In this issue of Blood, Alter and colleagues report on the spectrum of cancers occurring in 500 patients with DC as reported in the medical literature from 1910 to 2008 and in a prospective cohort of 50 DC patients followed at the National Cancer Institute (NCI). The study finds in both cohorts a cumulative incidence of cancer approximating 40% to 50%, as well as a shortened overall survival and a poor outcome after HSCT. Squamous cell carcinomas of the head and neck were the most frequently noted cancers in both study populations followed by skin, anorectal, and other cancers.
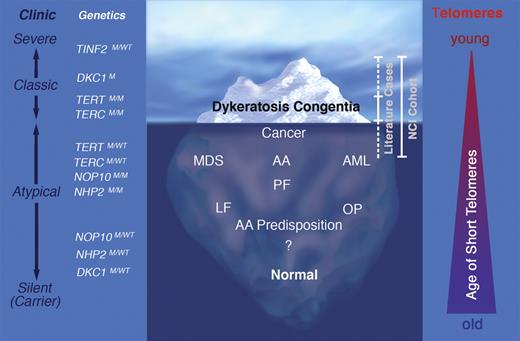
Dyskeratosis congenita (DC) is an inherited bone marrow failure syndrome (IBMFS) whose clinical spectrum has dramatically evolved over the past 10 years. Historically, patients were diagnosed with DC based on the association of BMF with the classic triad of mucocutaneous features including changes in skin pigmentation, dystrophic fingernails, and leukoplakia. Over the past decade, germline mutations in 6 distinct genes, DKC1, TERC, TERT, NHP2, NOP10, and TINF2, were found to account for approximately 50% of patients with DC.1 The products of the DC genes all participate in telomere maintenance, revealing that defective telomere maintenance is the primary factor underlying disease pathogenesis. By the time DC patients develop BMF, all have short telomeres.2-4 With the availability of genetic testing, the clinical spectrum of DC has broadened, and it has become clear that the initially described mucocutaneous manifestations are present in only a small proportion of patients, generally those with a more severe phenotype and an earlier onset of disease (see figure).
Clinical spectrum and genetics of dyskeratosis congenita (DC). Since the identification of the DC genes and the application of genetic testing, it is increasingly recognized that the classic DC clinical features represent the “tip of the iceberg” and are present in only a small proportion of patients. Instead, a substantial proportion of patients present without mucocutaneous manifestations, but with aplastic anemia (AA) or myelodysplastic syndrome (MDS). In other patients, extrahematopoietic manifestations may be the primary or only manifestations of disease, such as pulmonary fibrosis (PF), liver fibrosis (LF) or osteoporosis (OP). In rare cases, certain types of cancer occur at unexpectedly young ages or acute myeloid leukemia (AML) is the presenting feature. In addition to clinically affected individuals, a significant population of silent mutation carriers exists with no obvious disease manifestations. Clinic represents the clinical spectrum from silent mutation carriers to individuals with severe disease manifestations. The triangle on the right illustrates the age when telomeres become critically short; in severe disease, telomeres are short at a young age, whereas in individuals with mild disease, telomeres become critically short later in life. M indicates mutant; and WT, wild type. Professional illustration by Marie Dauenheimer.
Clinical spectrum and genetics of dyskeratosis congenita (DC). Since the identification of the DC genes and the application of genetic testing, it is increasingly recognized that the classic DC clinical features represent the “tip of the iceberg” and are present in only a small proportion of patients. Instead, a substantial proportion of patients present without mucocutaneous manifestations, but with aplastic anemia (AA) or myelodysplastic syndrome (MDS). In other patients, extrahematopoietic manifestations may be the primary or only manifestations of disease, such as pulmonary fibrosis (PF), liver fibrosis (LF) or osteoporosis (OP). In rare cases, certain types of cancer occur at unexpectedly young ages or acute myeloid leukemia (AML) is the presenting feature. In addition to clinically affected individuals, a significant population of silent mutation carriers exists with no obvious disease manifestations. Clinic represents the clinical spectrum from silent mutation carriers to individuals with severe disease manifestations. The triangle on the right illustrates the age when telomeres become critically short; in severe disease, telomeres are short at a young age, whereas in individuals with mild disease, telomeres become critically short later in life. M indicates mutant; and WT, wild type. Professional illustration by Marie Dauenheimer.
It has also become evident that the inheritance of DC is complex, with X-linked, autosomal dominant, and recessive pedigrees.1 Furthermore, DC may occur sporadically due to the presence of de novo germline mutations in a single allele of a DC-associated gene. Finally, in some families with autosomal dominant DC, the inheritance of successively shorter telomeres is associated with genetic “anticipation,” characterized by progressively more severe disease manifestations at younger ages with each generation.1,5,6 This genetic heterogeneity is associated with a wide clinical spectrum that ranges from intrauterine growth retardation or death in early childhood to no overt features of disease (see figure). The variability in clinical features and complexity of genetics presents a unique challenge to physicians and genetic counselors confronted with patients suffering from DC, silent mutation carriers, or family members of affected individuals.
The cancer risk assessment presented by Alter et al is based on patients reported in the literature since 1910,7 which primarily includes patients with a classic presentation and a relatively severe phenotype (see figure). In addition, the authors include the NCI cohort, which consists of families with at least one affected family member, persons with a very severe and early onset form of disease (recurrent TINF2 mutation carriers), or families with cancer who were later found to have DC (see figure). There is considerable ascertainment bias in both cohorts toward patients with a high disease penetrance, an early onset of disease, a more severe disease presentation, and an increased incidence of cancer.
Risk estimates depend on the characteristics of the studied population. While this study includes a large DC patient population, the genetic heterogeneity of DC was not taken into account. Despite these limitations, the study has many important findings, highlighting the need for careful cancer surveillance and the spectrum of cancers characteristic of patients with DC. In addition, the study outlines the need to identify new DC-specific hematopoietic stem cell transplantation (HSCT) approaches to improve long-term survival, and the need to explore new therapeutic agents that modify the course of disease. However, DC is a disease with a wide spectrum of features that are likely linked to the presence of specific gene mutations and other as yet poorly understood genetic modifiers and environmental exposures. Thus, not all DC patients will develop manifestations as severe as those described in the current study. When counseling DC patients regarding survival or possible treatment outcomes, it is important that these issues be considered to avoid generating unnecessary fear of dying, suffering from cancer, or withholding medical treatments that might offer a cure for this disease.
Conflict-of-interest disclosure: K.E.N. declares no competing financial interests. M.B. is on the Scientific Advisory Committee of the International PNH Registry, the Diamond Blackfan Anemia North America Registry, the Shwachman Diamond Syndrome Foundation, and Alexion Pharmaceutical Inc. ■
Acknowledgments
We are grateful to all patients, families, and collaborating physicians for participation in our BMF Research Program at Washington University School of Medicine, St Louis, MO (http://bmf.im.wustl.edu) and the Pediatric Hereditary Cancer Predisposition Program at the Children's Hospital of Philadelphia. We thank Philip J. Mason for discussion. M. B. is supported by National Institutes of Health grant R01 CA10532.
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal