Abstract
CD45 is the most prominent membrane protein on lymphocytes. The function and regulation of this protein tyrosine phosphatase remain largely obscure, mainly because of the lack of a known ligand, and it still remains unknown whether such tyrosine phosphatases are subject to extracellular control at all. We report that an anti-CD45RB antibody (Ab) that prevents rejection and induces tolerance activates CD45RB tyrosine phosphatase enzymatic activity in T lymphocytes, allowing us to directly monitor the effects of increased CD45RB activity on signal transduction. Using both kinase substrate peptide arrays as well as conventional biochemistry, we also provide evidence of the various kinases involved in bringing about the inhibitory effect of this Ab on CD3-induced T-cell receptor signaling. Furthermore, we report that activated CD45RB translocates to lipid rafts and interferes with lipid raft localization and activation state of CD45 substrate Lck. Thus, these findings indeed prove that CD45 is subject to extracellular control and also define a novel mechanism by which receptor tyrosine phosphatases control lymphocyte biology and provide further insight into the intracellular signaling pathways effected by anti-CD45RB monoclonal Ab treatment.
Introduction
Protein phosphorylation plays a crucial role in regulating almost every aspect of cellular physiology, tyrosine phosphorylation even more so, as it is extensively used only in eukaryotes acting as a critical control point for important cellular processes, such as communication between and within cells, proliferation or differentiation, cell death, motility, and transportation of molecules into or out of cells.1-3 The phosphorylation status of these control points is tightly regulated by the antagonistic activities of protein kinases and protein phosphatases. Abnormal functioning of protein kinases and phosphatases is implicated in various human diseases, ranging from cancer to immune deficiencies.4 Strikingly, whereas the action of many different protein kinases is now fairly well understood for a large variety of biologic events, the role of most protein phosphatases remains unclear
CD45 was one of the first protein tyrosine phosphatases found to be expressed on all nucleated hematopoietic cells as a major high molecular weight leukocyte cell surface antigen.5-8 Structural analysis of the CD45 cytoplasmic region has shown that the intracellular tail of CD45 contains 2 homologous domains.7 The first membrane, proximal phosphatase domain, has phosphatase activity, whereas the second domain is thought to be required for proper folding and substrate recruitment.9 Six different isoforms, varying in molecular weight (180-220 kDa), antigenicity, and glycosylation have been identified and characterized.10 Different isoforms may exert different functions in cellular physiology; however, this issue is still under debate. Little evidence for clear functional differences between isoforms has emerged from experiments using transgenic mice expressing single isoforms of CD45 on a null background11-13 ; thus, tools to specifically stimulate the activity of different isoforms are recommended.
The function of CD45 in lymphocyte biology remains largely unclear because of the lack of a known ligand. It may act either as a positive or a negative regulator of T-cell receptor (TCR)– and B-cell receptor–mediated signaling, via the dephosphorylation of phosphotyrosine residues either associated with reduced Src-family protein tyrosine kinase activity14,15 or enhanced activity of these kinases, respectively.16,17 In addition, CD45 may down-regulate Src family kinase activities during integrin-mediated adhesion in macrophages.18 Furthermore, CD45 was recently shown to suppress Janus kinases (Jaks) and negatively regulate cytokine receptor signaling.19 Thus, CD45 may interact at multiple levels with tyrosine kinase signal transduction, and the exact roles in signaling are yet to be elucidated.
In view of the prominence of CD45 protein levels in the T-cell membrane, immunomodulation of CD45 has been proposed as an attractive strategy to prevent T cell–mediated graft rejection. Indeed, antibodies (Abs) to CD45 have been effective in preventing rejection of transplants in several different animal models.20-23 We have reported earlier that a mouse anti–human CD45RB Ab (6G3) that also recognizes monkey CD45RB has similar effects on monkey and human cells in vitro and also prevents rejection in an allogeneic nonhuman primate renal transplantation model,24 although the molecular mechanism behind this action is unclear. This consideration prompted us to directly assess the effects of antihuman CD45RB Ab (6G3) treatment on CD45 enzymatic activity, and we observed direct stimulation of CD45 tyrosine phosphatase activity. Thus, provided with the unique opportunity to investigate the effects of CD45 activation on cellular signal transduction, we performed a screen of these effects using a peptide array consisting of 1176 different kinase substrates. We have shown earlier that kinome profiling using such arrays25-30 is a particularly powerful tool to generate descriptions of cellular signaling without a priori assumptions as to the pathways affected. In the present study, we provide first evidence that CD45 tyrosine phosphatase activity can be extracellularly regulated and, using kinome profiling combined with conventional technology, reveal a CD45-mediated inhibition of lipid raft–dependent TCR signal transduction, thus defining a novel mode of regulation in lymphocyte biology.
Methods
Cell culture and in vitro stimulations
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood of healthy volunteers using standard density gradient centrifugation over Ficoll-Paque Plus (GE Healthcare, Little Chalfont, United Kingdom), followed by washing and resuspension in RPMI 1640 supplemented with 10% fetal bovine serum, penicillin, and streptomycin. This study received Institutional Review Board approval from the University Medical Center Groningen, and informed consent was obtained in accordance with the Declaration of Helsinki.
Reagents and Abs
Abs directed against p38Thr180/Tyr182, LckTyr505, SrcTyr416, PKBSer308, p70S6 kinaseThr389, mTORSer2448, JNK Thr183/Tyr185, ZAP70Tyr493, phosphotyrosine (pY100), pan-actin, and horseradish peroxidase (HRP)–labeled secondary reagents, cell lysis buffer, and kinase buffer were purchased from Cell Signaling Technology (Danvers, MA). Total Lck, Fyn, and Cbp/PAG Abs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-CD45RB (6G3) and pan-CD45 Abs were produced in-house.31 Antimouse IgG1 was purchased from DakoCytomation Denmark (Glostrup, Denmark). Protein-G Sepharose and the PTP101 Phosphatase Activity Kit were from Sigma-Aldrich (St Louis, MO). [γ-33P]Adenosine triphosphate was purchased from GE Healthcare. Lysis buffer was supplemented with protease and phosphatase inhibitors (except for the phosphatase assays where the vanadate was excluded from the buffer), obtained from Merck (Darmstadt, Germany). Kinase array slides (PepChip) were purchased from Pepscan Systems (Lelysrad, The Netherlands). CD45 phosphatase inhibitor, rapamycin, pp1, and p38 inhibitor SB-203580 were purchased from Calbiochem (San Diego, CA).
Cytokine measurements
PBMCs of healthy volunteers (106 cells/mL) were activated with CD3 monoclonal Ab (mAb) OKT3 (ATCC, Manassas, VA) at a low concentration (1 ng/mL) with or without 6G3 (1 μg/mL). Cell supernatants were collected after 24 hours and tested for IL-2, interferon-γ (IFN-γ), IL-4, and IL-10 by enzyme-linked immunosorbent assay (R&D Systems Europe, Abingdon, United Kingdom). Cytokine measurements were also carried out in the presence of various inhibitors after 24 hours. Cell viability was checked after 24 hours by cell counting with trypan blue.
Kinome array analysis
The protocol of the kinome array is described in detail on the website (http://www.pepscanpresto.com/files/PepChip%20Kinase%20Lysate%20Protocol_v5.pdf). Stimulations were terminated by an ice-cold phosphate-buffered saline wash, and the cells were lysed as described by Diks et al.25 The experiments were performed using 3 donors.
Data acquisition and statistical analysis of PepChip array
After drying, the glass slides were exposed to a phosphor imager plate for 72 hours. Acquisition of the peptide array was performed using a phosphor imager (Storm; GE Healthcare). The level of incorporated radioactivity, which corresponds to the phosphorylation status, was quantified by array software (ScanAlyze; Eisen Software, Berkeley, CA) and as described elsewhere.25-28 Different kinase activities in lysates from anti-CD45RB, anti-CD3, anti-CD3 plus anti-CD45RB, and untreated controls were determined by significant fold change ratios of the combined values of phosphorylated peptides as described by Löwenberg et al.27
Western blot analysis
Total cell extracts were supplemented with sodium dodecyl sulfate (SDS) sample buffer and heated at 90°C for 5 minutes. Samples were loaded on 10% SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Billerica, MA). The membranes were blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline supplemented with 0.1% Tween 20 (TBST) and incubated overnight at 4°C with the indicated primary Abs diluted in 5% BSA TBST. The membranes were subsequently incubated for 1 hour at room temperature with HRP-conjugated secondary Abs diluted in 5% BSA TBST. All Abs were used in accordance with the supplier's protocol, and images were revealed with a Kodak X-OMAT 1000 processor (Kodak Nederland BV, Bunschoten, The Netherlands) using ECL Western Blotting Detection Reagents (GE Healthcare).
Immunoprecipitations and in vitro phosphatase assay
Total cellular proteins were extracted from cells treated with anti-CD45RB mAb (6G3) at the mentioned time points in a phosphate-free buffer (10 mM Tris/HCl, pH 7.6, 100 mM NaCl, 2 mM MgCl2, 1 mM CaCl2, 2 mM ethyleneglycoltetraacetic acid, 1% Triton X-100, 0.1 mM MnCl2, 0.1 mg/mL BSA, and protease inhibitors) and sonicated for 10 seconds. Cell lysates were then centrifuged for 5 minutes at 14 000g at 4°C. Aspecific binding was blocked by pre-incubation for 2 hours with protein-G Sepharose beads (Sigma-Aldrich). After 5 minutes of centrifugation at 14 000g at 4°C, supernatants were incubated overnight at 4°C with pan-CD45 Abs (1:10). This was followed by incubation with protein-G Sepharose beads for 2 hours at 4°C. After centrifugation for 5 minutes at 14 000g at 4°C, the beads were washed 3 times in ice-cold phosphate-free buffer before the addition of 200 μL phosphate-free buffer. Immunoprecipitates were used for the activity assay. Similarly, Lck was immunoprecipitated from untreated cells in the same buffer with the addition of phosphatase inhibitors using Catch and Release Reversible Immunoprecipitation System (Upstate Biotechnology, Charlottesville, VA).
Immunoprecipitated Lck was incubated with CD45 immunoprecipitates in phosphate-free buffer for 20 minutes at 30°C. The immunoprecipitates were dissolved in sample buffer, loaded on 10% SDS-PAGE, blocked with 5% BSA in TBST (0.05 M Tris, 100 mM NaCl, 0.05% Tween 20), and immunoblotted using p-Tyr 100 Ab and a secondary HRP-conjugated Ab.
Isolation of detergent-resistant membranes
Detergent-resistant membrane (DRM) fractions were isolated from cells as described.32 For each isolation, 20 × 106 were lysed in 2 mL ice-cold Tris-NaCl-ethylenediaminetetraacetic acid buffer (TNE; 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, and protease inhibitors) containing 1% (wt/vol) Triton X-100. After 30 minutes of incubation on ice, cells were homogenized further by passing the lysate at least 10 times through a 21-gauge needle; 2 mL of the lysate was transferred to a centrifuge tube and mixed with 2 mL 80% (wt/vol) sucrose in TNE. On top of this, 4 mL 35% (wt/vol) and 3 mL 5% (wt/vol) sucrose in TNE were loaded successively, resulting in a discontinuous gradient. Gradients were centrifuged in a Beckman SW41 swing-out rotor (Beckman Coulter, Fullerton, CA) at 40 000 rpm for 18 to 20 hours at 4°C. Eleven fractions of 1 mL each were collected (from top to bottom), vortexed, and stored at −80°C. The protein content of all fractions was measured using BSA as a standard.33
Calcium flux measurements
Isolated PBMCs were labeled with Indo-1-AM in the presence of pluronic acid (both from Invitrogen, Carlsbad, CA) for 1 hour at 37°C. After washing 3 times, the cells were resuspended in RPMI with 10% fetal calf serum. Fluorescence was measured with a Varioskan reader (Thermo Electron, Waltham, MA) at an excitation of 352 nm and emissions of 400 nm (bound Ca2+) and 475 nm (free Ca2+). Abs against CD3 and CD45RB were added; and at the end of the experiment, the cells were lysed with 10% Triton to determine the minimal (addition of ethyleneglycoltetraacetic acid) and maximal (addition of CaCl2) fluorescence intensity ratios. Ca2+ concentrations for each time point were calculated according to Grynkiewitz et al.34
Measurement of phosphatase activity
Tyrosine phosphatase activity measurements were done on treated raft and nonraft fractions (isolated the same way as mentioned in “Isolation of detergent-resistant membranes” except that phosphatase inhibitors were not added to the lysis buffer) using the PTP101 Tyrosine Phosphatase Assay Kit following the manufacturer's instructions (Sigma-Aldrich) either in the presence or absence of CD45 phosphatase inhibitor.
Results
Anti-CD45RB Ab increases CD45 phosphatase activity in resting cells
Anti-CD45RB mAb (6G3) has been shown to counteract T cell–mediated pathology in a model of transplantation.24 The underlying mechanism remains largely unclear. Based on the hypothesis that the Ab might influence the activity of the enzyme, we performed an in vitro phosphatase assay to investigate the action of anti-CD45RB mAb (6G3) on the enzymatic activity of CD45. To this end, we incubated Lck, a well-known substrate of CD45, with CD45RB immunoprecipitated from lysates obtained from either untreated PBMCs or treated with the anti-CD45RB Ab (6G3) for different time periods. Figure 1A shows dynamic regulation of CD45 enzymatic activity; an evident increase in CD45 tyrosine phosphatase enzymatic activity present in lysates obtained after 5, 10, and 20 minutes of PBMC treatment with the 6G3 Ab is seen. In apparent agreement, immunoblotting for phospho-Lck (Y-505) (Figure 1B) in whole-cell lysates of lymphocytes stimulated with the 6G3 Ab demonstrates a gradual decrease in tyrosine phosphorylation, confirming that the increased CD45 phosphatase activity seen in the in vitro assays is reflected by increased dephosphorylation of this CD45 substrate in a cellular context. Interestingly, stimulation of enzymatic activity shows a strict correlation with translocation of the phosphatase to lipid raft fraction of the cell (Figure 1C), suggesting that activated CD45RB localizes to this subcellular compartment. We conclude that CD45 in particular, and by inference receptor tyrosine phosphatases in general, may be subject to dynamic regulation by external cues with respect to their activation status and their subcellular localization.
In vitro phosphatase assay showing increased CD45RB phosphatase activity and translocation of CD45RB to lipid rafts. (A) CD45RB immunoprecipitated from PBMC lysates treated with anti-CD45RB mAb (6G3) treated at various time points. Cells were lysed in lysis buffer without any phosphatase inhibitors. Lck, immunoprecipitated from untreated cells lysed in buffer containing phosphatase inhibitors. The last lane of pTyr blot shows phosphorylated Lck in resting cells. Immunoprecipitated CD45RB was then incubated with immunprecipitated Lck in a phosphatase reaction buffer for 20 minutes at 30°C. SDS sample buffer was added, and the samples were boiled and run on an 8% SDS-PAGE gel, transferred, and probed with a phospho-tyrosine Ab. Reduced tyrosine phosphorylation of Lck observed over time compared with phosphorylated Lck in resting cells. (B) Tyrosine 505 on Lck shows a decrease in phosphorylation over a period of 20 minutes after treatment of whole-cell lysates of mononuclear cells with anti-CD45RB mAb (6G3). (C) Pooled raft (R) and nonraft (NR) fractions of control and anti-CD45RB (6G3) mAb-treated cells as obtained by sucrose density gradient show translocation of CD45RB to the raft fraction of treated cells. We also stimulated using antimouse IgG1, which was used as an isotypic control where CD45RB is found only in the nonraft fractions.
In vitro phosphatase assay showing increased CD45RB phosphatase activity and translocation of CD45RB to lipid rafts. (A) CD45RB immunoprecipitated from PBMC lysates treated with anti-CD45RB mAb (6G3) treated at various time points. Cells were lysed in lysis buffer without any phosphatase inhibitors. Lck, immunoprecipitated from untreated cells lysed in buffer containing phosphatase inhibitors. The last lane of pTyr blot shows phosphorylated Lck in resting cells. Immunoprecipitated CD45RB was then incubated with immunprecipitated Lck in a phosphatase reaction buffer for 20 minutes at 30°C. SDS sample buffer was added, and the samples were boiled and run on an 8% SDS-PAGE gel, transferred, and probed with a phospho-tyrosine Ab. Reduced tyrosine phosphorylation of Lck observed over time compared with phosphorylated Lck in resting cells. (B) Tyrosine 505 on Lck shows a decrease in phosphorylation over a period of 20 minutes after treatment of whole-cell lysates of mononuclear cells with anti-CD45RB mAb (6G3). (C) Pooled raft (R) and nonraft (NR) fractions of control and anti-CD45RB (6G3) mAb-treated cells as obtained by sucrose density gradient show translocation of CD45RB to the raft fraction of treated cells. We also stimulated using antimouse IgG1, which was used as an isotypic control where CD45RB is found only in the nonraft fractions.
Anti-CD45RB inhibits cytokine production in activated PBMCs
The capacity of the 6G3 Ab to stimulate CD45 enzymatic activity provides us with the opportunity to directly assess the effects of CD45 enzymatic activity on cellular signaling. To assess these consequences, we activated PBMCs with a low concentration of anti-CD3 and investigated the effect of the 6G3 mAb thereon with respect to the production of cytokines. Supernatants of CD3-activated PBMCs were harvested, and IL-2, IL-10, and IFN-γ levels were determined at 24 hours. Anti-CD45RB treatment results in a 40% to 60% reduction in IFN-γ production, whereas a 25% to 50% reduction in IL-2 levels was observed (Figure 2). Thus, incubation of activated human peripheral blood cells with anti-CD45RB mAb strongly targets CD3-dependent signaling, and further experiments were initiated to determine the effects of increased CD45 tyrosine phosphatase activity in CD3-dependent signal transduction. In addition, 95% of the cells were still viable after 24 hours.
Cytokine production at 24 hours. Production of cytokines IL-2, IFN-γ, and IL-10 at 24 hours of CD3 activation and the effects of anti-CD45RB mAb. *P < .005, Student t test; **P < .01, Student t test.
Cytokine production at 24 hours. Production of cytokines IL-2, IFN-γ, and IL-10 at 24 hours of CD3 activation and the effects of anti-CD45RB mAb. *P < .005, Student t test; **P < .01, Student t test.
Anti-CD45RB treatment rapidly alters kinomic profiles in resting and activated PBMCs
Defining the effects of CD45 activation on cellular physiology is difficult in the absence of a priori assumptions as to the pathways targeted by this phosphatase. Recently, however, kinome profiling using peptide arrays exhibiting kinase consensus sequences across the entire mammalian kinome has emerged as a powerful tool for generating comprehensive descriptions of cellular kinase activity.25-30 Hence, we incubated PBMCs from 3 healthy donors for 20 minutes with and without the anti-CD45RB Ab 6G3 and/or anti-CD3 and analyzed cellular responses using a peptide array containing 1176 different kinase consensus substrates spotted in duplicates. In vitro phosphorylation of peptide arrays by these differently treated cells revealed that the various treatments caused substantial changes in kinase activity. A complete listing of the peptide substrates with significantly altered phosphorylation on anti-CD45 mAb treatment can be found in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Analysis of kinomic profiles of resting PBMCs from 3 healthy persons revealed 128 different kinase substrates with either significantly increased or decreased phosphorylation on anti-CD45RB treatment of resting cells. Comparison of the kinase activity patterns of activated PBMCs from the same 3 persons treated with anti-CD45RB mAb identified 155 kinase substrates that were significantly differentially phosphorylated (P < .05; Document S1) compared with untreated cells. The results show that a short treatment with anti-CD45 mAb treatment significantly influences kinase activities on TCR ligation and reveal that the rapid immunosuppressive effect of anti-CD45RB mAb treatment correlates with profound alterations in cellular signaling.
Validation of kinome profile results
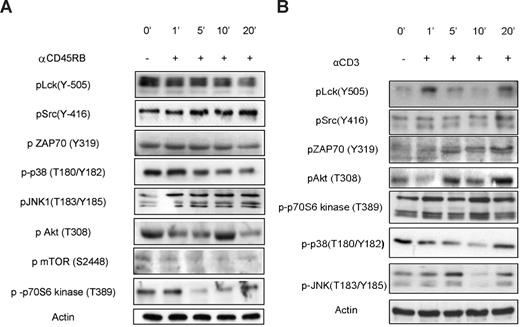
Predictably, among the most prominent effects of anti-CD45RB treatment observed on the PepChip was significantly increased phosphorylation of Lck autophosphorylation substrates and simultaneous reduction in phosphorylation of the Lck regulatory site (Tyr-505), suggesting an increased activity of CD45 on Lck in resting cells. The PepChip data were complemented by Western blotting using phospho-specific Abs; and as seen in Figure 3, the effects on Lck enzymatic activity, as measured using peptide arrays, are reflected in increased phosphorylation of Lck at its autophosphorylation site and reduced phosphorylation at its negative regulatory site. Interestingly, Western blots of pLck (Y-505) in cells treated with the combination of anti-CD3 (henceforth called activated) and anti-CD45RB 6G3 did not reveal any differences from basal levels, and a gradual decrease in phosphorylation was seen for the autophosphorylation site (Figure 4). This was confirmed by blotting for pZAP70 (Figure 4), which also shows no changes over basal levels, suggesting that there may be a block at the level of the Src family kinase Lck itself. Other substrates validated include JNK and p38 mitogen-activated protein kinase (Figures 3,4). Finally, one of the substrates that was consistently seen to be modulated in the peptide array experiments was p70S6 kinase. The ribosomal S6 protein kinase, p70 S6 kinase, is known for its role in modulating cell-cycle progression, cell size, and cell survival. We also tested upstream regulators of p70S6 kinase, namely, protein kinase B (PKB), and Figures 3 and 4 illustrate phosphorylation patterns for the same, thereby indicating their activation states as well. We also used anti-CD3 treated cells as a control for comparisons with anti-CD3/CD45-treated cells, which show the activation of Src family, mitogen-activated protein kinase, and PKB pathways (Figure 3B).
Validation of kinome profile results for anti-CD45RB treatment on resting cells. (A) Cells were incubated for various time points with or without anti-CD45RB. The phosphorylation status of Lck, Src, ZAP70, PKB, mTOR, p70S6 kinase, p38, and JNK in whole-cell lysates was assayed using phosphospecific mAbs on Western blot. (B) Cells were incubated for various time points with or without anti-CD3. The phosphorylation status of Lck, Src, ZAP70, PKB, p70S6 kinase, p38, and JNK in whole-cell lysates was assayed using phospho-specific mAbs on Western blot.
Validation of kinome profile results for anti-CD45RB treatment on resting cells. (A) Cells were incubated for various time points with or without anti-CD45RB. The phosphorylation status of Lck, Src, ZAP70, PKB, mTOR, p70S6 kinase, p38, and JNK in whole-cell lysates was assayed using phosphospecific mAbs on Western blot. (B) Cells were incubated for various time points with or without anti-CD3. The phosphorylation status of Lck, Src, ZAP70, PKB, p70S6 kinase, p38, and JNK in whole-cell lysates was assayed using phospho-specific mAbs on Western blot.
Validation of kinome profile results for anti-CD45RB treatments on anti-CD3 activated cells and IL-2 measurements in the presence of various inhibitors. (A) Cells were incubated for various time points with or without anti-CD45RB and simultaneously stimulated with anti-CD3 Ab, and phosphorylation states of the same proteins as in Figure 3 were tested. Actin was used as the loading control. (B) IL-2 was measured 24 hours after CD3 activation and anti-CD45RB mAb treatments in the presence of Src (pp1), p38 (SB203580), and mTOR inhibitors (n = 5). *P < .05.
Validation of kinome profile results for anti-CD45RB treatments on anti-CD3 activated cells and IL-2 measurements in the presence of various inhibitors. (A) Cells were incubated for various time points with or without anti-CD45RB and simultaneously stimulated with anti-CD3 Ab, and phosphorylation states of the same proteins as in Figure 3 were tested. Actin was used as the loading control. (B) IL-2 was measured 24 hours after CD3 activation and anti-CD45RB mAb treatments in the presence of Src (pp1), p38 (SB203580), and mTOR inhibitors (n = 5). *P < .05.
Mechanistic importance of CD45-induced changes in cellular kinome profiles
The identification of a multitude of changes in cellular kinase activities raises obvious mechanistic questions as to the importance of these events for the long-term physiologic consequences of CD45 ligation, in this case, diminished production of T cell–derived cytokines. Hence, we determined the effects of pharmacologic inhibitors of the pathways effected by such ligation on 6G3-dependent attenuation of IL-2. As expected, pharmacologic inhibition of Src-like kinases abolished the capacity of T cells to respond to TCR stimulation to produce IL-2 (although IFN-γ and IL-10 production was much less affected, and production of the latter 2 cytokines is thus only partly dependent on Src family kinase activity; not shown). In addition, diminished activation of p38MAP kinase cannot explain the effects of 6G3 on IL-2 generation. Inhibiting activity of the pro-inflammatory kinase does not inhibit CD3-dependent generation of IL-2; so at best, inhibition of p38MAP kinase constitutes an accessory part of 6G3 effects on cytokine production.
Importantly, however, in addition to the Src family, inhibition of the mammalian target of rapamycin (mTOR) pathway was just as effective as 6G3 in inhibiting IL-2 production. Furthermore, in the presence of 6G3, rapamycin is not very potent in further diminution of IL-2 production, suggesting that, after CD45 ligation, the PKB/mTOR/p70 S6 kinase pathways become rate-limiting. Hence, among the multitude of changes in kinase activities detected after application of 6G3, its effects on the Src and mTOR pathway seem most relevant with respect to cytokine production.
Activation of CD45RB rapidly affects localization of CD45RB
The broad inhibition of anti-CD3-induced signaling by the 6G3 Ab suggest that increased CD45 enzymatic activity influences a very fundamental property of the lymphocyte, essential for this CD3-dependent signaling. Many groups have now shown that the translocation of signaling elements to lipid rafts is essential for this signaling, opening the possibility that activation of CD45 interferes with raft localization of these elements, especially as ligation of CD45 induces translocation of the phosphatase to lipid rafts (Figure 1C). Accordingly, association to TX100 detergent-resistant cell membranes was used as the operational criterion to define raft-associated proteins, and an analysis of these effects was performed on CD45 substrate Lck and CD45RB itself. After a 20-minute incubation at 37°C with anti-CD45RB (6G3), or anti-CD3, or a combination of both, anti-CD3 and anti-CD45RB (6G3), untreated cells served as controls, cells were lysed for 30 minutes at 4°C with TX-100 and subjected to sucrose density gradient centrifugation to isolate the low-density, TX-100–resistant membrane rafts (DRMs). We investigated the consequences of catalytic activation of CD45 on its membrane localization. Importantly, unstimulated cells, displaying little CD45 enzymatic activity, only exhibited nonraft localized CD45. On stimulation, however, CD45 translocated into the raft fraction (Figure 1C) within 20 minutes. The functional consequences of this translocation were investigated using the Src family tyrosine kinase and CD45 substrate Lck as a readout. Figure 5B shows that, in 3 independent experiments, the total amounts of Lck translocating to lipid rafts after anti-CD45RB treatments were significantly lower than untreated rafts within 20 minutes of stimulation. After 60 minutes, the results reveal a dichotomous effect that includes dynamic regulation of both the total amount of Lck protein as well as regulation of its activation state (as deduced from the faster migration through SDS page of the unphosphorylated inactive form of the kinase compared with the active form). Both CD3 and CD45 ligation are capable of up-regulation of Lck levels (clearly visible after 60 minutes of stimulation); however, an induction of a faster migrating form of Lck (probably as a consequence of dual dephosphorylation) is observed in the samples in which CD45 enzymatic activity is increased by treatment with the 6G3 Ab (but not by anti-CD3 Ab alone). Similar effects are seen for Fyn. However, the adaptor protein Cbp/PAG, which is constitutively present in lipid rafts, remains largely unchanged in both the raft and nonraft fractions of treated and control cells (Figure 5B), suggesting that the effects observed are a consequence of CD45 enzymatic activity and do not represent physical displacement of Lck/Fyn from rafts as a consequence of CD45RB translocating into the rafts.
Localization of CD45 substrates. (A) Lipid raft isolation shown using GM1 as a marker. Fractions 3 to 6 were pooled and termed raft fractions and fractions 7 to 10 pooled and termed nonraft fractions. Equal proteins loaded for Western blotting. (B) Western blot data for Lck were quantified and ratio of raft to nonraft measured. Pooled data from 3 separate experiments are represented. Significant changes in amounts of Lck moving into rafts seen compared with untreated samples using a heteroscedastic the Student t test. Western blots showing amounts of p56Lck, p59Fyn, and Cbp/PAG after 20-minute stimulations and 60-minute stimulations are also shown. (C) Calcium flux measurements in the presence and absence of anti-CD45RB for 3 donors.
Localization of CD45 substrates. (A) Lipid raft isolation shown using GM1 as a marker. Fractions 3 to 6 were pooled and termed raft fractions and fractions 7 to 10 pooled and termed nonraft fractions. Equal proteins loaded for Western blotting. (B) Western blot data for Lck were quantified and ratio of raft to nonraft measured. Pooled data from 3 separate experiments are represented. Significant changes in amounts of Lck moving into rafts seen compared with untreated samples using a heteroscedastic the Student t test. Western blots showing amounts of p56Lck, p59Fyn, and Cbp/PAG after 20-minute stimulations and 60-minute stimulations are also shown. (C) Calcium flux measurements in the presence and absence of anti-CD45RB for 3 donors.
Effects of CD45 ligation on TCR-induced calcium transients
Altered Lck localization and phosphorylation would be expected to alter the strength of signal induced by anti-CD3. Among the most direct cellular events that quantitatively represent the strength of TCR activation are the calcium transients. To understand whether alteration of localization and phosphorylation can alter the strength of the signal induced by anti-CD3, we assessed the T cell–induced calcium flux in the presence and absence of anti-CD45RB (Figure 5C). The results show that CD45 ligation results in a strong attenuation of TCR-dependent signals.
CD45 tyrosine phosphatase activity increases in the rafts
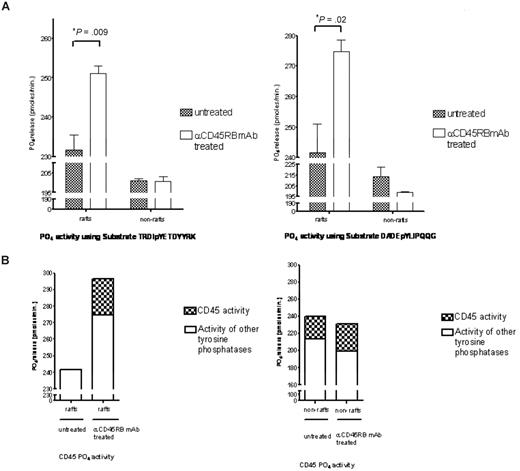
Subsequently, on translocation of CD45RB to the rafts, its phosphatase activity within these rafts was studied. Figure 6A shows significant increases in total tyrosine phosphatase activity in treated raft fractions compared with the untreated raft fractions carried out using 2 different tyrosine phosphorylated peptides. To determine how much of the total activity in these rafts was contributed by CD45RB, a specific inhibitor that targets the CD45 phosphatase activity was used at a concentration of 5 μM, and Figure 6B shows the total contribution of CD45 phosphatase activity to the total tyrosine phosphatase activity found in the untreated and treated raft fractions. This raft-restricted action of catalytically active CD45 defines a novel mode of CD45 action in T lymphocyte biology.
Tyrosine phosphatase activity measurements. (A) Raft and nonraft fractions from treated and control cells were subject to tyrosine phosphatase activity measurements based on the malachite green assay using 2 different tyrosine phosphorylated peptides, TRDIpYETDYYRK and DADEpYLIPQQG. Significant increases in phosphatase activity were seen in the raft fractions for both these peptides. (B) Furthermore, to determine how much of this activity was contributed by CD45, we used an inhibitor to CD45 (Calbiochem) at a concentration of 5 μM and show that approximately 17% of the total tyrosine activity in the raft fraction is contributed by CD45.
Tyrosine phosphatase activity measurements. (A) Raft and nonraft fractions from treated and control cells were subject to tyrosine phosphatase activity measurements based on the malachite green assay using 2 different tyrosine phosphorylated peptides, TRDIpYETDYYRK and DADEpYLIPQQG. Significant increases in phosphatase activity were seen in the raft fractions for both these peptides. (B) Furthermore, to determine how much of this activity was contributed by CD45, we used an inhibitor to CD45 (Calbiochem) at a concentration of 5 μM and show that approximately 17% of the total tyrosine activity in the raft fraction is contributed by CD45.
Discussion
In contrast to their enzymatic counterpart receptor tyrosine kinases, the action of receptor tyrosine phosphatases remains obscure. A major reason for this is that external stimuli capable of activating enzymatic activity have not been identified and understanding the action of receptor tyrosine phosphatases in cellular signaling remains one of the most important problems in mammalian cell biology.
CD45 is highly expressed (> 106 molecules/cell) on all hematopoietic cells and their precursors except mature erythrocytes and platelets.35-37 CD45 is a critical positive regulator of TCR- and B-cell receptor–mediated signaling required for the activation and development of lymphocytes and the basis for this regulation seems to lie in the cytoplasmic region, composed of tandem repeats of 2 potential phosphatase domains, which has been found to be conserved between species.7,38 Immunomodulation of CD45RB has been shown to be effective in several animal models.20-23 The molecular mechanisms underlying the effects of these Abs are poorly understood. Several mechanisms such as the depletion of functionally important subsets of T cells, B-cell activation, CTLA-4 transcription, and intercellular adhesion molecule/leukocyte function-associated antigen-1 interaction via shedding are thought to play a role in bringing about this effect.39-42 The present study identified increased CD45 enzymatic activity as a possible contributor to the effects of anti-CD45 as it results in the attenuation of cytokine production and reduced calcium transients. Further work needs to be done to establish the exact contribution of the various molecular effects of anti-CD45 on its immunomodulatory effects in vivo, but it is tempting to assume that many of these effects involve altered enzymatic activity of the phosphatase, albeit with cell specific effects on signaling.
We observed that ligation of CD45RB increased its enzymatic activity, by dephosphorylation of its substrate Lck. This finding provides the first evidence that enzymatic activity of receptor tyrosine phosphatases is under the control of external cues. This is an important observation because its logical extrapolation would be that in vivo receptor tyrosine phosphatase activity would be under the control of external cues, serving as ligands for these receptor phosphatases (which, by this measure, would then truly be receptors); although until the nature of these natural ligands is established, other possibilities should be kept in mind. It should prove interesting to investigate whether Ab-dependent ligation can also stimulate enzymatic activity of other receptor tyrosine phosphatases.
The observation that CD45 enzymatic activity can be dynamically regulated using the 6G3 Ab was exploited to obtain an insight into the cellular effects of increased CD45 enzymatic activity on lymphocyte signal transduction. This possibility is of substantial value as hitherto no good insight exists as to the functioning of receptor tyrosine phosphatases in mammalian signal transduction. Using an array of kinase pseudo-substrates, a tool recently demonstrated to be useful for delineating signal transduction in the absence of a priori assumptions,25-30 we demonstrated that the activities of multiple kinases are rapidly altered in resting and activated PBMCs after short-term treatment with anti-CD45RB mAb (6G3). The results reveal reduced Lck activity that may have an important role in the immunosuppressive effects of anti-CD45RB Ab in immune cells. The observed diminished anti-CD3-induced Lck activity after 6G3 activity correlates well with the appearance of hypophosphorylated Lck as observed in Figure 4.
PepChip data reveal the rapid effects of this Ab on the S6 kinase (S6K) substrates that are down-regulated. p70S6 kinase is involved in regulating protein synthesis, cell size, cell-cycle progression, and glucose homeostasis. S6 is also one of the most studied readouts of mTOR function, which is the master regulator of cellular physiology.43-46 In apparent agreement, on both the arrays and on the conventional Western blot, where kinase activating phosphorylation for mTOR and p70S6 kinase is down-regulated in both resting and activated cells treated with CD45RB. In line with this notion, decreased activation of various signaling pathways downstream of the TCR was observed. The inhibitor experiments indicate that modulation of the Src and PKB/mTOR/S6 kinase signaling cassette are mechanistically the most important of these changes, at least with respect to cytokine production. Furthermore, in this context, it is important to note that the specificity of the kinase changes identified is demonstrated by the observation that JNK, known to be dispensable for T-cell signaling, was increased rather than decreased and that these observations do not represent a general effect by the Ab on T-cell physiology but are a bona fide manifestation of CD45-dependent effects on TCR-induced signaling.
One recently published report also proposes a central role for mTOR in integrating the environmental signals that determine whether Ag recognition leads to activation or tolerance.47 Thus, it should be interesting to test whether the 6G3 Ab has the capacity to induce anergy in our experimental system, possibly explaining its strong suppressive effects on T cell–mediated immunity. In any case, the broad inhibition of anti-CD3-induced signaling events by 6G3 Ab suggests that increased CD45 enzymatic activity influences a very fundamental property of the lymphocyte, essential for this CD3-dependent signaling.
One of the other ways of regulation of CD45 PTPase activity is localization, and there is ample evidence that the ability of CD45 to access specific substrates depends on its location within the plasma membrane. Many groups have now shown that the translocation of signaling elements to lipid rafts is essential for signaling.48-51 The plasma membrane domain localization of CD45 has remained controversial, with some groups observing that approximately 10% of CD45 is associated with detergent-insoluble microdomains in resting cells52-54 and Janes et al showing microscopically that CD45 is excluded from lipid rafts in resting cells.51 Interestingly, the 6G3 Ab allows us to investigate CD45 localization after stimulation of its enzymatic activity. In agreement with Janes et al,51 in our experimental system CD45 is excluded from raft fractions in untreated cells. Importantly, on stimulating its catalytic activity, CD45RB translocates to the raft fraction. The dephosphorylation of Lck correlates well with the appearance of CD45RB within the raft fractions of these cells within 20 minutes. Localization of certain Srcs, such as Lck and Fyn, is also reduced in raft fractions within 20 minutes on treatment with 6G3, which again correlates well with the appearance of CD45 in the raft fractions. It is known that targeting to raft domains is crucial for Lck function.55 On stimulation of these cells with anti-CD3, we do not see a significant amount of CD45RB migrating to the raft fractions (data not shown), which is in agreement with Edmonds and Oostergaard,53 who also show that the amount of CD45 found associating with lipid rafts reduces on stimulation of T cells with anti-CD3. Accordingly, partitioning of CD45 into DRMs depended on the CD45's extracellular domains, and it was found that failure of CD45 variants to partition into DRMs correlated with their incapability to activate Lck.56 The increased levels of CD45 in the lipid raft fraction of the membrane may in turn lead us to hypothesize that it may cause physical displacement of Src family members out of the lipid raft, a phenomenon known to be incompatible with the catalytic action of the nonreceptor tyrosine kinases and thus expected to lead to reduced Src family activity. Alternatively, CD45 activity has recently been shown to be capable of dephosphorylating both allosteric tyrosine phosphorylation sites in Src family members and thus inactivation of these kinases. Hence, increased recruitment of CD45 to the lipid rafts and its subsequent engagement with the active variants of Src family kinase members may lead to deactivation of these kinases, which is a more favored hypothesis (Figure 7). Obviously, further work is needed to distinguish between these possibilities. It should be kept in mind, however, that both mechanisms may also be operative at the same time, a synergistic effect being the result. In addition, in this study, PBMCs were studied; however, it is possible that the responses on isolated T cells would differ marginally and requires further studies. Disregarding the exact mechanisms underlying CD45 ligation-dependent deactivation of Src family members, in the present study we have shown that such ligation activates receptor protein tyrosine phosphatase activity of CD45 and results in a lipid raft-specific action of these phosphatases, influencing a multitude of cellular signal transduction processes. Whether this novel paradigm in cellular signaling is also true for other receptor protein tyrosine phosphatases is currently under investigation in our laboratory.
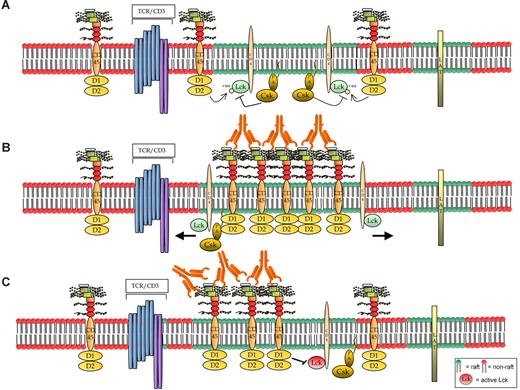
Hypothetical models of CD45RB signaling. (A) The resting cells contain small, dynamic lipid rafts, each with a limited number of associated molecules, probably including the TCR. Maybe because of the small size of the rafts, CD45 is not known to be raft-associated.51 However, (B) on activation using anti-CD45RB mAb, one hypothesis is that CD45 translocates to the raft fraction where its activity increases; thereby rafts act as signaling platforms for amplifying its phosphatase activity and also may result in the movement of its substrates out of the rafts. However, a specific induction of a faster migrating form of Lck (probably as a consequence of dual dephosphorylation) is observed in the samples in which CD45 enzymatic activity was increased by treatment with the 6G3 Ab, suggesting that the effects observed are a consequence of CD45 enzymatic activity and do not represent physical displacement of Lck from the rafts. Hence, we favor model C where (C) Ab ligation causes CD45RB to translocate into and remain in the rafts longer, thereby keeping it in the vicinity of the activated kinase and, because of its increased phosphatase activity, would result in the negative regulation of raft-specific signal transduction.
Hypothetical models of CD45RB signaling. (A) The resting cells contain small, dynamic lipid rafts, each with a limited number of associated molecules, probably including the TCR. Maybe because of the small size of the rafts, CD45 is not known to be raft-associated.51 However, (B) on activation using anti-CD45RB mAb, one hypothesis is that CD45 translocates to the raft fraction where its activity increases; thereby rafts act as signaling platforms for amplifying its phosphatase activity and also may result in the movement of its substrates out of the rafts. However, a specific induction of a faster migrating form of Lck (probably as a consequence of dual dephosphorylation) is observed in the samples in which CD45 enzymatic activity was increased by treatment with the 6G3 Ab, suggesting that the effects observed are a consequence of CD45 enzymatic activity and do not represent physical displacement of Lck from the rafts. Hence, we favor model C where (C) Ab ligation causes CD45RB to translocate into and remain in the rafts longer, thereby keeping it in the vicinity of the activated kinase and, because of its increased phosphatase activity, would result in the negative regulation of raft-specific signal transduction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Karin Klappe for her help with all the lipid raft work.
Authorship
Contribution: K.P. and L.V. designed the study, carried out the experiments, analyzed the data, and wrote the manuscript; and M.P.P. and S.P. contributed to designing the study and writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kaushal Parikh, Department of Cell Biology, Section Immunology, University Medical Center Groningen, University of Groningen, A Deusinglaan 1, 9713 AV Groningen, The Netherlands; e-mail: k.parikh@med.umcg.nl.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal