Abstract
The Epstein-Barr virus (EBV) causes infectious mononucleosis, establishes latency in resting memory B lymphocytes, and is involved in oncogenesis through poorly understood mechanisms. The EBV lytic cycle is initiated during plasma cell differentiation by mRNAs transcripts encoded by BZLF1, which induce the synthesis of EBV proteins such as the immediate-early antigen ZEBRA and the late membrane antigen gp350. Therefore, we assessed the capacity of circulating EBV-infected B lymphocytes from healthy EBV-seropositive subjects to enter and complete the EBV lytic cycle. Purified B lymphocytes were polyclonally stimulated and BZLF1- or gp350-secreting cells (BZLF1-SCs or gp350-SCs) were enumerated by ELISpot assays. The number of BZLF1-SCs ranged from 50 to 480/107 lymphocytes (median, 80; 25th-75th percentiles, 70-150) and gp350-SCs from 10 to 40/107 lymphocytes (median, 17; 25th-75th percentiles, 10-20). gp350-SCs represented only 7.7% to 28.6% of BZLF1-SCs (median, 15%; 25th-75th percentiles, 10.5%-20%). This EBV functional reservoir was preferentially restricted to plasma cells derived from CD27+ IgD− memory B lymphocytes. In 9 of 13 subjects, EBV DNA quantification in B-cell culture supernatants gave evidence of completion of EBV lytic cycle. These results demonstrate that EBV proteins can be secreted by EBV-infected B lymphocytes from healthy carriers, a majority generating an abortive EBV lytic cycle and a minority completing the cycle.
Introduction
The Epstein-Barr virus (EBV) is a widespread human γ-herpes virus that infects more than 90% of the world's population. Primary infection is usually asymptomatic in children, but in adolescents and in young adults EBV can cause infectious mononucleosis, a benign, self-limiting lymphoproliferative disease.1 EBV is also associated with several hematologic neoplasias, such as Burkitt lymphomas and also with nonhematologic undifferentiated nasopharyngeal carcinoma.2-4 In immunosuppressed individuals, transplant recipients, or AIDS patients, EBV may lead to lymphoproliferative disorders as non-Hodgkin lymphomas.5,6 EBV also could contribute to migration and invasion of metastasis cells in primary nonkeratinizing nasopharyngeal carcinoma,7 and recently it has been reported that a Plasmodium falciparum protein interacts with memory B cells as a potent cell activator leading to EBV reactivation in latently infected B cells, thus increasing the risk of Burkitt lymphoma.8,9 Nevertheless, the mechanisms involving EBV in oncogenesis remain largely unknown
During the EBV life cycle, viral genes encoding enzymes and proteins involved in nucleotide biosynthesis, RNA processing, viral DNA replication, capsid, and viral envelope synthesis are expressed. BZLF1 and BRLF1 genes encode transcriptional activator proteins and mediate the switch between the latency and the EBV lytic cycle. BZLF1 codes for the first productive cycle protein that is expressed during reactivation of viral replication in B cells and referred as ZEBRA, EB1, Z, or Zta.10-13 These genes initiate an ordered cascade of viral lytic gene expression culminating in release of infectious virus, usually leading to the death of the host cell.14 The viral glycoprotein genes that have been identified so far are all late genes and encoded many EBV lytic antigens. Membrane antigen gp350/220 is the most abundant viral protein in the lytically infected cell membrane and in the outer surface of the virus,15 but many others glycoproteins are encoded by different EBV genes.16
EBV persists in vivo in peripheral blood and tonsil resting B lymphocytes expressing the surface phenotype of memory cells.17-19 Memory B cells are long-living cells that recirculate in the body throughout the blood and lymphatic tissue, and migrate to the bone marrow and mucosal epithelium, the homing site of antibody (Ab)–secreting cells (SCs) after B cells encounter antigens.20 Memory B cells, which represent 15% to 40% of the circulating B cells,21 are somatically mutated on variable region genes and selected into the germinal centers of peripheral lymphoid organs for their antigen (Ag) high affinity.22-24 The progress in B-cell analysis makes it clear that circulating B cells include 3 main cell subsets on the basis of CD27 and IgD receptor expressions: (1) IgD− CD27+ memory B cells, class-switched lymphocytes that express and secrete IgG or IgA, a minority bearing only IgM receptors and secreting IgM25-28 ; (2) IgD+ CD27+ B cells generated by IgM memory B cells produce high affinity IgM and are in charge of T-independent responses29,30 ; and (3) IgD+ CD27− naive B cells. Peripheral Ag-specific memory B cells ensure lifelong humoral immunity through their clonal expansion capacity after Ag stimulation and plasma-cell differentiation.31
It has been established that, during the terminal differentiation into plasma cells (CD38high, CD10−, CD19+, CD20low, surface Ig−, cytoplasmic Ig+), EBV latently infected tonsil memory B cells, initiated EBV replication and lytic cycle in vivo, and expressed the immediate-early gene BZLF1.19 The presence of BZLF1- and gp350-mRNA transcripts indicates the initiation of the EBV lytic cycle and implicates synthesis of viral proteins and ultimately the cell death,32 but to date BZLF1 product- and gp350-cell secretions through plasma cell differentiation have not been reported. Moreover, the ability of EBV-infected peripheral blood B cells from healthy subjects to complete the EBV lytic cycle and produce viral Ag and viral particles after plasma cell differentiation remains unclear. Therefore, we hypothesized that molecular expression of BZLF1 and EBV-envelope glycoprotein genes in CD38hi plasma cells could be followed by the plasma cell secretion of BZLF1 products and gp350. To enumerate cells secreting these 2 proteins, we developed BZLF1- and gp350-enzyme-linked immunospot (ELISpot) assays using EBV-positive B95-8 and EBV-negative BJAB cell lines.
The ELISpot assay is based on cell synthesized protein immune capture by a solid phase and ELISA revelation. This assay has been developed for enumeration of cells producing cytokines,33 Ig- and Ab-SCs,34-37 and Ag such as HIV-1.38-40 Moreover, this approach makes it possible to characterize and enumerate Abs- or Ag-specific memory B cells in peripheral blood, providing a powerful tool to evaluate the full scope of Ag-specific B-cell responses.31
In the present study, the capacity of latent EBV-infected peripheral blood B lymphocytes to enter and complete the EBV lytic cycle was assessed in 13 healthy EBV carriers. The secretion of BZLF1 products and gp350 as well as the replication of EBV DNA were assessed using a combination of B-cell isolation method, in vitro polyclonal stimulation of B lymphocytes by CD40L ligation plus IL-2 and IL-10, sensitive BZLF1- and gp350-ELISpot assays, and EBV DNA quantification in culture supernatants by polymerase chain reaction (PCR). We identify rare EBV latently infected circulating memory B cells that are able to synthesize viral proteins and EBV genomes, highlighting a functional EBV reservoir preferentially restricted to circulating CD27+ IgD− memory B cells.
Methods
Subjects
Thirteen healthy adults with serologic evidence of previous EBV infection were included after written informed consent. All procedures were conducted and informed consent was obtained in accordance with the Declaration of Helsinki, and this study received Institutional Review Board–approval from the Montpellier Research Ethical Committee. A total of 25 mL of blood per person was collected in ethylenediaminetetraacetic acid tubes and B lymphocytes isolated within 18 hours.
Cell lines
BJAB is an EBV-negative B-cell lymphoma cell line that was used as a negative control for all experiments. The B95-8 and Mutu-I EBV-positive cell lines were used for the development of BZLF1- and gp350-ELISpot assays. Cell lines were cultured in RPMI 1640 culture medium supplemented with 15% fetal calf serum (FCS), 2 mM l-glutamine, and 100 U/mL penicillin (Laboratories Eurobio, Les Ulis, France). They were fed twice every week by replacing two-thirds of the cell-free supernatants with fresh medium. Mutu-I cells were incubated in absence or in presence of the cytokine transforming growth factor β1 (TGF-β1; PeproTech, Rocky Hill, NJ) at the concentration of 5 ng/mL for 48 hours before testing. B95-8 cells were untreated or treated for 15 days with acyclovir (Merck, Darmstadt, Germany) at a concentration of 100 μM, and tested for BZLF1 and gp350 expressions.
Isolation, stimulation, and culture of peripheral blood B lymphocytes
Blood samples were incubated with a RosetteSepTM B-cell enrichment cocktail, a cyclic tetramolecular complex of monoclonal Abs directed to CD2, CD3, CD4, CD8, CD14, CD16, CD56 cell surface markers and red blood cell glycophorine A, according to the manufacturer's instructions (StemCell Technologies, Vancouver, BC). Unwanted cells cross-linked to red blood cells with tetrameric complexes were discarded by sedimentation through a Ficoll-Hypaque density gradient (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Purified memory CD19+ B cells, as well as purified memory B cells, IgM memory B cells, and naive B cells were resuspended in a RPMI 1640 culture medium supplemented with 10% FCS, 2 mM l-glutamine, and 100 U/mL of penicillin (Laboratories Eurobio). Then, 105 cells per well were cultured in 96-well tissue culture plates (BD Biosciences, San Jose, CA) with or without 25 × 103 CD40L-expressing CDw32L mouse fibroblasts previously treated with mitomycin C (Sigma-Aldrich, St Louis, MO) at concentration of 50 μg/5 × 106 fibroblasts plus 0.5 ng IL-2, and 1 ng IL-10 (Tebu, Le Perray-en-Yvelines, France).41 At day 5 of culture, the cells were delicately recovered and seeded in 96-microwell plates of ELISpot assays (Figure S1B).
Flow cytometric analysis and Abs
The number and percentage of B cells in blood samples and enriched B-cell preparations were determined by flow cytometry (FC500 apparatus; Beckman Coulter, Fullerton, CA). The B cells were characterized as previously described.29,30,41 The cells were incubated 10 minutes with a mixture of phycoerythrin cyanine-7-conjugated antihuman CD19 and phycoerythrin cyanine-5–conjugated antihuman CD27 Abs (Beckman Coulter), phycoerythrin-conjugated antihuman IgM, and fluorescein isothiocyanate-conjugated antihuman IgD Abs (Tebu). B lymphocytes were defined on the basis of size/structure, CD19, CD27, IgD, and IgM membrane receptor expressions.
The efficiency of the polyclonal activation was also assessed by flow cytometry over the period of the in vitro activation. Activated B cells were stained with a combination of antihuman Abs directed against CD19, CD20, CD27, CD38, and CD138 receptors (all reagents from Beckman Coulter). The number of B cells was determined using calibrated Flow-Count fluorospheres (Beckman Coulter) according to the manufacturer's instructions. Adjustments for compensation and positive thresholds for each sample were made using isotopic controls (Beckman Coulter). Cells drawing phenotype of CD19+, CD27+, CD20low, CD38high, and CD138+ were considered as plasma cells.
B-cell cycle was analyzed in unstimulated and stimulated purified B cells, as previously described.42 The percentage of B cells that entered into the cell cycle (S or G2/M) was determined and represented 8.5% to 31.3% of stimulated B cells and 0.6% to 1.2% of unstimulated B lymphocytes. Furthermore, this polyclonal stimulation did not induce an increase of the number of cultured B cells all over the culture period (0.98-1.10 × 105 B cells/well at day 0, 0.45-0.61 × 105 B cells/well at day 6).
Fluorescence-activated cell sorting analysis
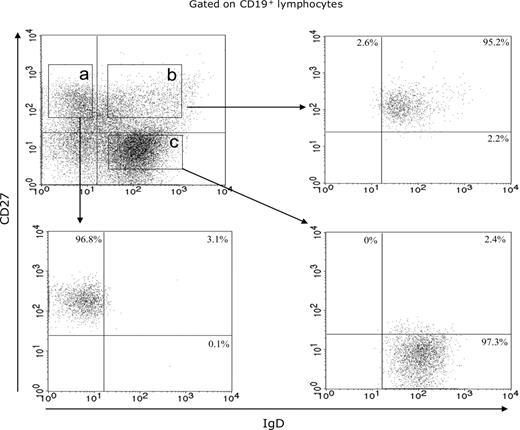
Blood B lymphocytes from 4 EBV healthy carriers (nos. 1-4) were obtained using the RosetteSepTM B-cell enrichment cocktail (StemCell Technologies) and stained with phycoerythrin cyanine-7-conjugated antihuman CD19 Abs, phycoerythrin cyanine-5-conjugated antihuman CD27 Abs (Beckman Coulter), and fluorescein isothiocyanate-conjugated antihuman IgD Abs (Tebu). CD19+ CD27+ IgD− (memory B cells), CD19+ CD27+ IgD+ (IgM memory B cells), and CD19+ CD27− IgD+ (naive B cells) were sorted with a fluorescence-activated cell sorting (FACS) Aria cell sorter (BD Biosciences) and stored in liquid nitrogen up to polyclonal B-cell activation. The purity of all isolated subsets was analyzed by flow cytometry with a FACS Aria cell sorter (BD Biosciences). CD19+ CD27+ IgD−, CD19+ CD27+ IgD+ and CD19+ CD27− IgD+ FACS-separated cell populations were 94.6% to 98.2%, 94.4% to 96.7%, and 93.9% to 97.3%, respectively. The IgM memory B-cell and naive B-cell subsets were contaminated by 3.3% plus or minus 1.4% and 0.1% plus or minus 0.1% memory B cells, respectively (Figure 1).
Purification control of enriched B-cell subsets. Flow cytometric fractionation of circulating B lymphocytes in memory B cell (area a), IgM memory B-cell (area b), and naive B-cell (area c) subsets, based on CD19, CD27, and IgD expression, from 1 representative case (no. 3).
Purification control of enriched B-cell subsets. Flow cytometric fractionation of circulating B lymphocytes in memory B cell (area a), IgM memory B-cell (area b), and naive B-cell (area c) subsets, based on CD19, CD27, and IgD expression, from 1 representative case (no. 3).
Enumeration of Ig-SCs
Ig-SCs were enumerated using a 2-color ELISpot assay.43 Briefly, a 96-well plate using Immobilon-P (Millipore, Bedford, MA) was coated overnight at 4°C with mouse monoclonal antihuman λ- and κ-light (L)–chain Abs (Tebu) at a concentration of 250 ng/100 μL in phosphate-buffered saline (PBS) (pH 7.2). The remaining binding sites were saturated with RPMI 1640 plus 10% FCS in PBS for 2 hours at 37°C, 104 unstimulated B cells, or 103 activated B cells per well were added and incubated for 18 hours at 37°C in a humidified atmosphere containing 5% CO2. After 9 washings (3 × PBS, 3 × PBS-0.05% Tween 20, 3 × PBS), a mixture of alkaline phosphatase- and peroxidase-conjugated goat Abs to human λ- and κ-L-chains Ig (Tebu) were added. After 3 washings with PBS, 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (Sigma-Aldrich) was added, and after 15 minutes of incubation, 3-amino-9-ethyl carbazole peroxidase chromogenic substrate (Sigma-Aldrich) was also added. Insoluble blue- or red-colored immunospots were obtained within 20 minutes and then were thoroughly rinsed with distilled water. The spots were counted by the KS ELISpot reader (Carl Zeiss, Jena, Germany). The results were expressed as the mean of duplicates of Ig-SCs/106 CD27+ B lymphocytes.
Enumeration of BZLF1- and gp350-SCs by ELISpot assays
A 96-well plate using Immobilon-P (Millipore) was coated overnight at 4°C with a mixture of anti-EBV polyclonal Abs constituted by a pool of sera from patients with high titers of anti-VCA (< 1/2560) and anti-EA antibodies (1/320). This pool was absorbed on CEM cells at a concentration of 5 × 106 cells/mL for 60 minutes at 37°C under agitation and then used at a 1:150 dilution. The remaining binding sites were saturated with RPMI 1640 plus 10% FCS for 2 hours at 37°C. We compared cell lines in experiments using viable B95-8, Mutu-I, and BJAB cells that were counted in a hemocytometer after trypan blue dye exclusion staining and then serially diluted in growth medium at concentration of 104, 103, and 102 cells/200 μL/well. B95-8 and BJAB cells were incubated with 50 μg/mL of cycloheximide (Sigma-Aldrich) for 30 minutes at 37°C in 5% CO2 atmosphere as controls. In human B lymphocyte experiments, 105 B cells suspended in 100 μL were added to each well, and the cells were incubated for 24 hours at 37°C in a humidified atmosphere containing 5% CO2. After 9 washings (3 × PBS, 3 × PBS-0.05% Tween 20, 3 × PBS), either 100 μL of anti-ZEBRA or anti-MA mouse monoclonal Abs (Argene, Foix, France) at 1:100 dilution were added for 6 hours at 37°C. After 3 PBS washings, peroxidase-conjugated antimouse monoclonal Ab (Tebu) was added for 3 hours at 37°C, washed 3 times, and a mixture of 3-amino-9-ethyl carbazole peroxidase chromogenic substrate (Sigma-Aldrich) was added. Insoluble red stainings were obtained within 20 minutes and the reaction stopped with distilled water. Red immunospots were fingerprints of BZLF1- or gp350-SCs that were enumerated by the KS ELISpot reader (Carl Zeiss). Results were expressed as the mean of duplicates for 104 line cells or 107 B lymphocytes.
EBV DNA quantification
Highly purified peripheral blood B cells were stimulated by CD40L ligation plus IL-2 and IL-10 to induce the differentiation of memory B cells into plasma cells (Figure S1B). Culture supernatants were tested for the presence of EBV DNA copies. The MagNA Pure compact instrument (Roche Diagnostics, Indianapolis, IN), running the MagNA Pure Compact Nucleic Acid isolation kit I, was used for automated DNA purification from culture supernatants. EBV DNA from culture supernatants was isolated by manual extraction using the QIamp DNA mini kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. Total DNA level in sample extracts was measured using the Light Cycler control DNA kit (Roche Diagnostics) according to the manufacturer's instructions.
The EBV DNA load was assessed by real-time PCR on a Light Cycler apparatus as previously described.44 An equivalent of 0.5 μg of extracted DNA was used in the PCR. Standard curves for the quantification of EBV DNA were generated using 10-fold serial dilution of Namalwa cells DNA diluted in 0.5 μg of EBV-negative human fibroblast DNA. The lower limit of detection was 50 copies/mL of culture supernatant, and the lower limit of quantification was 500 copies/mL.
Statistics
The results were compared using Wilcoxon rank sum test. The correlations between variables were analyzed by Spearman rank test. Data were presented as median values (25th to 75th percentiles). P values less than .05 were regarded as significant.
Results
Detection and enumeration of BZLF1- and gp350-SCs
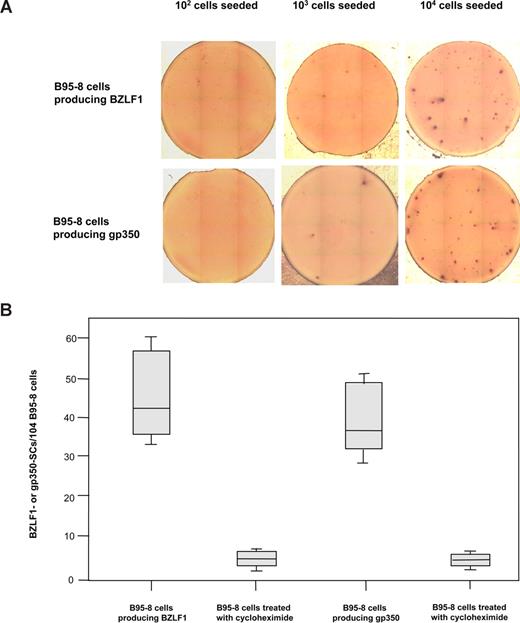
B95-8 cells were used to assess the capacity of the BZLF1- and gp350-ELISpot assays to detect cells secreting BZLF1 products and gp350 produced during the EBV lytic cycle. BZLF1- and gp350-SCs were detected in wells containing 103 and 104 EBV-positive B95-8 cells, whereas no immunospot was detected when wells were seeded with 102 to 104 EBV-negative BJAB cells (Figure 2A). As shown in Figure 2B, 30 to 60 BZLF1 immunospots/104 B95-8 cells (median, 42.5/104 B95-8 cells) and 28 to 50 gp350 immunospots/104 B95-8 cells (median, 36.5/104 B95-8 cells) were enumerated. B95-8 cells were also incubated with cycloheximide, an inhibitor of protein synthesis that dramatically decreased the number of immunospots because only 2 to 9 BZLF1-SCs/104 B95-8 cells (median, 4/104 treated B95-8 cells) and 3 to 7 gp350-SCs/104 B95-8 cells (median, 3.5/104 treated B95-8 cells) were detected. BZLF1-and gp350 expressions were explored in B95-8 cell line by ELISpot and intracytoplasmic immunofluorescence assays (Figure S2). The percentage of BZLF1-positive cells as well as gp350-positive cells were correlated to those of antigen SCs (R2 = 0.87 and 0.89, respectively). We also applied the ELISpot assays to acyclovir-treated B95-8 cells. Treatment with acyclovir partially reduced by nearly 50% the expression of BZLF1, whereas gp350 expression dramatically decreased (Figure S3). In addition, Mutu-I cell lines were tested by ELISpot and intracytoplasmic immunofluorescence assays. These cells were treated by TGF-β1 to induce the viral lytic cycle. BZLF1 secreting Mutu-I cells represented 70 to 110/104 Mutu-I cells versus 9% to 12% intracytoplasmic BZLF1-positive Mutu-I cells. Similar results were obtained with gp350-SCs (60-90/104 induced Mutu-I cells vs 7%-10% intracytoplasmic gp350-positive Mutu-I cells).
Secretion of BZLF1 products or gp350 by EBV-positive B95-8 cells. (A) Detection of BZLF1- and gp350-SCs by ELISpot assays in wells seeded with 102 to 104 EBV-positive B95-8 cells. (B) Enumeration of BZLF1- and gp350-SCs when cells were treated or not with cycloheximide. The ends of the boxes define the 25th and 75th percentiles, with a line at the median; error bars represent the 10th and 90th percentiles.
Secretion of BZLF1 products or gp350 by EBV-positive B95-8 cells. (A) Detection of BZLF1- and gp350-SCs by ELISpot assays in wells seeded with 102 to 104 EBV-positive B95-8 cells. (B) Enumeration of BZLF1- and gp350-SCs when cells were treated or not with cycloheximide. The ends of the boxes define the 25th and 75th percentiles, with a line at the median; error bars represent the 10th and 90th percentiles.
Rare plasma cells derived from circulating B cells synthesize BZLF1 product and gp350
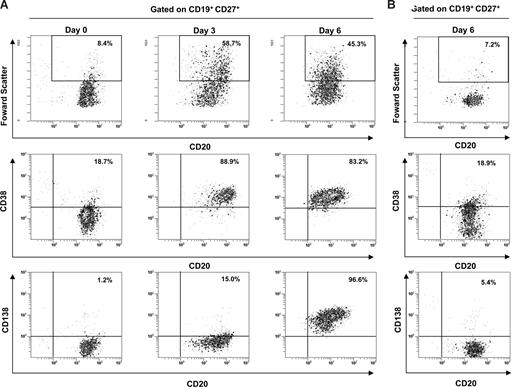
Highly purified peripheral blood B cells from 13 healthy EBV carriers were stimulated by CD40L ligation plus IL-2 and IL-10 to induce the differentiation of memory B cells into plasma cells (Figure S1B). As shown in Figure 3A, this polyclonal stimulation induced the plasmacytoid differentiation with a proportion of 81.6% to 97.2% of cultured memory and IgM memory B cells (CD19+, CD27+) responding by differentiation into plasma cells (CD19+, CD27+, CD20low, CD38high, and CD138+). In contrast, B cells cultured in standard media showed only 3.4% to 5.9% of plasma cells (Figure 3B). After 5 days of cell culture, Ig-SCs were also enumerated by an Ig 2-color ELISpot assay. Higher numbers of λ- plus κ-L-chains Ig-SCs were counted (median, 858,968/106 cultured CD27+ B cells, 25th-75th percentiles, 780,709-936,715) by comparison with unstimulated B cells (median, 1050/106 cultured CD27+ B cell; 25th-75th percentiles, 692-1583). This polyclonal activation induced the differentiation of most of the memory B cells into Ig-SCs (71.5%-98.5%).
Efficiency of B-cell polyclonal activation. CD19+ CD27+ B cells from 1 representative healthy control (no. 7) were analyzed by flow cytometry for size features and the expression of CD20, CD38, and CD138 over the culture period. Cells were gated on CD19 and CD27 membrane expression. (A) The phenotype of stimulated cells was shown at day 0, day 3, and day 6, which included the cell incubation of the ELISpot assay. (B) Histogram dot plots of unstimulated cells represented the level of spontaneous differentiation at day 6.
Efficiency of B-cell polyclonal activation. CD19+ CD27+ B cells from 1 representative healthy control (no. 7) were analyzed by flow cytometry for size features and the expression of CD20, CD38, and CD138 over the culture period. Cells were gated on CD19 and CD27 membrane expression. (A) The phenotype of stimulated cells was shown at day 0, day 3, and day 6, which included the cell incubation of the ELISpot assay. (B) Histogram dot plots of unstimulated cells represented the level of spontaneous differentiation at day 6.
EBV-infected peripheral blood B cells that were differentiated in vitro into plasma cells and able to initiate the EBV lytic cycle were enumerated. We distinguished EBV-infected SCs that were able to synthesize BZLF1 product from those secreting gp350 (Figure S4). As shown in Table 1, BZLF1-SCs were detected in 13 of 13 healthy EBV carriers and ranged from 50 to 480/107 B lymphocytes (median, 80/107; 25th-75th percentiles, 70-150/107). When B cells were cultured without polyclonal stimulation, BZLF1-SCs were only detected in 8 of 13 healthy EBV carriers and represented 20/107 B cells (nos. 2, 5, 7, 9, 10, and 12), 30/107 B cells (no. 8), and 50/107 B cells (no. 3; median 20/107, 25th-75th percentiles, 20-22.5/107), indicating that the rate of spontaneous reactivation ranged from 10% to 40%. Gp350-SCs were also detected in the 13 healthy carriers, and the number of immunospots ranged from 10 to 40/107 B lymphocytes (median, 17/107; 25th-75th percentiles, 10-20/107) and represented 7.7% to 28.6% of plasma cells initiating the EBV lytic replication as indicated by BZLF1-SCs (median, 15%; 25th-75th percentiles, 10.5%-20%). Gp350-SCs were detected in only 3 of 13 healthy controls (nos. 3, 8, and 12) when cultured B cells were unstimulated, and represented 10, 7, and 5 gp350-SCs/107 B cells, respectively.
Determination of EBV-infected B cells producing BZLF1, gp350, or EBV DNA
| EBV healthy carriers . | BZLF1-SCs/107 B cells . | gp350-SCs/107 B cells . | EBV-DNA copies/107 B cells . |
|---|---|---|---|
| 1 | 200 | 20 | 900 |
| 2 | 190 | 20 | 1300 |
| 3 | 480 | 40 | 53 200 |
| 4 | 130 | 10 | 960 |
| 5 | 60 | 10 | 18 580 |
| 6 | 80 | 12 | 2100 |
| 7 | 80 | 20 | 9360 |
| 8 | 150 | 21 | 40 200 |
| 9 | 50 | 10 | ND |
| 10 | 70 | 20 | ND |
| 11 | 70 | 10 | ND |
| 12 | 83 | 17 | 500 |
| 13 | 80 | 14 | ND |
| Median | 80 | 17 | 2100 |
| 25th percentile | 70 | 10 | 960 |
| 75th percentile | 150 | 20 | 18 580 |
| EBV healthy carriers . | BZLF1-SCs/107 B cells . | gp350-SCs/107 B cells . | EBV-DNA copies/107 B cells . |
|---|---|---|---|
| 1 | 200 | 20 | 900 |
| 2 | 190 | 20 | 1300 |
| 3 | 480 | 40 | 53 200 |
| 4 | 130 | 10 | 960 |
| 5 | 60 | 10 | 18 580 |
| 6 | 80 | 12 | 2100 |
| 7 | 80 | 20 | 9360 |
| 8 | 150 | 21 | 40 200 |
| 9 | 50 | 10 | ND |
| 10 | 70 | 20 | ND |
| 11 | 70 | 10 | ND |
| 12 | 83 | 17 | 500 |
| 13 | 80 | 14 | ND |
| Median | 80 | 17 | 2100 |
| 25th percentile | 70 | 10 | 960 |
| 75th percentile | 150 | 20 | 18 580 |
SCs indicates secreting cells; and ND, not detected.
Thus, if EBV-infected plasma cells initiating lytic viral replication induced BZLF1 expression, most of them do not complete the cycle, the frequency of cells achieving the EBV lytic cycle was estimated to 1 to 4 × 10−6 peripheral blood B cells.
In parallel, we also assessed the capacity of EBV-infected B cells to produce viral particles by measurement of EBV DNA in culture supernatants. EBV DNA copies were detected in 9 of 13 healthy EBV carriers and ranged from 500 to 53 200 EBV DNA copies/107 cultured B lymphocytes (median, 2100; 25th-75th percentiles, 960-18 580). However, when cultured B cells were unstimulated, EBV DNA copies were detected in 3 of 13 healthy EBV carriers (nos. 3, 5, and 8) and represented 620, 600, and 4340 EBV DNA copies/107 cultured B lymphocytes, respectively.
Finally, we performed the quantification of EBV DNA copies in highly purified peripheral blood B cells using real-time PCR, and detected EBV DNA copies in circulating B cells of 7 of 13 healthy carriers (nos. 1-4, 7, 12, and 13) that ranged from 200 to 23 200 EBV DNA copies/107 B lymphocytes (median, 740; 25th-75th percentiles, 270-6950).
The EBV functional reservoir is mainly restricted to memory B-cell subset in the peripheral blood
Resting B lymphocytes of 4 healthy EBV carriers (nos. 1-4) were investigated for the expression of BZLF1, gp350, and the quantification of EBV DNA according to the 3 main subsets of peripheral blood B cells. The blood memory B cells, IgM memory B cells, and naive B cells were highly purified with FACS and ranged from 13.40% to 27.90%, 12.50% to 32.10%, and 44.60% to 62.70%, respectively, and the B-cell purification method conserved these circulating cell subset proportions (data not shown). After B-cell polyclonal stimulation, the activation and differentiation of each subset were checked by flow cytometry (Figures S5,S6). The cells were tested by BZLF1- and gp350-ELISpot assays (Figure S7). In the memory B-cell subset, BZLF1-SCs ranged from 51 to 407/107 B cells (median 142; 25th-75th percentiles 118.5-209) and gp350-SCs ranged from 10 to 30/107 B cells (median 15; 25th-75th percentiles 10-22.5) as shown in Table 2. The number of BZLF1-SCs was significantly higher than gp350-SCs (P = .021). For the CD19+ CD27+ IgD+ cells, the number of BZLF1 immunospots ranged from 6 to 17/107 B cells (median, 6.5; 25th-75th percentiles, 6-9.5), whereas gp350-SCs were only detected in one subject (no. 3). Naive B cells did not generate immunospots (data not shown).
Enumeration of EBV-infected B cell subsets producing BZLF1, gp350, or EBV DNA
| B-cell subsets . | EBV healthy carriers . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
| IgM memory B cells | ||||
| BZLF1-SCs/107 B cells | 17 | 7 | 6 | 6 |
| gp350-SCs/107 B cells | ND | ND | 3 | ND |
| EBV DNA copies/107 B cells* | ND | 36 | 1940 | ND |
| Memory B cells | ||||
| BZLF1-SCs/107 B cells | 143 | 156 | 407 | 51 |
| gp350 SCs/107 B cells | 20 | 10 | 30 | 10 |
| EBV DNA copies/107 B cells* | 353 | ND | 23100 | ND |
| B-cell subsets . | EBV healthy carriers . | |||
|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | |
| IgM memory B cells | ||||
| BZLF1-SCs/107 B cells | 17 | 7 | 6 | 6 |
| gp350-SCs/107 B cells | ND | ND | 3 | ND |
| EBV DNA copies/107 B cells* | ND | 36 | 1940 | ND |
| Memory B cells | ||||
| BZLF1-SCs/107 B cells | 143 | 156 | 407 | 51 |
| gp350 SCs/107 B cells | 20 | 10 | 30 | 10 |
| EBV DNA copies/107 B cells* | 353 | ND | 23100 | ND |
SCs indicates secreting cells; and ND, not detected.
EBV DNA copies were quantified in culture supernatants of circulating B cells of each subset.
In parallel, EBV DNA copies were quantified in supernatants of cultured B cells by PCR assay in 4 healthy EBV carriers. EBV DNA copies were detected in supernatants of cultured CD19+ CD27+ IgD− cells from subjects 1 and 3 ranged from 353 to 23 100 DNA copies/107 B cells, and in supernatants of CD19+ CD27+ IgD+ cells from subjects nos. 2 and 3, ranged from 36 to 1940 DNA copies/107 B cells (Table 2). EBV DNA copies were not detected in supernatants of naive B cells subset for all healthy EBV carriers tested (data not shown). These results confirm that the functional EBV reservoir is mainly restricted to plasma cells derived from EBV-infected CD27+ IgD− memory B cells.
Discussion
Cells expressing EBV late proteins are considered as being extremely rare, and to date, assays enumerating cells synthesizing EBV viral proteins, particles, or infectious virus were not available. In the present study, using a combination of an efficient purification method of circulating B cells, BZLF1- and gp350-ELISpot assays, and EBV DNA quantification in cell culture supernatants, we demonstrate that EBV latently infected peripheral blood B lymphocytes constitute a functional reservoir able to secrete BZLF1 products and gp350 and achieve the EBV lytic cycle.
Our results indicated that cells stemming from the B95-8 cell line secreted BZLF1 products with a frequency comparable with that of cells secreting gp350. These results were in agreement with previously published data.19 Furthermore, the inhibition of the immunospot appearance by cycloheximide indicates that BZLF1 products and gp350 were synthesized de novo. Therefore, the immunospots corresponded to EBV-infected cells that secreted EBV antigens in vitro at early or late virus cycle.
Our strategy, applied to the exploration of memory B lymphocytes, suggests that all healthy EBV carriers have rare circulating cells that are able to differentiate into plasma cells secreting BZLF1 products and gp350. The frequency of BZLF1-SCs in healthy carriers was found to be similar to that previously observed in blood B cells with a limiting dilution EBV-specific DNA PCR.47 However, the number of plasma cells producing gp350 is significantly lower than that of cells secreting BZLF1. This is not the result of a technical artifact because the experiments performed with B95-8 cells, which are immortalized lymphoblastoid cells, showed no difference between cells secreting BZLF1 or gp350. Another possibility is that plasma cells secreting gp350 could be frailer than plasma cells secreting BZLF1, and so would be preferentially lost during transfers into ELISpot plates. However, cultured and stimulated B cells were delicately recovered and seeded in culture wells without cell stress, such as centrifugation and washing. Finally, EBV-Ag production seems to be dependent on signals inducing plasmacytoid differentiation because only few EBV-infected B cells enter lytic cycle spontaneously, as observed after 5 days of cell culture. Previous reports evidenced a 2-step process where peripheral EBV-infected cells undergo lytic infection and release EBV particles, which infect normal B cells early in the culture period, and then immortalize cocultured normal B cells.48,49 The B cells expressing spontaneously the EBV lytic cycle, as observed in our study, could be the same cells as those observed in the first step of the process, leading B cells to continuous lymphoblastoid cell line expansion. Thus, we conclude that EBV-infected plasma cells can initiate the lytic viral replication and synthesize BZLF1 products, although only a minority of them, estimated to be 15% of the total B cells expressing BZLF1, achieves the EBV lytic cycle. These results obtained with peripheral blood B lymphocytes by our new functional methodology provide an interesting feature because the viral replicative cycle is initiated when the differentiation into plasma cells is induced, but it appears to be aborted before expression of late antigen proteins for most EBV-infected plasma cells. Furthermore, this reservoir is productive, given that the quantification of EBV DNA in B-cell supernatants confirms the viral cycle completion and that cells secreting gp350 are potentially able to release EBV DNA. Plasma cells expressing late lytic antigens are extremely rare in tonsils, and the dramatic decrease of cells expressing late transcripts (BcLF1) compared with those producing immediate-early (BZLF1), early (BHRF1) transcripts could be linked to the destruction of these cells by EBV-specific cytotoxic T lymphocytes exerting an efficient immunologic control through the lytic cycle.19 In our study, this in vivo physiologic process does not interfere because our experiments were performed with cultures of highly enriched B cells depleted in T lymphocytes and thus were independent of cytotoxic T lymphocyte immunosurveillance.
When the detection of cells secreting BZLF1 products and gp350 and the quantification of EBV DNA in culture supernatants were extended to the 3 main subsets of circulating B-cell population, BZLF1- and gp350-SCs were mainly enumerated in CD27+ IgD− memory B cells. We confirm by our functional method that EBV infection is really restricted to the peripheral blood CD27+ IgD− memory B lymphocytes subset as reported,50,51 and the very rare CD27+ IgD+ cells that were able to enter viral lytic cycle could be the result of a contamination by the CD27+ IgD− B cells. Plasma cells synthesizing EBV proteins and EBV DNA are produced through the terminal differentiation of EBV latently infected memory B cells, and a minority complete the EBV lytic cycle. Our data are consistent with the concept, proof that EBV-infected cells have to differentiate through a germinal center before becoming latent memory B cells and establish a long-term persistence in the peripheral blood compartment. These cells circulate back to Waldeyer's ring and undergo viral replication to release infectious virus directly into the saliva or through EBV lytic infection of epithelial cells.51
We have shown that a very rare population of the latently EBV-infected peripheral blood resting memory B cells is able to complete the viral cycle and secrete viral particles, reinforcing the notion of a functional EBV reservoir. To our knowledge, this study is the first attempt to characterize, using ELISpot assay, the ability of EBV-infected peripheral blood memory B-cell reservoir to produce viral antigens and EBV DNA, in the normal carrier state. The capacity of this reservoir to produce viral lytic proteins cannot be directly observed52 because EBV maintains persistence in memory B lymphocytes by down-regulating the expression of all of the latent proteins so that the EBV-infected peripheral blood cells are neither immunogenic nor pathogenic to the host.53 However, when latently infected memory B cells are activated in vitro and driven to become plasma cells, they initiate EBV replication. Our strategy gives access to the measurement of this functional reservoir in the blood compartment after in vitro activation. In plasma cells derived from EBV-infected memory B cells, the EBV lytic cycle frequently aborted before expression of the late gene products because the viral strategy restricts BZLF1 and lytic reactivation by gene regulatory mechanisms, which include transcriptional54,55 and posttranscriptional controls,56 and posttranslational regulations by protein-protein interactions.57-59
In conclusion, these results suggest that our strategy based on the detection of the functional EBV reservoir can be applied to the exploration of B cells from compartments other than blood, as well as the setting-up and behavior of the functional EBV reservoir in EBV acute infection and EBV-associated malignancies. Further investigations using the same methods should assess the production of EBV proteins in clinical situations with potential viral reactivation observed in immunosuppressed patients or in EBV-related lymphomas.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Wendy Charles-Pickard of AMVF-Direct English for the English language review of this manuscript and Marie-France Huguet for her technical assistance.
This work was supported by the University Medical Center (Montpellier, France).
Authorship
Contribution: Y.A.T. performed research, analyzed and interpreted data, and wrote the paper; E.T. performed flow cytometric analysis and wrote the paper; K.B. performed research; V.F. performed PCR analysis and wrote the paper; G.P. and J.-M.S. wrote the paper; C. Duperray performed FACS analysis; C. Desgranges contributed reagents and wrote the paper; and J.-P.V. designed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Pierre Vendrell, Department of Virology, University Medical Center, 291 Avenue du Doyen Giraud, 34295 Montpellier, France; e-mail: jp-vendrell@chu-montpellier.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal