Abstract
Immunization with recombinant lentivector elicits higher frequencies of tumor antigen-specific memory CD8+ T cells than peptide-based vaccines. This finding correlates with our observation that, upon recombinant lentivector immunization, a higher fraction of antigen-specific effector CD8+ T cells does not down-regulate the expression of the survival/memory marker interleukin-7 receptor α chain (IL-7Rα). Here we show that, surprisingly, higher expression of IL-7Rα on recombinant lentivector-induced effector CD8+ T cells does not result in the up-regulation of survival molecules, such as Bcl-2. We thus hypothesized that physiologic levels of IL-7 might be limiting in vivo for delivering survival signals to the expanding population of effector cells. To test this hypothesis, we administered recombinant IL-7 during the effector phase of the response. We observed an up-regulation of Bcl-2 and a strong expansion of antigen-specific effector CD8+ T cells, and of naive CD8+ T cells. Strikingly, IL-7 treatment elicited also a significant increase in the number of antigen-specific memory CD8+ T cells in recombinant lentivector-immunized mice, but not in peptide-immunized mice. Altogether, these data show that IL-7 adjuvant treatment can enhance long-term antigen-specific CD8+ T-cell responses. However, its efficacy depends on the expression of IL-7Rα at the surface of effector CD8+ T cells.
Introduction
Upon antigen encounter, CD8+ T lymphocytes undergo clonal expansion and differentiate into effector cells endowed with lytic capacities. After the expansion phase, when the antigen is cleared, a contraction phase follows. During this phase, most effector cells die, and only few cells are selected to become long-lived memory T cells that can provide protection against subsequent antigen challenges. T cell–based vaccines against tumor antigens aim at improving the development of a strong and protective memory T-cell population. Therefore, it is crucial to understand the parameters influencing the generation of such a memory T-cell population. The magnitude and quality of a memory CD8+ T-cell response are influenced by several factors. These include the strength and duration of T-cell receptor (TCR) signaling during the priming phase,1,2 the costimulatory signals and the cytokine milieu provided by accessory cells,3-5 and the amplitude of the effector T-cell pool from which memory cells are selected.6,7 Recently, it has been proposed that high levels of expression of the interleukin-7 receptor α chain (IL-7Rα or CD127) on a small subset of effector CD8+ T cells confer them survival advantages through the up-regulation of Bcl-2 family members and can therefore be considered as a marker identifying the early precursors of long-lived memory T cells.8,9
IL-7Rα, expressed at high levels in naive T cells, is usually down-regulated in the population of antigen-activated cells and reexpressed on memory T cells. IL-7/IL-7R–mediated signaling pathways play a crucial role in regulating peripheral T-cell proliferation and survival.10 IL-7 represents the main homeostatic cytokine for the maintenance of the naive T-cell pool, and, to certain extent, for the survival of memory T cells.10-12 IL-7 promotes T-cell survival by directly counteracting the signaling pathways promoting apoptosis.13,14 Despite all the recent work on the importance played by IL-7 in the regulation of T-cell immunity, few reports have evaluated its efficacy as an adjuvant treatment in vaccination settings.15-18
We have previously shown that CD8+ T-cell responses elicited by immunizing human leukocyte antigen (HLA)–A2 transgenic mice with third-generation recombinant lentiviral vectors encoding the melanoma-associated antigen Melan-A26-35 (recombinant lentivector/Melan-A26-35) were longer lasting than those induced by peptide-based vaccines.19,20 In contrast to peptide immunizations, high frequencies of Melan-A–specific effector CD8+ T cells elicited by recombinant lentivector/Melan-A26-35 did not down-regulate IL-7Rα expression at the peak of the primary response. This suggested a role for the receptor in counteracting programmed cell death, thus eliciting higher numbers of memory CD8+ T cells. Surprisingly, we find here that cell-surface expression of IL-7Rα during the effector phase of the response does not result in the up-regulation of the survival molecule Bcl-2. In contrast, Bcl-2 is up-regulated among IL-7Rαhi antigen-specific CD8+ T cells present at the effector-to-memory transition and during the memory phase. These results suggest that physiologic levels of IL-7 might be limiting for delivering optimal survival signals to IL-7Rαhi effector CD8+ T cells during the expansion phase of the response. We have thus provided IL-7 in vivo as a vaccine adjuvant during the effector phase of the response. IL-7 treatment increases proliferation of both naive and Melan-A–specific effector CD8+ T cells in recombinant lentivector and peptide immunized mice, and induces the up-regulation of Bcl-2. Importantly, IL-7 treatment significantly increases the number of circulating Melan-A–specific memory CD8+ T cells and enhances their functionality only in mice vaccinated with recombinant lentivectors, but not with peptides.
Altogether, these results demonstrate that IL-7 adjuvant treatment can increase the pool of effector CD8+ T cells independently on the levels of IL-7Rα expression in those cells. However, the benefit of IL-7 treatment on the enhancement of the memory CD8+ T-cell pool requires high IL-7Rα expression on effector CD8+ T cells.
Methods
Mice
Immunizations were done using HLA-A*0201/H-2Kb transgenic mice.21 Animal experiments were carried out in pathogen-free facilities and in compliance with the rules of the local veterinary office. All animal experiments were approved by the ethics committee at Ludwig Institute for Cancer Research at Lausanne (Lausanne, Switzerland).
Construction of recombinant lentivector
All Melan-A sequences contained the Ala to Leu substitution at position 27 to increase the affinity of the peptide to HLA-A2. The Melan-A26-35 (ELAGIGILTV) sequence was expressed from a ubiquitin/protein/reference (UPR)–based construct as a linear fusion with enhanced green fluorescent protein–ubiquitin as previously described.20,22 The production of recombinant lentiviruses has been described previously.19,23,24
Vaccination
Peptide vaccination was carried out by subcutaneous injections at the base of the tail of 50 μg peptide admixed with 50 μg CpG oligodeoxynucleotide (TCCATGACGTTCCTGACGTT) optimized for mouse vaccination (oligodeoxynucleotide 1826, Coley Pharmaceutical Group), emulsified in 50 μL incomplete Freund adjuvant (IFA; Sigma-Aldrich, St Louis, MO). Recombinant lentivectors were administered by subcutaneous injections of 4 × 106 expression-forming units at the base of the tail. Mice were rechallenged with 50 μg peptide admixed with 50 μg CpG and 50 μL IFA.
In vivo IL-7 treatment
Recombinant lentivector- or peptide-immunized mice received daily intraperitoneal injections of 5 μg human recombinant IL-7 (rIL-7; Cytheris, Issy-les-Moulineaux, France) during the priming phase, as indicated. The absolute number of CD8+ T cells in peripheral blood mononuclear cells (PBMCs) was calculated by normalizing the number of cells with a fix number of bacteria counting beads (Invitrogen, Carlsbad, CA) added to each sample before the fluorescence-activated cell sorter (FACS) analysis.
BrdU administration
Mice received one intraperitoneal injection of 180 μg 5′-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich), and thereafter BrdU was given in the drinking water at a concentration of 800 μg/mL supplemented with 5% glucose. Mice immunized with recombinant lentivector and peptides received BrdU from day 10 to day 23 and from day 5 to day 13, respectively. BrdU incorporation into newly synthesized DNA of PBMCs was monitored in conjunction with cell-surface markers by flow cytometry (“Flow cytometry”).
Flow cytometry
PBMCs obtained by tail bleedings were incubated with phycoerythrin (PE)–conjugated Melan-A26-35/A2Kb tetramers (prepared in our laboratory25 ) for 45 minutes at 20°C, washed, and incubated with PE–Cy-7–conjugated anti–mouse CD8α antibody (clone 53-6.7; eBioscience, San Diego, CA), allophycocyanin (APC)–conjugated anti-CD127 antibody (clone A7R34; eBioscience), and fluorescein isothiocyanate (FITC)–conjugated anti-CD62L antibody (clone Mel-14; prepared in our laboratory), for 20 minutes at 4°C. The detection limit for staining of CD8+ T cells with tetramers was 0.2%, as assessed by analysis of CD8+ T cells from naive mice. Erythrocytes were lysed using the FACS Lysing solution (BD Biosciences, San Jose, CA).
For the analysis of intracellular Bcl-2 expression, cells were surface stained with PE–Cy-7–conjugated anti–mouse CD8α antibody (clone 53-6.7; eBioscience), fixed 20 minutes at 4°C in Cytofix-Cytoperm buffer (BD Biosciences), washed twice with Permeabilization-Wash buffer (BD Biosciences), and incubated overnight with FITC-conjugated anti–mouse Bcl-2 antibody (clone 3F11; BD Biosciences) or with FITC-conjugated isotype control (clone A19-3; BD Biosciences).
For the analysis of BrdU incorporation, PBMCs were stained with cell- surface antibodies, before following the guideline of the APC BrdU flow kit (BD Biosciences, clone 3D4).
For the analysis of interferon-γ (IFN-γ) production, mice immunized with recombinant lentivector/Melan-A26-35 were bled 45 days after immunization. PBMCs were incubated at 37°C in the presence of 5 μM Melan-A26-35 peptide and GolgiPlug (BD Biosciences); they were surface stained with peridinin chlorophyll protein (PerCP)–conjugated anti–mouse CD8α antibody (clone 53-6.7; BD Biosciences), fixed, and then stained with FITC-conjugated anti–mouse IFN-γ antibody (BD Biosciences).
Cells were analyzed using a FACSCalibur or a FACSCanto (BD Biosciences), equipped with CellQuest or DiVa software (BD Biosciences), respectively.
Statistical analyses
Significance was assessed using the unpaired Student t test. P < .05 was considered significant.
Results
Immunizations with recombinant lentivectors induce long-lived tumor antigen-specific CD8+ T-cell responses
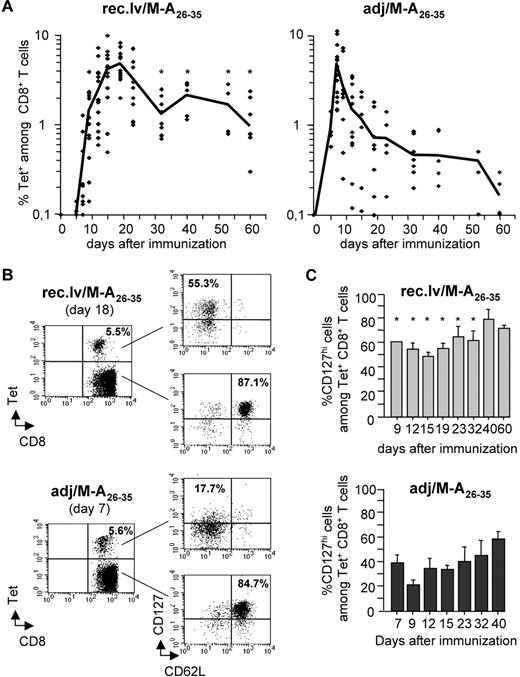
We monitored directly ex vivo the kinetics of antigen-specific CD8+ T-cell responses elicited by immunizing HLA-A2 transgenic mice (A2/Kb) with recombinant lentivectors encoding the HLA-A2–restricted Melan-A26-35 peptide (recombinant lentivector/Melan-A26-35) or with Melan-A26-35 peptides in adjuvants (adjuvant/Melan-A26-35; Figure 1A). The magnitude of the antigen-specific CD8+ T-cell response was assessed by ex vivo staining of PBMCs with Melan-A26-35/A2Kb tetramers (Tets). The CD8+ T-cell response induced by recombinant lentivector/Melan-A26-35 became detectable from day 10, peaked at day 18, and then slowly contracted. After the contraction phase, a significant proportion of Tet+CD8+ T cells developed into a long-lived memory population that remained detectable for several months. In contrast, peptide-induced CD8+ T-cell responses peaked at day 8 and then sharply contracted, yielding significantly lower frequencies of long-term memory T cells. Importantly, although all recombinant lentivector-immunized mice developed a measurable Tet+ memory CD8+ T-cell population, only 50% of peptide-immunized mice did so.
Immunizations with recombinant lentivectors elicit longer-lived Melan-A–specific CD8+ T-cell responses than peptide immunizations. (A) A2/Kb mice were immunized either with recombinant lentivectors encoding Melan-A26-35 (A27L) (rec.lv/M-A26-35), left panel, or with peptides Melan-A26-35 (A27L) in adjuvant (adj/M-A26-35), right panel. At the indicated time points after immunization, the frequency of Melan-A–specific CD8+ T cells (Tet+) was measured ex vivo by staining peripheral blood cells with Melan-A26-35/A2Kb tetramers. Each symbol represents an individual mouse. Note that, in the case of adjuvant/Melan-A26-35 immunizations, 2 mice at day 54 and 3 mice at day 60 showed a frequency of Tet+CD8+ T cells below the detection limit. Graphs represent the pool of 5 independent experiments. The black line connects mean values obtained at the indicated time points. *P < .05. (B) The expression of CD62L and CD127 (IL-7Rα) on Tet− and Tet+CD8+ T cells at the peak of the response (day 18 and day 7 for recombinant lentivector/Melan-A26-35 and for adjuvant/Melan-A26-35 immunizations, respectively) is shown for one representative experiment. (C) Bar graphs represent the percentage of CD127hi cells among Tet+CD8+ T cells at the indicated time points after immunization with recombinant lentivector/Melan-A26-35 (top panel) and with adjuvant/Melan-A26-35 (bottom panel). Error bars represent SD (n = 5). *P < .05.
Immunizations with recombinant lentivectors elicit longer-lived Melan-A–specific CD8+ T-cell responses than peptide immunizations. (A) A2/Kb mice were immunized either with recombinant lentivectors encoding Melan-A26-35 (A27L) (rec.lv/M-A26-35), left panel, or with peptides Melan-A26-35 (A27L) in adjuvant (adj/M-A26-35), right panel. At the indicated time points after immunization, the frequency of Melan-A–specific CD8+ T cells (Tet+) was measured ex vivo by staining peripheral blood cells with Melan-A26-35/A2Kb tetramers. Each symbol represents an individual mouse. Note that, in the case of adjuvant/Melan-A26-35 immunizations, 2 mice at day 54 and 3 mice at day 60 showed a frequency of Tet+CD8+ T cells below the detection limit. Graphs represent the pool of 5 independent experiments. The black line connects mean values obtained at the indicated time points. *P < .05. (B) The expression of CD62L and CD127 (IL-7Rα) on Tet− and Tet+CD8+ T cells at the peak of the response (day 18 and day 7 for recombinant lentivector/Melan-A26-35 and for adjuvant/Melan-A26-35 immunizations, respectively) is shown for one representative experiment. (C) Bar graphs represent the percentage of CD127hi cells among Tet+CD8+ T cells at the indicated time points after immunization with recombinant lentivector/Melan-A26-35 (top panel) and with adjuvant/Melan-A26-35 (bottom panel). Error bars represent SD (n = 5). *P < .05.
Antigen-specific CD8+ T cells elicited by recombinant lentivector immunizations express high levels of IL-7Rα
It has been proposed that the duration and the strength of T-cell priming on antigen encounter impact on the development of long-lived memory T cells.1 We therefore tested whether immunizations with recombinant lentivector/Melan-A26-35 and adjuvant/Melan-A26-35 differentially primed Tet+CD8+ T cells. We analyzed the expression of cellular markers defining the activation status of CD8+ T cells. The extent to which CD62L was down-regulated and CD44 up-regulated on Tet+CD8+ T cells did not differ between the 2 immunization modalities (data not shown). In addition, the degree of CD5 down-regulation, proposed to be correlated to the strength of TCR signal,26 was similar in Tet+CD8+ T cells from recombinant lentivector- and peptide-immunized mice (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Those results suggest that T-cell activation might not be different after recombinant lentivector and peptide immunization. We had previously observed that, after recombinant lentivector immunizations, a large fraction of antigen-specific CD8+ T cells expressed high levels of IL-7Rα (CD127) at the peak of the response.20 Here, we analyzed the expression of this receptor on Tet+CD8+ T cells during the course of the immune response after recombinant lentivector/Melan-A26-35 and adjuvant/Melan-A26-35 immunizations (Figure 1B,C). At every time point analyzed, a larger proportion of Tet+CD8+ T cells expressed high levels of IL-7Rα after recombinant lentivector/Melan-A26-35, than after adjuvant/Melan-A26-35 immunizations. At the peak of the response, more than 50% of Tet+CD8+ T cells induced by recombinant lentivector/Melan-A26-35 expressed IL-7Rα, whereas only 18% of peptide-induced CD8+ T cells did so, indicating that more than 80% of those cells had down-regulated its expression (Figure 1B,C). At later stages of the response, the proportion of IL-7Rαhi cells among Tet+CD8+ T cells increased in both immunization settings but remained significantly higher after recombinant lentivector/Melan-A26-35 than after adjuvant/Melan-A26-35 immunizations (Figure 1C). The majority (85%-90%) of naive Tet−CD8+ T cells expressed high levels of IL-7Rα at every time point analyzed (data not shown).
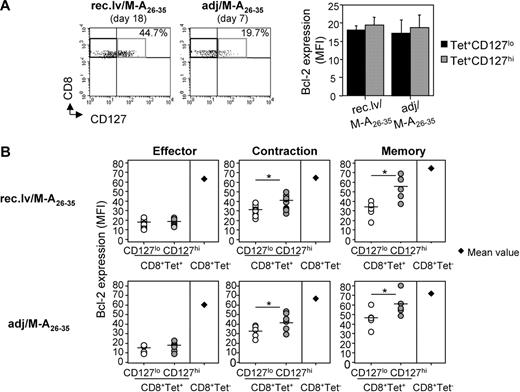
High cell-surface expression of IL-7Rα does not induce the up-regulation of Bcl-2 on Tet+ effector CD8+ T cells
It has been proposed that antigen-specific effector CD8+ T cells expressing high levels of IL-7Rα are selected to survive the contraction phase and become long-lived memory T cells, thus defining IL-7Rα as an early memory marker.8 Indeed, IL-7/IL-7R interaction has been shown to mediate the up-regulation of prosurvival molecules counteracting the programmed cell death that occurs during the contraction phase of the immune response. Therefore, we reasoned that the higher proportion of IL-7Rαhi effector CD8+ T cells induced by recombinant lentivector/Melan-A26-35 could account for higher frequencies of memory CD8+ T cells. Bcl-2 is the most important antiapoptotic molecule for T lymphocytes and can be induced on IL-7R engagement.10,13,27 We thus analyzed ex vivo the levels of Bcl-2 expression in IL-7Rαhi and IL-7Rαlo Tet+CD8+ T cells after recombinant lentivector/Melan-A26-35 and adjuvant/Melan-A26-35 immunizations (Figure 2). At the peak of the response, IL-7Rαhi Tet+CD8+ T cells did not express significantly higher levels of Bcl-2 than IL-7Rαlo cells (Figure 2A). Moreover, Bcl-2 expression in Tet+CD8+ T cells (both IL-7Rαhi and IL-7Rαlo) at the effector phase was lower than in naive Tet−CD8+ T cells (average mean fluorescence intensity [MFI], 20 vs 70, respectively; Figure 2B). In contrast to the effector phase, during the contraction and memory phases of the response, a higher expression of IL-7Rα on Tet+CD8+ T cells did correlate with higher Bcl-2 expression (Figure 2B). Concomitantly, the average MFI of Bcl-2 increased both in IL-7Rαhi and, to a lower extent, in IL-7Rαlo Tet+CD8+ T cells, reaching the levels of naive CD8+ T cells at the memory phase.
High expression of IL-7Rα does not induce up-regulation of Bcl-2 on Melan-A–specific CD8+ T cells at the effector phase, but it does it at the contraction and memory phases. (A) Melan-A–specific effector CD8+ T cells expressing low (■) and high ( ) levels of CD127 were analyzed for intracellular Bcl-2 expression upon recombinant lentivector and peptide immunizations. Error bars represent SD (n = 5 or 6). (B) Kinetics of Bcl-2 expression ex vivo in CD127lo and CD127hi Tet+CD8+ T cells, and in Tet−CD8+ T cells upon recombinant lentivector (top panels) and peptide (bottom panels) immunizations. The indicated effector, contraction, and memory phases are shown, respectively, at days 18, 35, and 60 after immunization with recombinant lentivectors, and at days 7, 19, and 40 after immunization with peptides. Round symbols represent individual mice; horizontal bars represent mean values. For Tet−CD8+ T cells, the mean value of 5 mice for each time point is shown. *P < .05.
) levels of CD127 were analyzed for intracellular Bcl-2 expression upon recombinant lentivector and peptide immunizations. Error bars represent SD (n = 5 or 6). (B) Kinetics of Bcl-2 expression ex vivo in CD127lo and CD127hi Tet+CD8+ T cells, and in Tet−CD8+ T cells upon recombinant lentivector (top panels) and peptide (bottom panels) immunizations. The indicated effector, contraction, and memory phases are shown, respectively, at days 18, 35, and 60 after immunization with recombinant lentivectors, and at days 7, 19, and 40 after immunization with peptides. Round symbols represent individual mice; horizontal bars represent mean values. For Tet−CD8+ T cells, the mean value of 5 mice for each time point is shown. *P < .05.
High expression of IL-7Rα does not induce up-regulation of Bcl-2 on Melan-A–specific CD8+ T cells at the effector phase, but it does it at the contraction and memory phases. (A) Melan-A–specific effector CD8+ T cells expressing low (■) and high ( ) levels of CD127 were analyzed for intracellular Bcl-2 expression upon recombinant lentivector and peptide immunizations. Error bars represent SD (n = 5 or 6). (B) Kinetics of Bcl-2 expression ex vivo in CD127lo and CD127hi Tet+CD8+ T cells, and in Tet−CD8+ T cells upon recombinant lentivector (top panels) and peptide (bottom panels) immunizations. The indicated effector, contraction, and memory phases are shown, respectively, at days 18, 35, and 60 after immunization with recombinant lentivectors, and at days 7, 19, and 40 after immunization with peptides. Round symbols represent individual mice; horizontal bars represent mean values. For Tet−CD8+ T cells, the mean value of 5 mice for each time point is shown. *P < .05.
) levels of CD127 were analyzed for intracellular Bcl-2 expression upon recombinant lentivector and peptide immunizations. Error bars represent SD (n = 5 or 6). (B) Kinetics of Bcl-2 expression ex vivo in CD127lo and CD127hi Tet+CD8+ T cells, and in Tet−CD8+ T cells upon recombinant lentivector (top panels) and peptide (bottom panels) immunizations. The indicated effector, contraction, and memory phases are shown, respectively, at days 18, 35, and 60 after immunization with recombinant lentivectors, and at days 7, 19, and 40 after immunization with peptides. Round symbols represent individual mice; horizontal bars represent mean values. For Tet−CD8+ T cells, the mean value of 5 mice for each time point is shown. *P < .05.
From these results, we concluded that cell-surface expression of IL-7Rα was not inducing preferential survival signals in Tet+CD8+ T cells during the priming and effector phases of the immune response, but it did so during the contraction and memory phases.
IL-7 adjuvant treatment induces Bcl-2 up-regulation in Tet+CD8+ T cells in vivo
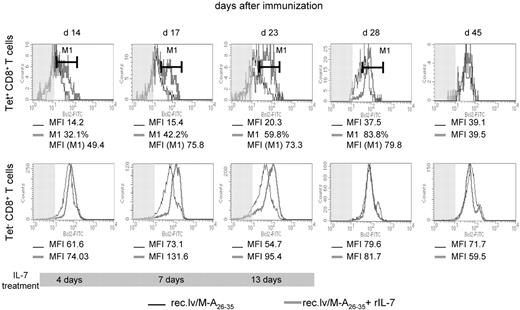
The lack of Bcl-2 up-regulation in IL-7Rαhi Tet+ effector CD8+ T cells in vivo could be the result of limiting amounts of physiologic IL-7 available for the expanding population of antigen-specific cells. We thought to improve the survival of those cells by providing exogenous IL-7 as an adjuvant treatment. We thus injected rIL-7 (5 μg/day) during the expansion phase of the recombinant lentivector-induced CD8+ T-cell response (days 10-23). As readout of the efficacy of IL-7 treatment, Bcl-2 expression was measured ex vivo, at different time points, among circulating Tet+ and Tet−CD8+ T cells (Figure 3). Four days of IL-7 therapy (14 days after immunization) resulted in the up-regulation of Bcl-2 in 32% of Tet+CD8+ T cells. This fraction increased to 42% after 7 days of IL-7, and after 13 days of treatment approximately 60% of the Tet+CD8+ T-cell population had up-regulated Bcl-2 (Figure 3 top panels). Administration of IL-7 also induced the up-regulation of Bcl-2 expression in naive CD8+ T cells (Figure 3 bottom panels). Here, the totality of the cells showed a progressive up-regulation of Bcl-2 in response to IL-7 starting from day 4 until the end of the treatment. Once IL-7 treatment had been interrupted, Bcl-2 expression in both Tet+ and Tet−CD8+ T cells from IL-7–treated mice had returned to the levels of their untreated counterparts.
IL-7 adjuvant treatment elicits up-regulation of Bcl-2 in Tet+ and Tet−CD8+ T cells. Histogram plots show ex vivo expression of Bcl-2 in Tet+ (top panels) and Tet− (bottom panels) CD8+ T cells upon recombinant lentivector/Melan-A26-35 immunizations without (black line) or with rIL-7 treatment (gray line; days 10-23), at the indicated time point after immunization. The shaded area represents isotype control staining. The percentage of CD8+ T cells (M1) that up-regulated Bcl-2 expression upon rIL-7 treatment, and Bcl-2 expression levels (MFI) in those cells, as well as in cells from untreated mice, are indicated for every time point. The gray bar at the bottom of the plots represents the duration of IL-7 treatment.
IL-7 adjuvant treatment elicits up-regulation of Bcl-2 in Tet+ and Tet−CD8+ T cells. Histogram plots show ex vivo expression of Bcl-2 in Tet+ (top panels) and Tet− (bottom panels) CD8+ T cells upon recombinant lentivector/Melan-A26-35 immunizations without (black line) or with rIL-7 treatment (gray line; days 10-23), at the indicated time point after immunization. The shaded area represents isotype control staining. The percentage of CD8+ T cells (M1) that up-regulated Bcl-2 expression upon rIL-7 treatment, and Bcl-2 expression levels (MFI) in those cells, as well as in cells from untreated mice, are indicated for every time point. The gray bar at the bottom of the plots represents the duration of IL-7 treatment.
These data demonstrated that IL-7/IL-7R signaling was functional in vivo in antigen-specific effector CD8+ T cells. Moreover, they supported the hypothesis that, at the peak of the response, Bcl-2 expression was not up-regulated in effector cells expressing high levels of IL-7Rα because of the limiting amount of physiologic IL-7 in vivo.
IL-7 adjuvant treatment enhances antigen-specific effector and memory CD8+ T-cell responses after recombinant lentivector/Melan-A26-35 immunization
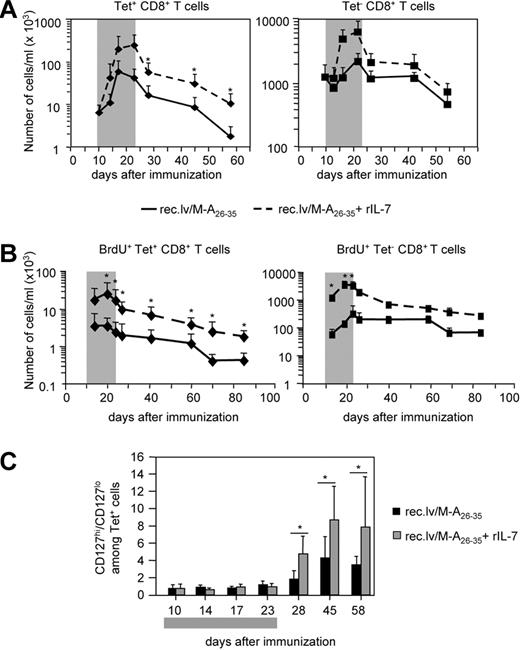
We next analyzed the effect of in vivo IL-7 administration on the expansion and contraction of antigen-specific CD8+ T cells after recombinant lentivector/Melan-A26-35 immunization (Figure 4). During IL-7 treatment, we observed a strong increase in the number of circulating Tet+ and Tet−CD8+ T cells (Figure 4A left and right panels, respectively). One week after the cessation of IL-7 treatment, Tet−CD8+ T cells had returned to baseline levels. In sharp contrast, the population of Tet+CD8+ T cells, although contracting, remained, for at least 2 months, 5 to 10 times more abundant than the CD8+ T-cell population from immunized mice that had not received IL-7. This difference was maintained also at later time points (day 85; Figure S2). IL-7R–mediated signals, besides regulating T-cell survival, also promote T-cell proliferation.10 Therefore, we decided to analyze whether IL-7 treatment raised the number of Melan-A–specific effector CD8+ T cells by increasing their proliferation. Thus, we performed BrdU incorporation experiments (Figure 4B). A2/Kb mice were immunized with recombinant lentivector/Melan-A26-35 and either left untreated or treated with rIL-7 (5 μg/day) during the effector phase of the response (days 10-23). Concomitantly with IL-7 treatment, they received BrdU in the drinking water. At different time points after immunization, we measured the number of naive and antigen-specific CD8+ T cells incorporating BrdU during the effector phase. The results clearly showed that IL-7 adjuvant treatment significantly increased proliferation of Melan-A–specific CD8+ T cells (as well as of naive CD8+ T cells) as long as the treatment was provided (Figure 4B). Once BrdU was removed from the drinking water, we were able to follow the turnover of BrdU+ antigen-specific CD8+ T cells. The number of those cells decreased at the same rate in untreated and in IL-7–treated mice (Figure 4B). In a separate experiment, we have measured proliferation of antigen-specific cells during the contraction phase of the response, and we have observed very little proliferation in both untreated and IL-7–treated mice (data not shown). Thus, the progressive loss of BrdU+ antigen-specific CD8+ T cells was mainly the result of cell death. Altogether, these results indicated that Melan-A–specific CD8+ T cells contracted at a similar rate in mice that have received or not IL-7 treatment during the effector phase of the response.
IL-7 adjuvant treatment increases Melan-A–specific effector and memory CD8+ T-cell responses on recombinant lentivector immunizations. (A) Mice immunized with recombinant lentivector/Melan-A26-35 were either treated with rIL-7 ( ) or left untreated (−) during the effector phase of the immune response (days 10-23). The number of Tet+CD8+ T cells (left panel) and the number of Tet−CD8+ T cells (right panel) per milliliter of blood are shown at different time points after immunization. Error bars represent SD (n = 12). (B) In a separate experiment, mice were treated as in panel A. All mice received BrdU in the drinking water (days 10-23). Graphs represent the kinetics of BrdU incorporation in Tet+ (left panel) and in Tet− (right panel) CD8+ T cells. The number of BrdU+ cells per milliliter of blood at the indicated time points is shown. Error bars represent SD (n = 7). (C) Ratio between CD127hi and CD127lo Tet+CD8+ T cells in untreated (■) and IL-7–treated (
) or left untreated (−) during the effector phase of the immune response (days 10-23). The number of Tet+CD8+ T cells (left panel) and the number of Tet−CD8+ T cells (right panel) per milliliter of blood are shown at different time points after immunization. Error bars represent SD (n = 12). (B) In a separate experiment, mice were treated as in panel A. All mice received BrdU in the drinking water (days 10-23). Graphs represent the kinetics of BrdU incorporation in Tet+ (left panel) and in Tet− (right panel) CD8+ T cells. The number of BrdU+ cells per milliliter of blood at the indicated time points is shown. Error bars represent SD (n = 7). (C) Ratio between CD127hi and CD127lo Tet+CD8+ T cells in untreated (■) and IL-7–treated ( ) mice. Error bars represent SD (n = 12). The gray boxes in panels A and C represent the IL-7 treatment period; and in panel B, the IL-7 and BrdU treatment period. *P < .05.
) mice. Error bars represent SD (n = 12). The gray boxes in panels A and C represent the IL-7 treatment period; and in panel B, the IL-7 and BrdU treatment period. *P < .05.
IL-7 adjuvant treatment increases Melan-A–specific effector and memory CD8+ T-cell responses on recombinant lentivector immunizations. (A) Mice immunized with recombinant lentivector/Melan-A26-35 were either treated with rIL-7 ( ) or left untreated (−) during the effector phase of the immune response (days 10-23). The number of Tet+CD8+ T cells (left panel) and the number of Tet−CD8+ T cells (right panel) per milliliter of blood are shown at different time points after immunization. Error bars represent SD (n = 12). (B) In a separate experiment, mice were treated as in panel A. All mice received BrdU in the drinking water (days 10-23). Graphs represent the kinetics of BrdU incorporation in Tet+ (left panel) and in Tet− (right panel) CD8+ T cells. The number of BrdU+ cells per milliliter of blood at the indicated time points is shown. Error bars represent SD (n = 7). (C) Ratio between CD127hi and CD127lo Tet+CD8+ T cells in untreated (■) and IL-7–treated (
) or left untreated (−) during the effector phase of the immune response (days 10-23). The number of Tet+CD8+ T cells (left panel) and the number of Tet−CD8+ T cells (right panel) per milliliter of blood are shown at different time points after immunization. Error bars represent SD (n = 12). (B) In a separate experiment, mice were treated as in panel A. All mice received BrdU in the drinking water (days 10-23). Graphs represent the kinetics of BrdU incorporation in Tet+ (left panel) and in Tet− (right panel) CD8+ T cells. The number of BrdU+ cells per milliliter of blood at the indicated time points is shown. Error bars represent SD (n = 7). (C) Ratio between CD127hi and CD127lo Tet+CD8+ T cells in untreated (■) and IL-7–treated ( ) mice. Error bars represent SD (n = 12). The gray boxes in panels A and C represent the IL-7 treatment period; and in panel B, the IL-7 and BrdU treatment period. *P < .05.
) mice. Error bars represent SD (n = 12). The gray boxes in panels A and C represent the IL-7 treatment period; and in panel B, the IL-7 and BrdU treatment period. *P < .05.
We hypothesized that the effect of IL-7 in increasing the number of long-lived Tet+CD8+ T cells was the result of a selective effect of the cytokine on effector T cells expressing high levels of IL-7Rα. We thus analyzed the relative frequency of IL-7Rαhi cells during and after the cessation of IL-7 treatment (Figure 4C). It has been shown that, in T cells, IL-7 stimulation induces the down-regulation of IL-7Rα expression.28 In line with this, our data showed that, during the IL-7 treatment period, the expression level of IL-7Rα on Tet+CD8+ T cells was indeed lower in IL-7–treated than in untreated mice (Figure 4C). However, after the cessation of the treatment, a significantly larger fraction of Tet+CD8+ T cells was found to express high levels of IL-7Rα in IL-7–treated mice compared with the untreated mice.
Altogether, these results demonstrated that administration of IL-7 in vivo elicited proliferation of effector CD8+ T cells and increased long-term antigen-specific CD8+ T-cell responses after recombinant lentivector/Melan-A26-35 immunization. We suggest that IL-7 stimulated the expansion of the large fraction of antigen-specific effector CD8+ T cells expressing high levels of IL-7Rα. Those cells, once the treatment was interrupted, could overcome, at least in part, the contraction phase giving rise to the increased memory population.
IL-7 adjuvant treatment does not increase long-term Tet+CD8+ T-cell responses after peptide immunization
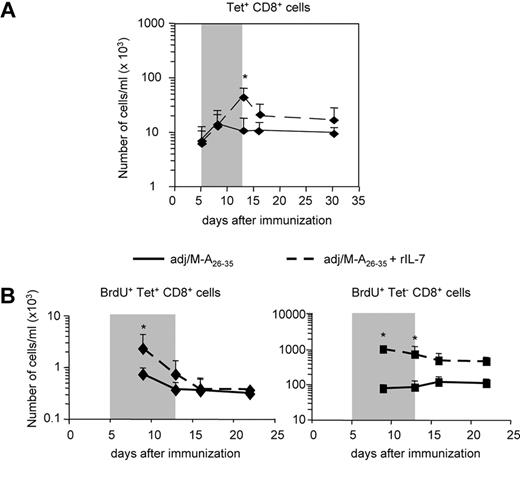
As shown in Figure 1, the majority of Tet+ effector CD8+ T cells elicited by immunization with adjuvant/Melan-A26-35 had down-regulated surface expression of IL-7Rα. We have previously hypothesized that the positive effect of IL-7 treatment on the number of memory T cells was mediated by high expression of IL-7Rα on effector T cells. We thus predicted that IL-7 treatment of peptide-immunized mice would not increase the pool of Tet+ memory CD8+ T cells. Treatment of peptide-immunized mice with IL-7 during the effector phase (days 5-13) increased the number of Tet+CD8+ T cells to the same extent as it did in mice immunized with recombinant lentivector/Melan-A26-35 (Figure 5A). However, after the cessation of IL-7 administration, the population of Tet+CD8+ T cells contracted and, after 2 weeks, returned to levels not significantly higher than that observed in untreated mice. As for recombinant lentivector, we analyzed whether IL-7 was able to increase proliferation of antigen-specific effector CD8+ T cells by measuring the number of Tet+ cells incorporating BrdU (Figure 5B). We observed a significant increase in the proliferation of Tet+ effector cells in mice that received IL-7 treatment. However, after IL-7 was removed from the system, BrdU+Tet+ cells contracted and returned to the levels of untreated mice.
IL-7 adjuvant treatment does not increase Melan-A–specific memory CD8+ T-cell responses on peptide immunization. Mice immunized with adjuvant/Melan-A26-35 were either treated with rIL-7 ( ) or left untreated (−) during the effector phase of the immune response (days 5-13). (A) Kinetics of Melan-A–specific CD8+ T-cell responses. The number of Tet+CD8+ T cells per milliliter of blood is shown. (B) In a separate experiment, mice were treated as in panel A. All mice received BrdU in the drinking water (days 5-13). Graphs represent the kinetics of BrdU incorporation in Tet+ (left panel) and in Tet− (right panel) CD8+ T cells. The number of BrdU+ cells per milliliter of blood at the indicated time points is shown. Error bars represent SD (n = 5). The gray box in panel A indicates the IL-7 treatment period; and in panel B, the IL-7 and BrdU treatment period. *P < .05.
) or left untreated (−) during the effector phase of the immune response (days 5-13). (A) Kinetics of Melan-A–specific CD8+ T-cell responses. The number of Tet+CD8+ T cells per milliliter of blood is shown. (B) In a separate experiment, mice were treated as in panel A. All mice received BrdU in the drinking water (days 5-13). Graphs represent the kinetics of BrdU incorporation in Tet+ (left panel) and in Tet− (right panel) CD8+ T cells. The number of BrdU+ cells per milliliter of blood at the indicated time points is shown. Error bars represent SD (n = 5). The gray box in panel A indicates the IL-7 treatment period; and in panel B, the IL-7 and BrdU treatment period. *P < .05.
IL-7 adjuvant treatment does not increase Melan-A–specific memory CD8+ T-cell responses on peptide immunization. Mice immunized with adjuvant/Melan-A26-35 were either treated with rIL-7 ( ) or left untreated (−) during the effector phase of the immune response (days 5-13). (A) Kinetics of Melan-A–specific CD8+ T-cell responses. The number of Tet+CD8+ T cells per milliliter of blood is shown. (B) In a separate experiment, mice were treated as in panel A. All mice received BrdU in the drinking water (days 5-13). Graphs represent the kinetics of BrdU incorporation in Tet+ (left panel) and in Tet− (right panel) CD8+ T cells. The number of BrdU+ cells per milliliter of blood at the indicated time points is shown. Error bars represent SD (n = 5). The gray box in panel A indicates the IL-7 treatment period; and in panel B, the IL-7 and BrdU treatment period. *P < .05.
) or left untreated (−) during the effector phase of the immune response (days 5-13). (A) Kinetics of Melan-A–specific CD8+ T-cell responses. The number of Tet+CD8+ T cells per milliliter of blood is shown. (B) In a separate experiment, mice were treated as in panel A. All mice received BrdU in the drinking water (days 5-13). Graphs represent the kinetics of BrdU incorporation in Tet+ (left panel) and in Tet− (right panel) CD8+ T cells. The number of BrdU+ cells per milliliter of blood at the indicated time points is shown. Error bars represent SD (n = 5). The gray box in panel A indicates the IL-7 treatment period; and in panel B, the IL-7 and BrdU treatment period. *P < .05.
Altogether, these results demonstrated that, in peptide-immunized mice, administration of IL-7 could induce a transient expansion of effector populations expressing high and low levels of IL-7Rα. However, once the treatment was interrupted, the Tet+IL-7Rαlow cells, representing the majority of the effector population, could not survive the contraction phase. These data confirm that the development of a larger memory T-cell population after IL-7 treatment requires that a high frequency of antigen-specific cells expresses IL-7Rα during the effector phase, as it occurs on recombinant lentivector immunization.
IL-7 treatment improves the functionality of Melan-A–specific memory CD8+ T cells
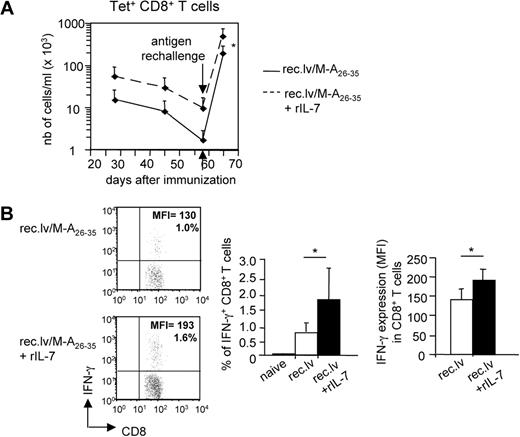
We next wondered whether the increased number of memory CD8+ T cells induced by IL-7 adjuvant treatment in recombinant lentivector-immunized mice translated into improved secondary responses. To answer this question, mice previously immunized with recombinant lentivector/Melan-A26-35, and treated or not with IL-7, were rechallenged with adjuvant/Melan-A26-35 at the memory phase (day 58; Figure 6A). Upon peptide rechallenge, the magnitude of the secondary Tet+CD8+ T-cell response was higher in mice that had received IL-7 treatment, reflecting the higher numbers of antigen-specific memory CD8+ T cells present in those mice. Next, we studied the functionality of the memory CD8+ T cells derived from recombinant lentivector/Melan-A26-35–immunized mice that received or not IL-7 adjuvant treatment. To this purpose, we analyzed the production of IFN-γ by those cells upon short in vitro Melan-A– specific stimulation (Figure 6B). The results showed that a higher percentage of CD8+ T cells from IL-7–treated mice produced IFN-γ upon stimulation. Importantly, based on the MFI of IFN-γ staining in those cells, we could conclude that antigen-specific CD8+ T cells from mice treated with IL-7 produced significantly higher amount of IFN-γ per cell compared with untreated mice.
IL-7 treatment improves the functionality of Melan-A–specific memory CD8+ T cells. A2/Kb mice were immunized with recombinant lentivector/Melan-A26-35 and either left untreated or treated with rIL-7 during the effector phase of the primary immune response (days 10-23). (A) At the memory phase (day 58 after immunization), mice were rechallenged with adjuvant/Melan-A26-35 immunizations. Eight days later, the number of Tet+ was measured ex vivo. Graphs represent the mean values. Error bars represent SD (n = 6). (B) At day 45 after primary immunization, PBMCs from immunized mice were shortly restimulated in vitro with Melan-A26-35 peptides. Intracellular content of IFN-γ on stimulation was measured. (Left panels) Dot plots show one representative experiment. (Right panels) Histogram plots show the frequency of IFN-γ+ among CD8+ T cells, and the expression level (MFI) of IFN-γ in those cells. Error bars represent SD (n = 7-9). *P < .05.
IL-7 treatment improves the functionality of Melan-A–specific memory CD8+ T cells. A2/Kb mice were immunized with recombinant lentivector/Melan-A26-35 and either left untreated or treated with rIL-7 during the effector phase of the primary immune response (days 10-23). (A) At the memory phase (day 58 after immunization), mice were rechallenged with adjuvant/Melan-A26-35 immunizations. Eight days later, the number of Tet+ was measured ex vivo. Graphs represent the mean values. Error bars represent SD (n = 6). (B) At day 45 after primary immunization, PBMCs from immunized mice were shortly restimulated in vitro with Melan-A26-35 peptides. Intracellular content of IFN-γ on stimulation was measured. (Left panels) Dot plots show one representative experiment. (Right panels) Histogram plots show the frequency of IFN-γ+ among CD8+ T cells, and the expression level (MFI) of IFN-γ in those cells. Error bars represent SD (n = 7-9). *P < .05.
These data demonstrated that IL-7 adjuvant treatment not only increased the numbers of circulating antigen-specific memory CD8+ T cells, but it also improved their functionality.
Discussion
In this study, we show the efficacy of IL-7 adjuvant therapy in increasing the populations of antigen-specific effector and memory CD8+ T cells after immunization with recombinant lentivector encoding the melanoma-associated antigens Melan-A26-35 and gp10025-33. We demonstrate that IL-7 adjuvant therapy not only increases the numbers of antigen-specific memory CD8+ T cells but also improves their functionality as assessed by secondary responses. Importantly, we propose that the impact of IL-7 treatment on the memory CD8+ T-cell pool depends on high levels of IL-7Rα expression in the population of effector cells, specifically observed on recombinant lentivector immunization.
Upon antigen stimulation, CD8+ T cells undergo vigorous clonal expansion and in several models of viral infection or peptide immunizations, activated T cells down-regulate the expression of IL-7Rα.8,9,11,30 This occurs mainly through changes in Il-7Rα gene transcription.28,31 Here we show that a high percentage of antigen-specific effector CD8+ T cells fails to down-regulate IL-7Rα expression on recombinant lentivector immunizations. Several factors could account for this phenomenon. TCR signaling has been shown to repress IL-7Rα expression.32 Thus, we can envisage that different strength of T-cell activation signals could be delivered after recombinant lentivector and peptide immunizations. However, our analysis of CD44, CD62L (data not shown), and CD526 (Figure S1) expression on antigen-specific CD8+ T cells after recombinant lentivector and peptide immunizations did not support this hypothesis. Besides TCR signaling, signals mediated by the common gamma chain (γc) cytokines IL-2, IL-4, and IL-7 have been proposed to influence the levels of IL-7Rα expression on effector T cells.28,33 We could therefore envisage that different levels of these cytokines are produced after recombinant lentivector and peptide immunizations. In particular, the highly inflammatory environment produced by the strong adjuvant formulation used in peptide vaccines could account for a higher level of IL-7Rα down-regulation. In addition, CD4+ T-cell help has been proposed to regulate IL-7Rα expression on antigen-specific CD8+ T cells.34 However, in our system, CD4+ T-cell depletion before recombinant lentivector immunizations led to a strong decrease in the magnitude of antigen-specific CD8+ T-cell responses but did not impact on the expression of IL-7Rα on the remaining effector CD8+ T cells (S.C. and F.L., unpublished data, March 2007).
Previous reports have proposed that the small fraction of antigen-specific CD8+ T cells expressing high levels of IL-7Rα at the effector phase of the response are preferentially selected to survive and become long-lived memory T cells. This occurs because they receive prosurvival signals leading to the up-regulation of Bcl-2 and Bcl-XL expression. By contrast, IL-7Rαlo effector CD8+ T cells that express lower levels of Bcl-2 will undergo programmed cell death characteristic of the contraction phase.8,9 Surprisingly higher expression of IL-7Rα on antigen-specific effector CD8+ T cells elicited by recombinant lentivector immunizations did not lead to higher levels of Bcl-2 (Figure 2). One possible explanation for the lack of Bcl-2 up-regulation in IL-7Rαhi effector CD8+ T cells is that in vivo, in the presence of the antigen, concomitant TCR-mediated signaling could inhibit physiologic IL-7R signaling to be properly transduced, as it has been already proposed.26
Despite the lack of Bcl-2 up-regulation in IL-7RαhiTet+ effector CD8+ T cells, we observed a lower level of contraction of Melan-A–specific CD8+ T cells in recombinant lentivector immunized mice compared with peptide-immunized mice. It could be that IL-7R signaling regulates the expression of other molecules involved in the control of T-cell survival. However, we did not observe any difference in Bcl-XL expression in effector cells expressing high and low levels of IL-7Rα (data not shown). We still cannot exclude that high levels of IL-7Rα could affect the expression of other molecules regulating cell viability versus cell death, such as the proapoptotic molecule Bim, a key factor in regulating programmed cell death of antigen-activated CD8+ T cells lacking IL-7Rα expression.35
In contrast to effector CD8+ T cells, high expression of IL-7Rα on CD8+ T cells during the contraction and memory phases of the response appeared to mediate the up-regulation of Bcl-2. This could be explained by the fact that, once the antigen is cleared and the contraction phase starts, TCR-mediated signals are no longer inhibiting IL-7R signaling in vivo as previously mentioned. Another possibility is that the physiologic amount of IL-7 cytokine available for the expanding population of Melan-specific effector CD8+ T cells could be limiting in vivo. Once the antigen-specific effector CD8+ T-cell pool shrinks during the transition to the memory phase, cells may become able to respond again to the available IL-7. Starting from this hypothesis, we thought of providing exogenous IL-7 as an adjuvant treatment during the course of the expansion phase of recombinant lentivector-induced CD8+ T-cell response. The first effect of IL-7 therapy that we observed was the up-regulation of Bcl-2 (Figure 3) and a strong increase in the number of both naive and Melan-A–specific effector CD8+ T cells (Figure 4A), mainly the result of increased proliferation, as assessed by BrdU incorporation experiments (Figure 4B). Indeed, IL-7/IL-7R–mediated signaling, besides regulating T-cell survival, also promotes T-cell proliferation through the activation of the phosphatidylinositol 3-kinase pathway.10 These data confirmed the integrity of the IL-7R signaling in vivo in activated cells. Importantly, when IL-7 administration was interrupted, the number of naive CD8+ T cells dropped to baseline levels, whereas the number of Melan-A–specific CD8+ T cells remained significantly higher than in untreated mice, for at least 2 months after immunization (Figure 4A). The same IL-7 therapy regimen in peptide-immunized mice only induced an increase in the effector T-cell numbers during administration of the cytokine but did not lead to a significant increase in the population of Melan-A–specific memory CD8+ T cells once the treatment was interrupted (Figure 5). From these findings we concluded that, even if IL-7 was able to increase the number of effector cells, the majority of these cells, expressing low level of IL-7Rα, were not able to survive the contraction phase, similar to that observed in untreated mice. The fact that IL-7 administration was able to trigger proliferation of IL-7Rαlo peptide-induced effector CD8+ T cells could be the result of the high dose of cytokine used.
Altogether, these findings support an important role for IL-7 cytokine as adjuvant therapy to increase vaccine-induced memory T-cell populations, as it has been previously proposed.18 Importantly, IL-7 treatment not only increases the number of antigen-specific CD8+ memory T cells on recombinant lentivector immunizations but also improves their functional responses (Figure 6). We are currently testing whether this increased functionality could provide IL-7–treated mice with higher protection against tumor challenges.
Given the strong effect of IL-7 administration in expanding the naive CD8+ T-cell compartment, however, it would be critical to provide the cytokine only for limited periods of time, in order not to affect the general homeostatic T-cell balance and eventually to avoid possible unwanted autoimmune disorders. Our results suggest that IL-7 therapy, provided during the expansion phase of the CD8+ T-cell response, could be highly effective in increasing the effector and memory CD8+ T-cell populations. However, the specific effect on memory CD8+ T cells would only occur in particular conditions of effector CD8+ T-cell activation. Specifically, the long-term efficacy of IL-7 therapy seems to strongly depend on high levels of IL-7Rα expression on antigen-specific effector CD8+ T cells. We think that administration of IL-7 therapy during the effector phase of the CD8+ T-cell response induces the expansion of both IL-7Rαhi and IL-7Rαlo cells. However, once the therapy is interrupted, only the cells that were expressing high levels of IL-7Rα can be selected to survive and become long-lived memory T cells. In vaccination settings, such as recombinant lentivector immunizations, where effector IL-7Rαhi T cells are in high numbers, the surviving population selected at the end of the therapy will obviously be larger compared with other immunizations settings, such as peptide immunizations, where the initial number of IL-7Rαhi cells is lower.
As mentioned previously, IL-7Rα expression is tightly regulated at a transcriptional level. Recently, it has been proposed that different balance of transcription factors and epigenetic changes at the level of the IL-7Rα gene promoter sustain a stable repression of the receptor expression in short-lived effector CD8+ T cells and an activation of its expression in memory precursor effector CD8+ T cells.36 We could thus hypothesize that IL-7Rαhi cells, which transiently down-regulate the expression of the IL-7Rα upon IL-7 administration, are committed to reacquire its expression after cytokine removal and can be selected to survive. In contrast, IL-7Rαlo effector cells might have a stable repression status of the receptor gene expression and could fail to up-regulate it again once IL-7 treatment is interrupted, thus dying after cytokine removal.
In conclusion, IL-7 treatment should be beneficial in vaccinations settings1,37 in which high frequencies of effector CD8+ T cells do not down-regulate IL-7Rα expression upon priming, such as recombinant lentivector-based vaccines.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cytheris for the generous gift of recombinant IL-7 and Dr Sophie Sierro for critical reading of the manuscript.
This work was supported in part by the Swiss National Funds, the Cancer Research Institute, and the Leenaards Foundation.
Authorship
Contribution: S.C. and L.C. performed experiments, analyzed results, made the figures, and wrote the paper; and S.C., F.L., and L.C. designed the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sara Colombetti, Ludwig Institute for Cancer Research–Lausanne Branch, Ch des Boveresses 155, 1066 Epalinges, Switzerland; e-mail: sara.colombetti@licr.unil.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal