreted the data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: German Ott, MD, Department of Clinical Pathology, Robert-Bosch-Krankenhaus, Auerbachstrassa 110, 70376 Stuttgart, Germany; e-mail: german.ott@rbk.de.
References
Author notes
*A.R. and G.O. are joint senior authors of this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal