Abstract
Hematopoietic stem cells (HSCs) have a very low rate of cell division in the steady state; however, under conditions of hematopoietic stress, these cells can begin to proliferate at high rates, differentiate into mature hematopoietic cells, and rapidly reconstitute ablated bone marrow (BM). Previously, we isolated a novel evolutionarily conserved DNA replication factor, PSF1 (partner of SLD5-1), from an HSC-specific cDNA library. In the steady state, PSF1 is expressed predominantly in CD34+KSL (c-kit+/Sca-1+/Lineage−) cells and progenitors, whereas high levels of PSF1 expression are induced in KSL cells after BM ablation. In 1-year-old PSF1+/− mice, the pool size of stem cells and progenitors is decreased. Whereas young PSF1+/− mutant mice develop normally, are fertile, and have no obvious differences in hematopoiesis in the steady state compared with wild-type mice, intravenous injection of 5-fluorouracil (5-FU) is lethal in PSF1+/− mice, resulting from a delay in induction of HSC proliferation during ablated BM reconstitution. Overexpression studies revealed that PSF1 regulates molecular stability of other GINS components, including SLD5, PSF2, and PSF3. Our data indicate that PSF1 is required for acute proliferation of HSCs in the BM of mice.
Introduction
Tissue regeneration is one of the most tightly controlled processes requiring an ordered program involving induction of proliferation and differentiation of damaged-tissue stem cells. In the normal state, hematopoietic stem cells (HSCs) undergo cell division at a very low rate. However, if the bone marrow (BM) is ablated by an anticancer drug or radioactivity, HSCs that are in a quiescent state are stimulated to proliferate and restore the BM; self-renewal of HSCs is involved in this process.1 In a mouse experimental model, only one HSC was found to be sufficient to reconstitute the entire hematopoiesis.2 In this system, it has been suggested that daughter cells derived from HSCs can either commit to a program of differentiation that will eventually result in production of mature, nonproliferating cells or retain HSC properties.
Several proteins thought to be involved in HSCs for induction of self-renewal, including Wnt and Notch ligand families, have been isolated3-5 ; however, what lies downstream of them in the signaling pathway, especially molecules involved in DNA replication, is not known.
PSF1 (partner of SLD5-1) is evolutionarily conserved and is involved in DNA replication in lower eukaryotes,6-8 and human.9 PSF1 forms a tetrameric complex (GINS complex) with SLD5, PSF2, and PSF3. Recently, crystal structure of the human GINS complex was reported.10-13 In yeast, GINS complex associates with MCM2-7 complex and CDC45, and this C-M-G complex (CDC45-MCM2-7-GINS) regulates both the initiation and progression of DNA replication.14-17
Previously, we cloned the mouse ortholog of PSF1 from an HSC-specific cDNA library.18 PSF1 is predominantly expressed in highly proliferative tissues, such as testis and BM. Loss of PSF1 causes embryonic lethality around the implantation stage.18 PSF1−/− embryos revealed impaired proliferation of multipotent stem cells, ie, the inner cell mass. In mice, PSF1 is highly expressed in proliferating HSCs and the hematopoietic progenitor cells (HPCs). However, the biologic function of PSF1 in hematopoiesis is not understood.
In this study, we used PSF1+/− mice for studying the function of PSF1 in hematopoiesis. Here we show that haploinsufficiency of PSF1 causes loss of regeneration capacity resulting from a delay in induction of acute proliferation of HSCs after BM ablation. Our data suggest that both alleles of PSF1 are essential for acute proliferation of HSCs after BM ablation.
Methods
Mice
C57BL/6 mice were purchased from SLC (Shizuoka, Japan). PSF1 mutant mice and Runx1-deficient mice (Runx1-deficient mice were a gift from Dr T. Watanabe, Tohoku University, Sendai, Japan) were maintained and bred as described.18,19 All animal studies were approved by the Animal Care Committee of Kanazawa University and the Osaka University Animal Care and Use Committee. For BM ablation studies, 8-week-old wild-type and PSF1+/− mice were treated with a single tail vein injection of 5-fluorouracil (5-FU; 150 mg/kg body weight; Kyowa Hakko Kogyo, Tokyo, Japan).
Immunohistochemistry and FACS analysis
Tissue fixation, preparation of tissue sections, and staining of sections with antibodies were performed as described previously.18 For immunohistochemistry of fetal liver (FL), rabbit anti-PSF1 antibody was used.18 Horseradish peroxidase-conjugated secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). For immunocytochemistry, we used monoclonal anti-PSF1 antibody (Aho57.2; see next paragraph below) as a first antibody and Alexa 488–conjugated antirat IgG (Invitrogen, Carlsbad, CA) as a second antibody. Stained cells and the sections were observed using an Olympus IX-70 microscope equipped with UPlanFI 4/0.13 and LCPlanFI 20 /0.04 dry objective lenses (Olympus, Tokyo, Japan). Images were acquired with a CoolSnap digital camera (Roper Scientific, Trenton, NJ), and processed with Adobe Photoshop version 8.0.1 software (Adobe Systems, San Jose, CA).
For the generation of monoclonal anti-PSF1 antibodies, cDNA encoding the full-length protein sequence of PSF1 was amplified by polymerase chain reaction (PCR), and then cDNA was ligated into pGEX-4T-1 vector (GE Healthcare, Little Chalfont, United Kingdom) for the preparation of glutathione S-transferase (GST)–fusion proteins. PSF1-coding region was amplified from the mouse NIH3T3 cDNA using the primers 5′-GGA ATT CAT GTT CTG CGA AAA AGC TAT G-3′ (sense) and 5′-GGA ATT CTC AGG ACA GCA CGT GCT CTA GA-3′ (antisense) and was subsequently subcloned as a EcoRI-EcoRI fragment into the pGEX-4T-1 vector (GE Healthcare) in the correct reading frame to express the GST-PSF1 fusion protein. This construct was transformed into Escherichia coli JM109 strains (Toyobo Engineering, Osaka, Japan) to obtain GST-tagged fusion proteins. Recombinant GST-PSF1 was purified using glutathione-Sepharose 4B column (GE Healthcare) according to the manufacturer's instructions. Purified GST-fused proteins were used as antigen for immunization of rats, and rat/mouse hybridomas were established by standard procedures.20 A stable hybridoma cell line, aho57.2, was obtained. The specificities of all antibodies were determined by immunoblotting and immunocytochemistry.
Preparation of FL and BM cells and fluorescence-activated cell sorter (FACS) analysis was as described previously.21,22 The antibodies used in flow cytometric analysis for lineage marker (Lin) were fluorescein isothiocyanate– or phycoerythrin-conjugated Gr-1 (RB6-8C5), Mac-1 (M1/70), B220 (RA3-6B2), TER119, anti-CD4 (GK1.5), and anti-CD8 (53-6.72). Allophycocyanin-conjugated anti–c-kit (ACK2), and biotin-conjugated anti–Sca-1 and CD34 were also applied. Biotinylated anti–Sca-1 or CD34 was visualized with peridinin chlorophyll protein–streptavidin. These antibodies were purchased from BD Biosciences (San Jose, CA). The stained cells were analyzed by FACSCalibur (BD Biosciences) and sorted by JSAN (Bay Bioscience, Kobe, Japan). For immunocytochemistry of HSCs from 5-FU–treated BM, 300 CD34+ KSL cells were isolated by sorting from BM obtained from 5 mice 4 days after 5-FU treatment. The average number of total BM mononuclear cells and CD34+ KSL obtained from right and left femurs and tibias after treatment with 5-FU was 9.3 plus or minus 5.3 × 106 and 60 plus or minus 38, respectively.
For the analysis of apoptosis, cells were stained with anti–annexin V antibodies (eBioscience, San Diego, CA). For platelet analysis, blood from wild-type and mutant mice was obtained from the tail vein and collected in phosphate-buffered saline (PBS) containing 3.8 mM citric acid, 7.5 mM trisodium citrate, and 10 mM of dextrose (PBS–acid-citrate-dextrose). Cells were stained with fluorescein isothiocyanate–conjugated anti-CD41 antibody (eBioscience).
qRT-PCR
Total RNA was isolated using the RNAeasy Kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. RNA was reverse transcribed using the ExScript RT Reagent Kit (Takara, Kyoto, Japan). Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was performed using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) on an Mx3000 system (Stratagene, La Jolla, CA). Levels of the specific amplified cDNAs were normalized to the level of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) housekeeping control cDNA. We used the following primer sets: 5′-GAA GGG CTC ATG ACC ACA GT-3′ and 5′-GGA TGC AGG GAT GAT GTT CT-3′, for GAPDH, and 5′-CCG GTT GCT TCG GAT TAG AG-3′ and 5′-CTC CCA GCG ACC TCA TGT AA-3′ for PSF1.
Cell culture
The colony formation unit in culture (CFU-c) assay was performed as described previously.21 A total of 2 × 102 KSL-Mac-1−/lo cells that had been sorted from the BM of wild-type or PSF1+/− mice were placed in 1 mL semisolid medium (MethoCult; StemCell Technologies, Vancouver, BC). After 10 days of culture, the number of colonies was counted.
For the analysis of sensitivity of HSCs to 5-FU, 103 KSL cells derived from wild-type or PSF1+/− mice were seeded onto semisolid medium and continuously cultured for 10 days with or without 5-FU (1 ng/mL to 1 μg/mL). Each condition was represented by at least 3 wells, and each experiment was performed in triplicate. To examine the number of apoptotic cells in the population of colony-forming cells, colonies grown in methylcellulose semisolid medium were harvested 6 days after the CFU-c assay was initiated, and the cells were stained with Cy5-conjugated anti–annexin V antibody (eBioscience).
Cell-cycle analysis
Cells were sorted by FACS and fixed in 70% ethanol overnight. After treatment with RNase A (0.5 mg/mL; Sigma-Aldrich, St Louis, MO), cells were labeled with 5 μg/mL propidium iodide (PI) and analyzed by FACSCalibur.
Transfection and immunoblot analysis
NIH3T3 cells were transfected with pEF-BOSE, pEF-flag-PSF1, pEF-Myc-SLD5, pEF-HA-PSF2, and/or pEF-VSVG-PSF3, and immunoblotting was performed as previously described.23 GAPDH was detected with anti-GAPDH antibodies (Chemicon International, Temecula, CA) for endogenous protein control. Transfection efficiency of each plasmid was determined by qRT-PCR (see “qRT-PCR”). We used the following primer sets: 5′-ATG GAC TAC AAG GAC GAC GAT GAC-3′ and 5′-CTC CCA GCG ACC TCA TGT AA-3′ (for FLAG-PSF1); 5′-GAG ATG AAC CGA CTT GGA AAG GG-3′ and 5′-TCC TCA TCA CGC ATC TGT TC-3′ (for VSVG-PSF2); 5′-TAC GAT GTT CCA GAT TAC GCG GG-3′ and 5′-CAG GAT GTC GTC CAA AGA CA-3′ (for HA-PSF3); 5′-CTC ATC TCA GAA GAG GAT CTG GG-3′ and 5′-GTG TGG TCC ATA TAC TCT TTG-3′ (for Myc-SLD5).
Transplantation study
For the analysis of sensitivity of HSCs to 5-FU in vivo, 8-week-old mice were treated with 5-FU (see “Cell culture”), and after 1 day of 5-FU treatment, PSF1+/+ or PSF1+/− BM mononuclear cells (Ly5.2, 4 × 105) were transplanted into lethally irradiated (8.5 Gy) recipients (Ly5.1) together with untreated normal Ly5.1 BM cells (2 × 105). Four weeks after transplantation, donor contribution was determined by FACS using anti-Ly5.1 (eBioscience) and anti-Lin antibodies mixture.
Results
PSF1 is predominantly expressed in proliferating immature hematopoietic cells
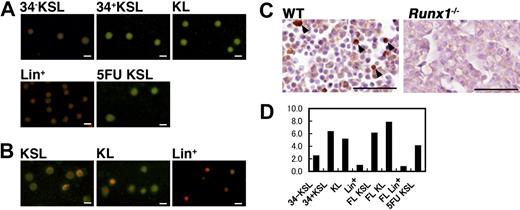
Previously, we reported that PSF1 expression was predominantly observed in immature cell populations in embryonic and adult tissues, such as blastocysts and spermatogonium in the adult.18 Moreover, PSF1 expression was observed in immature hematopoietic cells (HCs) designated as Lin− c-kit+ Sca-1+ (KSL) at the RNA expression level. To confirm whether PSF1 protein is expressed in HSCs or not, CD34− or CD34+ KSL, Lin−c-kit+Sca-1− (KL) or Lin+ cells from adult BM were sorted, and expression of PSF1 was determined in each fraction (Figure 1A). It has been reported that CD34− KSL cells in the adult mouse BM are dormant and represent HSCs with long-term marrow repopulating ability, whereas CD34+ KSL cells are progenitors with short-term reconstitution capacity.2,24 In the steady state, a high level of PSF1 expression was observed in CD34+ KSL cells and KL cells, whereas a very low level of PSF1 expression was observed in CD34− KSL cells. However, no PSF1 expression was detected in mature cells (Lin+; Figure 1A). It was reported that, after BM ablation, all HSCs express CD34 in a situation when BM is acutely reconstituted.25 Therefore, to know whether PSF1 expression in HSCs correlates with cell cycle of HSCs, CD34+ KSL cells were sorted from the BM 4 days after ablation of the BM by 5-FU. As expected, almost all CD34+ KSL cells were stained by anti-PSF1 antibody (Figure 1A).
PSF1 expression in proliferating HSC population. (A,B) Immunostaining of several BM (A) or FL-derived (B) HC population with anti-PSF1 antibody. (A) CD34−KSL (CD34−c-kit+Sca-1+Lin− cells), 34+KSL (CD34+c-kit+Sca-1+Lin− cells), KL (c-kit+Sca-1−Lin− cells), Lin+ (Lin+ cells), and 5-FU KSL (5-FU-treated mouse derived CD34+KSL cells). (B) KSL (c-kit+Sca-1+Lin− cells), KL (c-kit+Sca-1−Lin− cells), and Lin+ (Lin+ cells). Green color shows PSF1 staining. Nuclei were counterstained with PI (red). Bar represents 10 μm. (C) Sections of E12.5 FL from wild-type (WT) or Runx1−/− mice were stained with anti-PSF1 polyclonal antibody. Sections were counterstained with hematoxylin (original magnification ×400). Arrows indicate PSF1+ cells. Bars represent 50 μm. (D) PSF1 mRNA expression in various HC fractions of BM or FL cells as indicated in panel A. 5-FU KSL indicates KSL cells were sorted from BM of mice 4 days after treatment with 5-FU. The values were normalized to the amount of mRNA in Lin+ cells from BM.
PSF1 expression in proliferating HSC population. (A,B) Immunostaining of several BM (A) or FL-derived (B) HC population with anti-PSF1 antibody. (A) CD34−KSL (CD34−c-kit+Sca-1+Lin− cells), 34+KSL (CD34+c-kit+Sca-1+Lin− cells), KL (c-kit+Sca-1−Lin− cells), Lin+ (Lin+ cells), and 5-FU KSL (5-FU-treated mouse derived CD34+KSL cells). (B) KSL (c-kit+Sca-1+Lin− cells), KL (c-kit+Sca-1−Lin− cells), and Lin+ (Lin+ cells). Green color shows PSF1 staining. Nuclei were counterstained with PI (red). Bar represents 10 μm. (C) Sections of E12.5 FL from wild-type (WT) or Runx1−/− mice were stained with anti-PSF1 polyclonal antibody. Sections were counterstained with hematoxylin (original magnification ×400). Arrows indicate PSF1+ cells. Bars represent 50 μm. (D) PSF1 mRNA expression in various HC fractions of BM or FL cells as indicated in panel A. 5-FU KSL indicates KSL cells were sorted from BM of mice 4 days after treatment with 5-FU. The values were normalized to the amount of mRNA in Lin+ cells from BM.
It is known that HSCs in the FL contain cells that cycle at a higher rate than those in the adult BM and HSCs express the Mac-1 antigen.26 We sorted KSL (without Mac-1), KL, and Lin+ cells from the FL at embryonic day (E) 12.5 and determined PSF1 expression. High PSF1 expression was found in both KSL and KL cells (Figure 1B), and Lin+ cells did not express PSF1 as seen in the adult BM.
On immunohistochemistry, PSF1 expression was seen in a small population of round HCs in the FL at E12.5 (Figure 1C). However, in the adult liver, which is no longer a hematopoietic organ, PSF1 expression was not observed (data not shown). Furthermore, to test the specificity of this staining in the FL, we studied PSF1 expression in Runx1-deficient mice, which lack definitive hematopoiesis27 and could not detect PSF1-positive cells in the FL in this mutant embryo (Figure 1C). To confirm the specific expression of PSF1 in proliferative and immature HCs, we performed qRT-PCR (Figure 1D). PSF1 was highly expressed in BM-derived CD34+ KSL and Lin−Kit+, and FL-derived Lin−Kit+Sca1+ and Lin−Kit+ cells. KSL cells derived from BM at 4 day after 5-FU treatment also expressed higher amounts of PSF1 transcript than CD34− KSL cells. These results demonstrated that PSF1 is highly expressed in proliferating HSCs and progenitors.
Pool size of HSCs/HPCs is decreased in PSF1+/− old mice
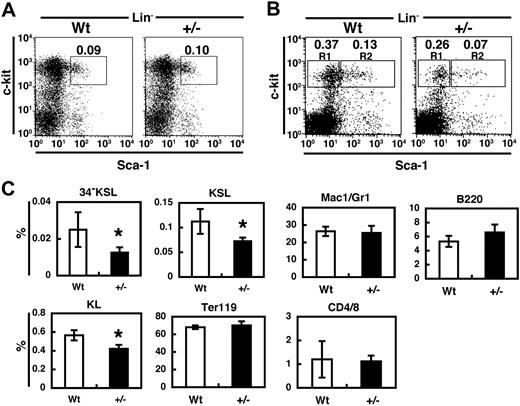
To investigate how haploinsufficiency of PSF1 affects hematopoiesis, we analyzed the BM of PSF1+/− mice.18 Although no significant differences were found in KSL cells (Figure 2A) and mature cells populations (data not shown) between wild-type and PSF1+/− at 8 weeks of age, the relative number of KSL cells was approximately 2-fold lower in one year-old mice compared with wild-type littermates (Figure 2B). In addition, the population of CD34− KSL cells (LT-HSCs), CD34+ KSL cells (KSL: ST-HSCs), and c-kit+Sca-1−Lin− (KL) were significantly decreased in PSF1+/− mice (Figure 2C). The relative cell number of each type of mature HC such as myeloid cells (Mac1/Gr-1+ cells), T cells (CD4/CD8 cells), B cells (B220+), or erythroid cells (TER119+) of the PSF1+/− BM was similar to that of the wild-type BM (Figure 2C). These data suggested that both alleles of PSF1 gene are essential for maintenance of the proper pool size of HSCs or HPCs throughout life.
Haploinsufficiency of PSF1 for hematopoiesis. (A,B) Cells of KSL populations in the BM from 8-week-old mice (A) and 1-year-old mice (B) were analyzed. Percentage of each fraction indicated by box was represented. R1 and R2 in panel B indicates fraction from c-kit+Sca-1−Lin− cells and c-kit+Sca-1+Lin− cells, respectively. (C) Quantitative evaluation in percentage of each fraction among all BM cells of 1-year-old wild-type (Wt) or PSF1+/− (+/−) mice as indicated. Mac-1/Gr-1 (myeloid), B220 (B cells), CD4/CD8 (T cells), or TER119 (erythroid). HSCs populations were studied in a CD34− KSL cell population. Populations of KSL (c-kit+Sca-1+Lin− cells) and KL (c-kit+Sca-1−Lin− cells) were also evaluated. *P < .05.
Haploinsufficiency of PSF1 for hematopoiesis. (A,B) Cells of KSL populations in the BM from 8-week-old mice (A) and 1-year-old mice (B) were analyzed. Percentage of each fraction indicated by box was represented. R1 and R2 in panel B indicates fraction from c-kit+Sca-1−Lin− cells and c-kit+Sca-1+Lin− cells, respectively. (C) Quantitative evaluation in percentage of each fraction among all BM cells of 1-year-old wild-type (Wt) or PSF1+/− (+/−) mice as indicated. Mac-1/Gr-1 (myeloid), B220 (B cells), CD4/CD8 (T cells), or TER119 (erythroid). HSCs populations were studied in a CD34− KSL cell population. Populations of KSL (c-kit+Sca-1+Lin− cells) and KL (c-kit+Sca-1−Lin− cells) were also evaluated. *P < .05.
PSF1+/− mice show hypersensitivity for fluorouracil in the BM
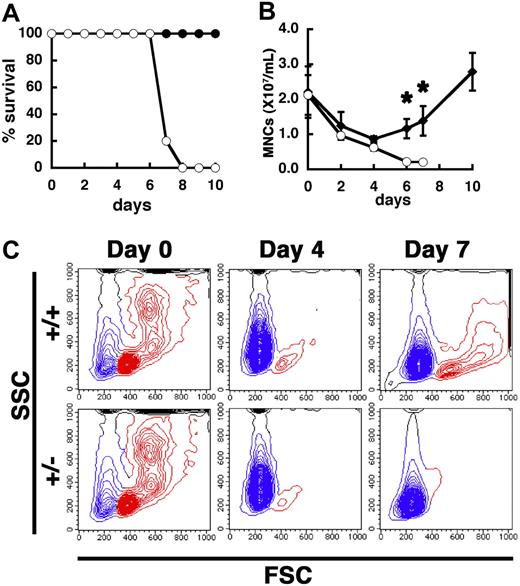
Next, we ablated the BM by 5-FU injection, which kills cycling HSCs/HPCs and forces dormant HSCs into cycle, and studied the ability of PSF1+/− mice to reconstitute BM hematopoiesis and analyzed the effect of haploinsufficiency of PSF1 on HSCs and HPCs (Figure 3A). Although the LD50 of 5-FU is 350 mg/kg in normal mice,28 the PSF1+/− mice died within 8 days after a single injection with a lower dose 5-FU (150 mg/kg), whereas wild-type mice did not die with this treatment. On 5-FU injection in PSF1+/− mice, the number of peripheral leukocytes decreased precipitously over 7 days; however, in wild-type mice, the number of peripheral leukocytes decreased over 4 days and then it began to increase by day 6 (Figure 3B). After 5-FU injection, the forward scatterdull/high population, which includes lymphocytes, granulocytes, and monocytes, were reconstituted in the BM of wild-type mice after approximately day 7; however, such reconstitution was not observed in the BM of PSF1+/− mice (Figure 3C red-colored population). We also calculated the number of peripheral red blood cells and platelets; however, no significant differences were found between PSF1+/+ and PSF1+/− mice in normal and acute phase (data not shown).
Hypersensitivity of PSF1+/− mice to 5-FU. (A) Survival curves after 5-FU injection. ○ indicate PSF1+/− 8-week-old mice (n = 10); and ●, wild-type 8-week-old mice (n = 10). (B) Number of mononuclear cells in the peripheral blood of wild-type (●) and PSF1+/− (○) mice over time after 5-FU injection. 5-FU was injected into 8-week-old mice on day 0. Means plus or minus SEM are shown (n = 10). *P < .05 vs those in PSF1+/− mice on days 2, 4, 6, 8, and 10 after BM ablation with 5-FU. (C) Kinetics of BM cells after 5-FU injection. +/+ indicates wild-type mice; and +/−, PSF1+/− mice. Results of FACS analysis are shown. SSC indicates side-scattered light; and FSC, forward-scattered light. Blue represents RBC; and red, leukocytes.
Hypersensitivity of PSF1+/− mice to 5-FU. (A) Survival curves after 5-FU injection. ○ indicate PSF1+/− 8-week-old mice (n = 10); and ●, wild-type 8-week-old mice (n = 10). (B) Number of mononuclear cells in the peripheral blood of wild-type (●) and PSF1+/− (○) mice over time after 5-FU injection. 5-FU was injected into 8-week-old mice on day 0. Means plus or minus SEM are shown (n = 10). *P < .05 vs those in PSF1+/− mice on days 2, 4, 6, 8, and 10 after BM ablation with 5-FU. (C) Kinetics of BM cells after 5-FU injection. +/+ indicates wild-type mice; and +/−, PSF1+/− mice. Results of FACS analysis are shown. SSC indicates side-scattered light; and FSC, forward-scattered light. Blue represents RBC; and red, leukocytes.
PSF1 is essential for HSC proliferation after 5-FU treatment
Before 5-FU injection, there were no obvious differences in the numbers of HSCs and HPCs in the BM between 8-week-old wild-type and PSF1+/− mice (Figures 2A, 4A). 5-FU injection induces HSCs into cycle, and it was reported that these cycling HSCs weakly express Mac-1.26,29 We therefore studied whether 5-FU injection promotes the proliferation of Mac-1lo–expressing HSCs. Before 5-FU injection, the number of HSC population designated as Mac-1− KSL was not significantly different between wild-type and PSF1+/− mice as shown in Figure 2A (see also Figure 4A,B). After 5-FU injection, the KSL cell population decreased in both wild-type and PSF1+/− mice during the first few days (Figure 4A). Four to 6 days after 5-FU injection, the population of cycling Mac-1lo–KSL cells increased dramatically in wild-type mice, whereas very few of the KSL cells except for the Mac-1lo–KSL population appeared in the PSF1+/− mice (Figure 4A). Because of this delay of recovery, the absolute number of HSCs in the BM was approximately 5.9-fold lower in PSF1+/− mice compared with that in wild-type mice at 6 days after 5-FU treatment (Figure 4B).
Both alleles of PSF1 are required for acute reconstitution after BM ablation. (A) Time course of c-kit and Mac-1 expression in Lin−Sca-1+ cells during BM reconstitution after 5-FU injection (+/+, 8-week-old wild-type mice; +/−, 8-week-old PSF1+/− mice). Results of FACS analysis are shown. Boxes indicate HSC-containing populations. Percentage of all BM cells corresponding to HSC population indicated by the box is shown in the right corner of each figure. (B) Total number of KSL cells derived from the femurs and tibias of wild-type (●) and PSF1+/− mice (○) on the indicated days before (day 0) and after 5-FU injection as described in panel A. *P < .05 versus that in PSF1+/− mice on the respective day.
Both alleles of PSF1 are required for acute reconstitution after BM ablation. (A) Time course of c-kit and Mac-1 expression in Lin−Sca-1+ cells during BM reconstitution after 5-FU injection (+/+, 8-week-old wild-type mice; +/−, 8-week-old PSF1+/− mice). Results of FACS analysis are shown. Boxes indicate HSC-containing populations. Percentage of all BM cells corresponding to HSC population indicated by the box is shown in the right corner of each figure. (B) Total number of KSL cells derived from the femurs and tibias of wild-type (●) and PSF1+/− mice (○) on the indicated days before (day 0) and after 5-FU injection as described in panel A. *P < .05 versus that in PSF1+/− mice on the respective day.
Loss of PSF1 leads to delayed HSC proliferation in the acute phase after BM ablation
Because it has been reported that the percentage of proliferating HSCs reaches a maximum on approximately day 6 in normal mice,29 we sorted the total population of HSCs (KSL–Mac-1−/lo) and HPCs (Lin−Sca-1−c-kit+Mac-1−/lo) on day 6 after 5-FU injection and analyzed the cell cycle of those HSCs and HPCs. The cells were analyzed for DNA content by PI staining (Figure 5A). The percentage of HSCs in the S/G2/M phase was approximately 50% lower in PSF1+/− mice than those in wild-type mice, whereas the percentage of HPCs in the S/G2/M phase was not significantly different between PSF1+/− and wild-type mice. These data suggest that both alleles of PSF1 are essential for acute BM reconstitution.
Loss of PSF1 leads to delay of HSC proliferation in the acute phase. (A) Percentage of KSL cells or HPCs (Lin−c-kit+Sca-1−) in the S/G2/M phase among the total number of HSCs or HPCs, respectively, on day 6 after 5-FU injection as described in Figure 4A. ■ indicates HPCs; □, KSL cells. Mean values plus or minus SEM are shown (n = 5). *P < .05. (B) CFU-c assay using KSL cells obtained from mice on day 6 after 5-FU injection. +/+ indicates wild-type mice; and +/−, PSF1+/− mice. ■ indicates CFU cluster (CLST; containing < 30 cells); □, CFU-C (CFU; containing > 30 cells). (C) Comparison between wild-type and PSF1+/− KSL cells for sensitivity to 5-FU toxicity. Sorted KSL cells from the BM of 8-week-old mice were seeded in semisolid medium with indicated concentration of 5-FU, and total CFU-C number was counted after 10 days of culturing. Results are expressed as a percentage compared with control condition (100%). (D) Rescue experiments. PSF1+/− mice were injected with 5-FU on day 0. One day after 5-FU injection (▲), Lin−CD45+c-kit+ cells (KL cells; 5 × 104/mice) that had been derived from wild-type BM were injected into PSF1+/− mice (n = 5).
Loss of PSF1 leads to delay of HSC proliferation in the acute phase. (A) Percentage of KSL cells or HPCs (Lin−c-kit+Sca-1−) in the S/G2/M phase among the total number of HSCs or HPCs, respectively, on day 6 after 5-FU injection as described in Figure 4A. ■ indicates HPCs; □, KSL cells. Mean values plus or minus SEM are shown (n = 5). *P < .05. (B) CFU-c assay using KSL cells obtained from mice on day 6 after 5-FU injection. +/+ indicates wild-type mice; and +/−, PSF1+/− mice. ■ indicates CFU cluster (CLST; containing < 30 cells); □, CFU-C (CFU; containing > 30 cells). (C) Comparison between wild-type and PSF1+/− KSL cells for sensitivity to 5-FU toxicity. Sorted KSL cells from the BM of 8-week-old mice were seeded in semisolid medium with indicated concentration of 5-FU, and total CFU-C number was counted after 10 days of culturing. Results are expressed as a percentage compared with control condition (100%). (D) Rescue experiments. PSF1+/− mice were injected with 5-FU on day 0. One day after 5-FU injection (▲), Lin−CD45+c-kit+ cells (KL cells; 5 × 104/mice) that had been derived from wild-type BM were injected into PSF1+/− mice (n = 5).
When HSCs are cultured in semisolid media, they divide and generate large colonies, including mature HCs. If PSF1 haploinsufficiency leads to cell death in HSCs, HSCs cannot form colonies in vitro. Therefore, next we investigated the in vitro colony-forming capacity of HSCs (KSL-Mac-1−/lo) that had been obtained on day 6 after 5-FU injection by cell sorting (Figure 5B). The HSCs from PSF1+/+ and PSF1+/− mice formed the same total numbers of colonies and clusters (data not shown); however, the HSCs from PSF1+/− mice formed a markedly higher number of CFU clusters (> 30 cells) compared with PSF1+/+ cells. In the case of HSCs derived from wild-type mice, approximately 89% of all colonies were large colonies; however, approximately 71% of colonies generated by HSCs from PSF1+/− mice were small colonies. To determine whether haploinsufficiency of PSF1 simply induced cell death resulting in reduced colony size in CFU-c assay, we examined the number of apoptotic cells in the colony-forming cell population by staining with anti–annexin V antibodies (Table 1). No significant differences were found in the apoptotic cells with respect to Lin−Kit+, Lin−Sca1+, and Lin− population between cells from CFU-c derived from PSF1+/+ and PSF1+/− HSCs. These data suggested that the decreased colony size in PSF1+/− CFU-c is not induced by activation of DNA damage checkpoint and cell death. These results indicated that PSF1+/− HSCs may not easily divide into daughter cells committed to a program of differentiation, but haploinsufficiency did not induce cell death. Furthermore, to test the possibility that PSF1+/− HSCs are simply more sensitive to 5-FU toxicity, KSL cells were sorted from BM of wild-type or PSF1+/− mice and seeded onto semisolid medium in the presence or absence of 5-FU. Figure 5C illustrates the effect of exposure to various concentrations of 5-FU on colony-forming activity of KSL cells. KSL cells survived and those from wild-type and PSF1+/− formed comparable number of colonies, suggesting that deletion of one PSF1 allele of HSCs does not cause hypersensitivity for 5-FU. To support this interpretation, BM of wild-type or PSF1+/− mice was collected after 1 day of 5-FU treatment and transplanted into lethally irradiated recipient mice together with untreated normal cells as competitor. After 4 weeks of transplantation, donor contribution was determined by FACS. The percentage of contributed cells (chimerism) was 56 plus or minus 12 and 24 plus or minus 14 in recipients, which were transplanted with wild-type or PSF1+/− BM cells, respectively, although the contribution of PSF1+/−-derived HSCs in recipient mice was slightly less. These data suggested that deletion of one PSF1 allele of HSCs does not cause hypersensitivity for 5-FU. Moreover, transplantation of HSCs and HPCs that had been obtained from wild-type mice completely rescued the lethality of 5-FU in PSF1+/− mice (Figure 5D). These data suggest that the defect in acute reconstitution of the BM in PSF1+/− mice is not caused by disruption of the BM microenvironment in these mice.
Percentage of apoptotic cells among CFU-c cell population
| Marker . | Genotype . | |
|---|---|---|
| PSF1+/+ . | PSF1+/− . | |
| Lin−Kit+ | 39 ± 33 | 36 ± 29 |
| Lin−Sca1+ | 3.6 ± 2.3 | 2.6 ± 1.6 |
| Lin− | 2.2 ± 1.1 | 1.8 ± 1.4 |
| Marker . | Genotype . | |
|---|---|---|
| PSF1+/+ . | PSF1+/− . | |
| Lin−Kit+ | 39 ± 33 | 36 ± 29 |
| Lin−Sca1+ | 3.6 ± 2.3 | 2.6 ± 1.6 |
| Lin− | 2.2 ± 1.1 | 1.8 ± 1.4 |
It was reported that PSF1 is essential for DNA replication in yeast.14-17 This observation raises the possibility that haploinsufficiency of PSF1 leads to abnormal DNA replication, activation of DNA damage checkpoint, S-phase arrest, and cell death in mice. To evaluate this possibility, apoptotic cells were quantified by FACS analysis after staining with annexin V in 5-FU–treated or untreated BM cells (Table 2). In PSF1+/− BM cells, apoptotic cells from Lin−, Lin−Kit+, or Lin−Kit+Sca+ cell populations were slightly increased compared with the analogous populations derived from wild-type mice; however, none of these differences was statistically significant. In addition, no obvious S-phase arrest was found in PSF1+/− HSCs (Figure 5A). We also examined the expression of ATM, ATR, XCCR1, Brca2, and p21 in both wild-type and PSF1+/− CD34-KSL cells by qRT-PCR; however, no obvious differences were found (data not shown). These data suggested that haploinsufficiency does not lead to abnormal DNA replication or increased activation of DNA damage checkpoint.
Percentage of apoptotic cells in various hematopoietic cell populations
| Marker . | Genotype . | |
|---|---|---|
| PSF1+/+ . | PSF1+/− . | |
| Normal state | ||
| Lin−Kit+Sca1+ | 5.4 ± 4 | 9.3 ± 0.4 |
| Lin−Kit+ | 4.5 ± 3 | 8.1 ± 0.2 |
| Lin− | 7.0 ± 5 | 12 ± 0.6 |
| 5-FU treated* | ||
| Lin−Kit+Sca1+ | 5.2 ± 2 | 7.4 ± 0.5 |
| Lin−Kit+ | 4.2 ± 2 | 5.1 ± 0.7 |
| Lin− | 4.7 ± 2 | 5.9 ± 1 |
| Marker . | Genotype . | |
|---|---|---|
| PSF1+/+ . | PSF1+/− . | |
| Normal state | ||
| Lin−Kit+Sca1+ | 5.4 ± 4 | 9.3 ± 0.4 |
| Lin−Kit+ | 4.5 ± 3 | 8.1 ± 0.2 |
| Lin− | 7.0 ± 5 | 12 ± 0.6 |
| 5-FU treated* | ||
| Lin−Kit+Sca1+ | 5.2 ± 2 | 7.4 ± 0.5 |
| Lin−Kit+ | 4.2 ± 2 | 5.1 ± 0.7 |
| Lin− | 4.7 ± 2 | 5.9 ± 1 |
BM cells were collected from mice 6 days after treatment with 5-FU.
Taken together, these data indicate that both alleles of PSF1 are essential for promoting HSC cycling and that this requirement is limited to HSCs.
PSF1 regulates molecular stability of other GINS components in mutual manner
To address whether the silencing of one of the GINS components, PSF1, affects the cellular stability of other GINS components, we performed ectopic expression of all GINS component with or without PSF1 (Figure 6). For the evaluation of the transfection efficiency by plasmids, the amounts of overexpressed gene transcripts were quantified by real-time PCR; no significant differences were found between GINS and G-NS condition for VSVG-PSF2, HA-PSF3, and Myc-SLD5 expression (Table 3). These data indicated that transfection efficiencies of all plasmids were almost equivalent between GINS and G-NS conditions. When all GINS components (PSF1, PSF2, PSF3, and SLD5) were cotransfected, a stable “GINS” complex was formed. However, lack of PSF1 led to destabilization of PSF2, PSF3, and SLD5 (G-NS; Figure 6A). These data suggest that lack of PSF1 results in the formation of an incomplete GINS complex, along with the destabilization of other GINS components in PSF1+/− HSCs. Finally, we concluded that PSF1 is expressed in proliferating HSCs and is essential for BM regeneration and regulation of stem cell pool size (Figure 6B).
PSF1 mutually regulates molecular stability of other GINS components. (A) Western blot analysis of GINS components ectopically expressed on NIH3T3 cells. Cells were cotransfected with VSVG-PSF2, HA-PSF3, and Myc-SLD5 in the presence (GINS) or absence (G-NS) of Flag-PSF1 or empty vector (Vector). The blots were probed with specific antibodies as indicated. GAPDH was used as a loading control. (B) Scheme of PSF1 expression in the course of HSC differentiation. The level of PSF1 expression is represented by the dark gray area. Both alleles of PSF1 are essential for populations in the light gray area.
PSF1 mutually regulates molecular stability of other GINS components. (A) Western blot analysis of GINS components ectopically expressed on NIH3T3 cells. Cells were cotransfected with VSVG-PSF2, HA-PSF3, and Myc-SLD5 in the presence (GINS) or absence (G-NS) of Flag-PSF1 or empty vector (Vector). The blots were probed with specific antibodies as indicated. GAPDH was used as a loading control. (B) Scheme of PSF1 expression in the course of HSC differentiation. The level of PSF1 expression is represented by the dark gray area. Both alleles of PSF1 are essential for populations in the light gray area.
Relative mRNA expression in transfected cells
| Average of relative expression . | Plasmid used for transfection . | |
|---|---|---|
| Flag-PSF1, VSVG-PSF2, HA-PSF3, Myc-SLD5 (GINS) . | VSVG-PSF2, HA-PSF3, Myc-SLD5 (G-NS) . | |
| VSVG-PSF2 | 1 | 1.05 |
| HA-PSF3 | 1 | 1.14 |
| Myc-SLD5 | 1 | 1.14 |
| Average of relative expression . | Plasmid used for transfection . | |
|---|---|---|
| Flag-PSF1, VSVG-PSF2, HA-PSF3, Myc-SLD5 (GINS) . | VSVG-PSF2, HA-PSF3, Myc-SLD5 (G-NS) . | |
| VSVG-PSF2 | 1 | 1.05 |
| HA-PSF3 | 1 | 1.14 |
| Myc-SLD5 | 1 | 1.14 |
Discussion
In this study, we showed that PSF1 is highly expressed in proliferating HSCs, and haploinsufficiency of PSF1 caused severe delay in induction of HSC proliferation during ablated BM reconstitution and disrupted pool size maintenance of HSCs throughout life. In addition, we showed that PSF1 regulates protein stability of other GINS components.
During embryogenesis, PSF1−/− embryos show severe growth defect in the inner cell mass, that is, the pluripotent stem cells.18 This observation raises the possibility that PSF1 could regulate the proliferation and/or pool size for other tissue stem cells. Recently, stem cells were identified in the small intestine.30 Crypt base columnar cells are stem cells and can be visualized by continuous bromodeoxyuridine incorporation study. Our preliminary experiment showed that the number of crypt base columnar cells also decreased in adult PSF1+/− mice compared with adult wild-type mice (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Further experiments may help establish the function of PSF1 in the regulation of cell proliferation and/or the pool size of various tissue stem cells.
So far, a multiplicity of molecules have been studied for their role in cell-cycle progression, including extrinsic factors, such as Notch and sonic hedgehog, Wnt3a, etc, and intrinsic factors, such as Bmi1, PTEN, p21, p18, and others.31 However, the mechanism of DNA replication in HSCs has not been elucidated. Although the essential role of PSF1 in DNA replication has been reported in yeast,8 its function in mammalian cells has not been clearly understood. We previously reported that PSF1 was essential for cell division of totipotential embryonic stem cells by gene-targeting studies and showed that PSF1 was highly expressed in adulthood in BM, testis, and ovary, where cell division of stem cells is actively induced in the adult. Here we reported that PSF1 is essential for acute proliferation of HSCs. Taken together, it is clear that PSF1 plays important roles in cell proliferation of the stem cell system. Moreover, we and other groups isolated mammalian PSF2, PSF3, and SLD5, which together make up the GINS complex, and the roles of these GINS component have been reported in cell division.7,8 In this report, we found that PSF1 expression was weak in slow cycling CD34− LT-HSCs and high in cycling CD34+ ST-HSC. Therefore, the GINS complex is likely to closely associate with cell cycle of HSCs. At present, molecules affecting PSF1 expression in dormant HSCs have not been isolated; however, proliferating HSCs after BM ablation by 5-FU almost exclusively express PSF1 at high levels. This suggested that PSF1 expression is inductively, but not intrinsically, regulated in HSCs affected by exogenous molecules produced from cells responding to BM suppression. At present, although it is not clear whether PSF1 plays a role in DNA replication of HSCs, isolation of molecules affecting PSF1 expression in HSCs may contribute to the understanding of process of self-renewal in HSCs.
It was reported that “GINS” replication complex, which is composed of PSF1, PSF2, PSF3, and SLD5, interacts with CDC45 and MCM complex and is involved in the initiation of DNA replication in lower eukaryote.14-17 To determine whether haploinsufficiency of PSF1 impairs DNA replication at stem cell level resulting in reduced pool size of HSCs, we examined the expression levels of DNA-damage checkpoint genes, such as ATM, ATR, XCCR1, BRCA2, and p21 in HSCs. No significant differences were found between CD34− KSL cells derived from young and old PSF1+/+ and PSF1+/− BM (data not shown). These data suggested that the decreased pool size of HSC population in PSF1+/− BM is not induced by activation of DNA damage checkpoint.
In this study, haploinsufficiency of PSF1 severely suppressed BM reconstitution by delaying the proliferation of the HSC population. Based on our result, there are 2 possibilities to explain this suppression not only by gene-dose effect, but also other processes. As one possibility, PSF1 may bring about the molecular stability of DNA replication proteins. Overexpression studies suggested that PSF1 regulates stable expression of other GINS components (Figure 6A). Thus, it is probable that lower expression of PSF1 in HSCs of PSF1+/− mice may lead to down-regulation of SLD5, PSF2, and PSF3 in HSCs. Therefore, incompletely formed GINS complex may have a dominant negative effect and/or induce instability of other DNA replication complexes, such as CDC45 or MCMs. Another possibility is that PSF1 may induce HSC specific gene expression for effective engraftment capacity. It was reported that HSCs shift gene expression and engraftment phenotype with cell cycle transit.32 Compared with HSCs from wild-type mice, HSCs obtained from 5-FU-injected PSF1+/− mice expressed a lower level of Mac-1, which appeared to be expressed in cycling stem cells and to be involved in cell adhesion (Figure 5A).26 Thus, haploinsufficiency of PSF1 may affect HSC properties. As it is thought that DNA replication of important genes for cell function occurs in the early period of the S phase, it is possible that PSF1 regulates the expression of several genes involved in the formation of the BM stem cell niche through DNA replication specifically in HSCs. In addition, loss of PSF1 causes abnormality of chromatin segregation in mice and nematodes (M.U., N.T., unpublished data, May 1, 2007). It has been also reported that PSF2 depletion inhibits the transition of metaphase to anaphase through the suppression of the attachment of tubulin to the kinetochore.33 These data suggest that the GINS complex might have roles in other biologic processes.
It is also known that, after chemotherapy with anticancer drug, some patients have prolonged BM suppression for unknown reasons.34 Moreover, BM dysfunction resulting in pancytopenia is observed with aging in elderly people for unknown reasons. Haploinsufficiency of PSF1 in mice induced delay of BM recovery after 5-FU treatment and attenuated the number of HSCs with aging. Therefore, attenuation of PSF1 expression in HSCs may cause prolonged BM suppression after chemotherapy and pancytopenia with aging. So far, the association of stem cell division with DNA replication proteins in hematopoietic disorders has not been reported. It is intriguing to analyze the relationship of hematopoietic diseases and DNA replication protein, such as PSF1. Based on our analysis, identification of PSF1-dependent genes probably sheds light on the mechanism of DNA replication in HSCs and ontogeny of hematopoietic disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr T. Watanabe (Tohoku University, Japan) for providing us the AML-1/Runx1 mutant mice, and Ms Y. Shimizu, Ms K. Ishida, Ms M. Sato, Mrs Y. Nakano, Mrs K. Fukuhara, and Mrs N. Fujimoto for technical assistance.
This work was supported in part by the Japanese Ministry of Education, Culture, Sports, Science and Technology and the Japan Society for Promotion of Science.
Authorship
Contribution: M.U. and N.T. designed the research, analyzed data, and wrote the paper; K.S. and M.A. helped generate PSF1 mutant mice; and M.I. helped generate anti-PSF1 antibody.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nobuyuki Takakura, Department of Signal Transduction, Research Institute for Microbial Diseases, Osaka University, 3-1 Yamada-oka, Suita, Osaka 565-0871, Japan; e-mail: ntakaku@biken.osaka-u.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal