MicroRNA profiles defined by signatures of normal B-cell subpopulations, genomic changes, and oncogene activity segregate diffuse large B-cell lymphomas into unique subgroups with potential clinical relevance.
Diffuse large B-cell lymphoma (DLBCL) is an aggressive, potentially fatal, mature B-cell lymphoid malignancy with considerable clinical, pathologic, and genetic heterogeneity. Global alterations of microRNA (miRNA) expression relative to normal lymphoid tissues have been observed in primary DLBCL cases,1 but the use of miRNA expression patterns to probe into the genetic heterogeneity of DLBCL has not been reported until now. Li and colleagues have developed a novel miRNA-based classification of DLBCL using unsupervised hierarchical clustering analysis.2 Subsets of miRNAs as few as 17 identified the presence of 3 unique subgroups in a cohort of 53 primary DLBCL. Previously, signatures of miRNAs have been identified by miRNA expression profiling in prognostic subgroups of chronic lymphocytic leukemia/small lymphocytic lymphoma defined by IgVH mutation status and ZAP70 expression.3 This elegant study by Li et al, however, represents the first demonstration of an miRNA-driven approach to explore the molecular heterogeneity of a lymphoid malignancy and provides convincing evidence that the miRNA profiles stratifying DLBCL are unique products of B-cell differentiation stage-specific miRNA signature and tumorigenesis-specific miRNA expression changes.
The miRNA profiles in DLBCL described in this study contain genetic fingerprints of normal B-cell subsets. These profiles overlap considerably with a miRNA signature of 39 miRNAs that can distinguish naive, centroblasts, and memory B cells.4 The DLBCL samples are clustered by the novel DLBCL miRNA classifiers and the normal B-cell miRNA classifier into 3 subgroups with a concordance rate of about 90%. Thus, the DLBCL miRNA classifiers may provide an alternative cell-of-origin classification in DLBCL besides the mRNA-based and immunohistochemical-based cell-of-origin classifications,5,6 potentially introducing an extra layer of complexity to the molecular heterogeneity of DLBCL. The latter 2 classification schemes, which have a high but not exact correlation with each other, have divided DLBCL into 2 established subgroups corresponding to 2 B-cell differentiation stages: germinal center B cell (GCB)–like, and non-GCB– or activated B cell–like. Quite unexpectedly, the miRNA-based classification, despite its strong association with the miRNA fingerprints of normal B cells, lacks correlation with the immunohistochemistry-based cell-of-origin classification in this study. This discrepancy can be conceivably due to a less than perfect correlation between the mRNA and immunophenotype-based classifications. However, it is not likely to fully account for the discrepancy. A more likely explanation is: although both the miRNA and mRNA expression profiles are influenced by signatures associated with certain B-cell subsets and tumor-associated aberrations, there may be differences in the relative impact of these factors on these 2 types of profiles. It is possible that miRNA-based genetic fingerprints of normal B cells are more resistant to being masked by tumor-specific alterations and are therefore more accurate in the determination of cell origin in DLBCL. Indeed, miRNA profiles have demonstrated to be highly reflective of the cell types and differentiation stages of human cancers and are superior to mRNA profiles in classifying them.7 Importantly, preliminary results have shown that miRNA-based subclassification of DLBCL may have prognostic value. Further validation using larger cohorts is necessary.
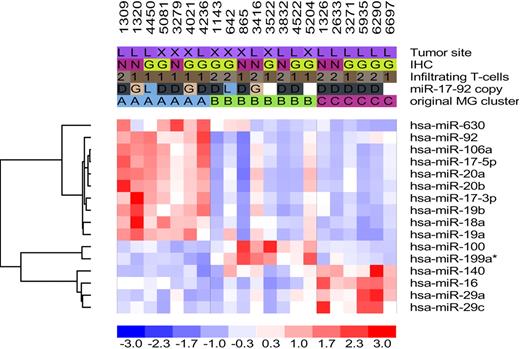
The authors also identify 2 tumor-associated alterations that can influence the miRNA expression profiles in DLBCL: miRNA gene copy number changes and increased c-MYC activity. These alterations have the potential to disrupt the normal expression of miRNAs during B-cell differentiation by deregulating expression of miRNAs, which may lead to ectopic expression in an inappropriate differentiation stage, and/or inability to coordinate miRNA expression with transition between differentiation stages. For example, miR-125b, a miRNA more highly expressed in centroblasts, becomes abnormally overexpressed in the “memory B cell–like” DLBCL subgroup because of gene amplification. Another example is miR-17-92, an oncogenic polycistronic cluster that is targeted by both gene amplifications and deregulated c-MYC, resulting in its overexpression in the “centroblast-like” subgroup of DLBCL. Normally, miR-17-92 is expressed at higher levels in centroblasts compared with naive and memory B cells.4 c-MYC is not normally expressed in GCB cells, suggesting that the differential expression of miR-17-92 in centroblasts may be regulated by transcription factors other than c-MYC. Increased copy numbers and transcription induction by a deregulated c-MYC may result in persistent expression of miR-17-92, which would otherwise be down-regulated during transition from GCB cells to memory B cells. This study highlights the importance of c-MYC as well as miR-17-92 deregulation in the pathogenesis of DLBCL. Undoubtedly, investigations to dissect the influence of other oncogenes or tumor suppressors on the miRNA expression profiles in DLBCL are necessary to further understand the role of these complicated gene regulatory networks in lymphomagenesis.
MicroRNA expression profiling in DLBCL: training and validation sets. Two rounds of one-way ANOVA testing identified subsets of 38 and 16 miRNAs whose expression could effectively discriminate DLBCLs into these 3 subsets. See the complete figure in the article beginning on page 6681.
MicroRNA expression profiling in DLBCL: training and validation sets. Two rounds of one-way ANOVA testing identified subsets of 38 and 16 miRNAs whose expression could effectively discriminate DLBCLs into these 3 subsets. See the complete figure in the article beginning on page 6681.
Above all, the generation of these miRNA profiles has provided us with a framework to delineate the roles of specific miRNAs in DLBCL lymphomagenesis. Careful comparative studies of these miRNA profiles with the normal B-cell subset signatures should reveal miRNAs that are deregulated during specific stages of B-cell differentiation. In addition, comparison of mRNA profiles of DLBCL with differential expression of a particular miRNA should facilitate the identification of target genes coordinately regulated by the miRNA and the molecular mechanisms by which it contributes to lymphomagenesis.8
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal