Abstract
Reconstitution of cytomegalovirus (CMV)–specific CD8+ T cells is essential to the control of CMV infection in CMV-positive recipients (R+) after allogeneic hematopoietic stem cell transplantation (HCT). Six-color flow cytometry was used to assess the functional profile of CMV-specific CD8+ T cells in 62 of 178 R+ HCT recipients followed virologically for CMV reactivation. R+ recipients receiving grafts from CMV-negative donors (D−; D−/R+) reconstituted fewer multifunctional CD8+ T cells expressing tumor necrosis factor-α (TNF-α), macrophage inflammatory protein-1β (MIP-1β), and CD107 in addition to interferon-γ (IFN-γ), compared with D+/R+ recipients. Unlike monofunctional CD8+ T cells secreting IFN-γ, which were abundantly generated during CMV reactivation in D−/R+ recipients, the relative lack of multifunctional CD8+ T cells persisted until at least 1 year post-HCT. D−/R+ recipients were more likely to require recurrent and prolonged use of antivirals. These findings were robust to statistical adjustment for pretransplant factors, as well as for posttransplant factors including graft-versus-host disease (GVHD) and its treatment by steroids. These analyses suggest that D+/R+ transplants, on average, generate higher levels of multifunctional CMV-specific T cells and require less antiviral therapy compared with D−/R+ HCT recipients. These results highlight the benefit of D+ donors in improving outcomes of R+ HCT recipients by reducing the duration and recurrent need of antiviral treatment, aided by increased levels of multifunctional CMV-specific T cells.
Introduction
Cytomegalovirus (CMV) reactivation remains a significant cause of morbidity and mortality due to the extended period of immunodeficiency after allogeneic hematopoietic stem cell transplantation (HCT)1-4 despite great strides in management of the infection in the past 2 decades.5-7 The CMV status of the recipient before HCT has a strong influence on HCT outcome.4,8-13 Key questions addressed in this study are the impact of donor CMV status on the reconstitution of effective CMV immunity or risk of CMV reactivation and ganciclovir (GCV) usage in CMV-positive recipients (R+). Previous animal studies using a murine CMV model demonstrated a major role of CMV-specific T cells in the control of viral replication,14,15 which concurs with clinical studies in recipients post-HCT.16,17 Establishment of minimal levels of donor-derived CMV-specific immunity accelerates control of CMV infection, which is substantiated by the heightened risk for CMV reactivation in T cell–depleted transplant recipients.18-21 Further direct evidence for the role of CMV-specific T-cell immunity in controlling CMV infection was obtained from adoptive transfer of donor-derived CD8+ T cells in HCT recipients.22-24 A report evaluating interferon-γ (IFN-γ) production in human leukocyte antigen-A2 (HLA-A2) HCT recipients after receiving grafts from CMV-negative donors (D−), noted a delay of CMV-specific T cell immune reconstitution in those with frequent CMV sequelae, while early recovery of T-cell immunity was linked to lower rates of CMV infection and disease.25 Despite improvements in monitoring techniques,26,27 the effect of donor CMV status is still a potential HCT risk factor, especially for unrelated donor (URD) transplants.10,12,28,29
Antigen-specific CD8+ T cells are functionally heterogeneous, with properties associated with the extent of CD8+ T-cell differentiation.30 IFN-γ+/tumor necrosis factor-α+ (TNF-α+) double-positive T cells are more prominent in the established T-cell memory pool than in the activated CD8+ T-cell population, which mainly produce IFN-γ during the acute antigen-driven phase.30,31 Consequently, evaluation of antigen-specific T-cell production of IFN-γ is necessary, but likely insufficient, as the sole marker of functional immunity.32 Limited data are available on multiple cytokine expression profiles of CMV-specific CD8+ T cells in HCT recipients.33 Betts and colleagues have reported that HIV-specific CD8+ T cells, which simultaneously degranulated and produced IFN-γ, TNF-α, macrophage inflammatory protein-1β (MIP-1β), and interleukin-2 (IL2), were associated with lower viral load (VL) and HIV long-term nonprogressor status.34 Analogous findings were reported in HIV patients with the HLA B*2705 allele, who also control HIV infection, and in the rectal mucosa of chronically infected HIV patients.35,36 These findings in the context of HIV infection motivated us to investigate whether levels of multifunctional CMV-specific CD8+ T cells in HCT recipients correlated with the CMV status of the donor and the differentiation state of transplanted CMV-specific memory T cells.
We used a pp65 peptide library as a stimulatory antigen to evaluate the ex vivo functional profile of pp65-specific CD8+ T cells from R+ recipients receiving a T cell–replete graft from either a D+ or D− donor. Four functional parameters were evaluated by flow cytometry, including antiviral cytokines IFN-γ and TNF-α, chemokine MIP-1β, and degranulation marker, CD107a/b. We hypothesize that a mature CD8+ T-cell functional profile leads to lower recurrent CMV infection and lower antiviral usage in D+/R+ recipients of HCT.
Methods
Prospective study subjects
Peripheral blood mononuclear cells (PBMC) from R+ HCT recipients were collected between day 40 and day 360 post-HCT (for details see Gallez-Hawkins et al37 ). R+ HCT recipients (178), whose CMV status was determined using latex agglutination (CMV-SCAN; Becton Dickinson, San Jose, CA), were enrolled in an observational trial approved by the City of Hope Institutional Review Board (IRB) with informed consent obtained in accordance with the Declaration of Helsinki. CMV reactivation is defined as the initial demonstration of CMV infection either by blood culture (BC) or by 2 consecutive quantitative serum polymerase chain reaction (PCR) assays of CMV DNA during a monitoring period that began at day 21 post-HCT and continued thereafter twice weekly until day 100 as previously described.37 After day 100, CMV in plasma was monitored in patients at high risk because of graft-versus-host disease (GVHD) or immunosuppressive medication. Subjects with PCR or BC-confirmed CMV reactivation were treated with GCV for 6 weeks.38 A subset (n = 135) of the subjects limited only by sample availability were evaluated for CMV-specific CD8+ T cells producing IFN-γ, and a smaller subset (n = 62) limited by levels of CMV-specific T cells and complexity of the assay was further evaluated for T-cell multifunctional profile. Patient characteristics of all 3 groups of subjects, including CMV and reactivation status are summarized in Table 1.
Demographic profiles of HCT recipients in groups 1-3
| . | Group 1 (N = 178), n (%) . | Group 2 (N = 135), n (%) . | Group 3 (N = 62), n (%) . | |||
|---|---|---|---|---|---|---|
| D+/R+ . | D−/R+ . | D+/R+ . | D−/R+ . | D+/R+ . | D−/R+ . | |
| Total n | 128 (72) | 50 (28) | 98 (73) | 37 (27) | 41 (66) | 21 (34) |
| Diagnosis/diseases | ||||||
| Acute myeloid leukemia | 43 (34) | 13 (26) | 33 (34) | 9 (24) | 11 (27) | 5 (24) |
| Acute lymphoid leukemia | 22 (17) | 11 (22) | 18 (18) | 10 (27) | 12 (29) | 7 (33) |
| Chronic myeloid leukemia | 20 (16) | 6 (12) | 17 (17) | 6 (16) | 7 (17) | 3 (14) |
| Chronic lymphoid leukemia | 0 (0) | 2 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hodgkin lymphoma | 3 (2) | 2 (4) | 3 (3) | 0 (0) | 0 (0) | 1 (5) |
| Non-Hodgkin lymphoma | 19 (15) | 7 (14) | 12 (12) | 3 (8) | 4 (10) | 0 (0) |
| Others | 21 (16) | 9 (18) | 15 (15) | 9 (24) | 7 (17) | 5 (24) |
| Source of stem cell | ||||||
| Bone marrow | 19 (15) | 11 (22) | 20 (20) | 14 (38) | 5 (12) | 4 (19) |
| Peripheral blood stem cell | 109 (85) | 39 (78) | 78 (80) | 23 (62) | 36 (88) | 17 (81) |
| Median recipient age, y | 44 y | 42 y | 42 y | 39 y | 44 y | 40 y |
| Male sex | ||||||
| Donor | 76 (59) | 31 (62) | 51 (52) | 18 (49) | 19 (46) | 10 (48) |
| Recipient | 69 (54) | 24 (48) | 47 (48) | 19 (51) | 22 (54) | 11 (52) |
| Conditioning | ||||||
| Myeloablative | 80 (63) | 30 (60) | 65 (66) | 21 (57) | 24 (59) | 14 (67) |
| Nonmyeloablative/reduced intensity | 48 (38) | 20 (40) | 33 (34) | 16 (43) | 17 (41) | 7 (33) |
| Total body irradiation | 56 (44) | 24 (48) | 45 (46) | 21 (57) | 14 (34) | 10 (48) |
| Donor type | ||||||
| URD | 31 (24) | 31 (62) | 23 (23) | 23 (62) | 11 (27) | 11 (52) |
| Sibling | 97 (76) | 19 (38) | 75 (77) | 14 (38) | 30 (73) | 10 (48) |
| Acute GVHD | ||||||
| O-I | 50 (39) | 14 (28) | 40 (41) | 11 (30) | 16 (39) | 6 (29) |
| > II | 78 (61) | 36 (72) | 58 (59) | 26 (70) | 25 (61) | 15 (71) |
| Chronic GVHD | ||||||
| None | 26 (20) | 12 (24) | 20 (20) | 10 (27) | 8 (20) | 5 (24) |
| Yes | 102 (80) | 38 (76) | 78 (80) | 27 (73) | 33 (80) | 16 (76) |
| Subjects received steroids | ||||||
| Day 90 | ||||||
| > 0 ≤ 1 mg/kg per day | 69 (54) | 30 (60) | 55 (56) | 24 (65) | 18 (44) | 15 (71) |
| > 1 mg/kg per day | 7 (5) | 5 (10) | 7 (7) | 4 (11) | 3 (7) | 2 (10) |
| Day 180 | ||||||
| > 0 ≤ 1 mg/kg per day | 59 (46) | 26 (52) | 49 (50) | 20 (54) | 18 (44) | 11 (52) |
| > 1 mg/kg per day | 10 (8) | 3 (6) | 7 (7) | 3 (8) | 4 (10) | 2 (10) |
| Day 360 | ||||||
| > 0 ≤1 mg/kg per day | 49 (38) | 20 (40) | 39 (40) | 13 (35) | 16 (39) | 8 (38) |
| > 1 mg/kg per day | 3 (2) | 2 (4) | 3 (3) | 2 (5) | 1 (2) | 0 (0) |
| CMV reactivation | ||||||
| ≥ 1 positive PCR | 73 (57) | 33 (66) | 56 (57) | 25 (68) | 17 (41) | 16 (76) |
| Median day of first positive PCR | 42.5 | 45.5 | 44 | 42 | 45 | 42 |
| Maximum viral load of PCR positives, gc/mL | 412 | 878 | 433 | 890 | 500 | 920 |
| > 1 positive BC | 49 (38) | 21 (42) | 38 (39) | 16 (43) | 12 (29) | 10 (48) |
| Any GCV treatment | 56 (44) | 28 (56) | 42 (43) | 23 (62) | 14 (34) | 15 (71) |
| Prolonged and/or recurrent GCV treatment | 18 (14) | 16 (32) | 12 (12) | 12 (32) | 5 (12) | 8 (38) |
| CMV disease | 7 (5) | 4 (8) | 5 (5) | 3 (8) | 2 (5) | 1 (5) |
| . | Group 1 (N = 178), n (%) . | Group 2 (N = 135), n (%) . | Group 3 (N = 62), n (%) . | |||
|---|---|---|---|---|---|---|
| D+/R+ . | D−/R+ . | D+/R+ . | D−/R+ . | D+/R+ . | D−/R+ . | |
| Total n | 128 (72) | 50 (28) | 98 (73) | 37 (27) | 41 (66) | 21 (34) |
| Diagnosis/diseases | ||||||
| Acute myeloid leukemia | 43 (34) | 13 (26) | 33 (34) | 9 (24) | 11 (27) | 5 (24) |
| Acute lymphoid leukemia | 22 (17) | 11 (22) | 18 (18) | 10 (27) | 12 (29) | 7 (33) |
| Chronic myeloid leukemia | 20 (16) | 6 (12) | 17 (17) | 6 (16) | 7 (17) | 3 (14) |
| Chronic lymphoid leukemia | 0 (0) | 2 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hodgkin lymphoma | 3 (2) | 2 (4) | 3 (3) | 0 (0) | 0 (0) | 1 (5) |
| Non-Hodgkin lymphoma | 19 (15) | 7 (14) | 12 (12) | 3 (8) | 4 (10) | 0 (0) |
| Others | 21 (16) | 9 (18) | 15 (15) | 9 (24) | 7 (17) | 5 (24) |
| Source of stem cell | ||||||
| Bone marrow | 19 (15) | 11 (22) | 20 (20) | 14 (38) | 5 (12) | 4 (19) |
| Peripheral blood stem cell | 109 (85) | 39 (78) | 78 (80) | 23 (62) | 36 (88) | 17 (81) |
| Median recipient age, y | 44 y | 42 y | 42 y | 39 y | 44 y | 40 y |
| Male sex | ||||||
| Donor | 76 (59) | 31 (62) | 51 (52) | 18 (49) | 19 (46) | 10 (48) |
| Recipient | 69 (54) | 24 (48) | 47 (48) | 19 (51) | 22 (54) | 11 (52) |
| Conditioning | ||||||
| Myeloablative | 80 (63) | 30 (60) | 65 (66) | 21 (57) | 24 (59) | 14 (67) |
| Nonmyeloablative/reduced intensity | 48 (38) | 20 (40) | 33 (34) | 16 (43) | 17 (41) | 7 (33) |
| Total body irradiation | 56 (44) | 24 (48) | 45 (46) | 21 (57) | 14 (34) | 10 (48) |
| Donor type | ||||||
| URD | 31 (24) | 31 (62) | 23 (23) | 23 (62) | 11 (27) | 11 (52) |
| Sibling | 97 (76) | 19 (38) | 75 (77) | 14 (38) | 30 (73) | 10 (48) |
| Acute GVHD | ||||||
| O-I | 50 (39) | 14 (28) | 40 (41) | 11 (30) | 16 (39) | 6 (29) |
| > II | 78 (61) | 36 (72) | 58 (59) | 26 (70) | 25 (61) | 15 (71) |
| Chronic GVHD | ||||||
| None | 26 (20) | 12 (24) | 20 (20) | 10 (27) | 8 (20) | 5 (24) |
| Yes | 102 (80) | 38 (76) | 78 (80) | 27 (73) | 33 (80) | 16 (76) |
| Subjects received steroids | ||||||
| Day 90 | ||||||
| > 0 ≤ 1 mg/kg per day | 69 (54) | 30 (60) | 55 (56) | 24 (65) | 18 (44) | 15 (71) |
| > 1 mg/kg per day | 7 (5) | 5 (10) | 7 (7) | 4 (11) | 3 (7) | 2 (10) |
| Day 180 | ||||||
| > 0 ≤ 1 mg/kg per day | 59 (46) | 26 (52) | 49 (50) | 20 (54) | 18 (44) | 11 (52) |
| > 1 mg/kg per day | 10 (8) | 3 (6) | 7 (7) | 3 (8) | 4 (10) | 2 (10) |
| Day 360 | ||||||
| > 0 ≤1 mg/kg per day | 49 (38) | 20 (40) | 39 (40) | 13 (35) | 16 (39) | 8 (38) |
| > 1 mg/kg per day | 3 (2) | 2 (4) | 3 (3) | 2 (5) | 1 (2) | 0 (0) |
| CMV reactivation | ||||||
| ≥ 1 positive PCR | 73 (57) | 33 (66) | 56 (57) | 25 (68) | 17 (41) | 16 (76) |
| Median day of first positive PCR | 42.5 | 45.5 | 44 | 42 | 45 | 42 |
| Maximum viral load of PCR positives, gc/mL | 412 | 878 | 433 | 890 | 500 | 920 |
| > 1 positive BC | 49 (38) | 21 (42) | 38 (39) | 16 (43) | 12 (29) | 10 (48) |
| Any GCV treatment | 56 (44) | 28 (56) | 42 (43) | 23 (62) | 14 (34) | 15 (71) |
| Prolonged and/or recurrent GCV treatment | 18 (14) | 16 (32) | 12 (12) | 12 (32) | 5 (12) | 8 (38) |
| CMV disease | 7 (5) | 4 (8) | 5 (5) | 3 (8) | 2 (5) | 1 (5) |
Distribution of HCT recipients in groups 1-3 by category of pretransplantation factors including underlying diagnosis, stem cell source, recipient age, sex, conditioning regimen, and donor type. Posttransplantation outcomes are tabulated for groups 1-3 including acute and chronic GVHD, steroid usage above and below 1 mg/kg for 3 time points, CMV reactivation defined by PCR, blood culture (BC), GCV treatment, and diagnosis of CMV disease. GVHD grades are based on the Keystone scale. HCT patient groups 1-3 are defined in “Prospective study subjects.”
URD indicates 6/6 antigen-matched unrelated donor; and gc, genome copies.
Peptides
Pools of overlapping 15-mer CMV pp65 peptides (PepMixes) were purchased from JPT Peptide Technologies (Berlin, Germany).
Stimulation of PBMC for ex vivo analysis of CMV-pp65 T cells and functional markers
CMV pp65 PepMixes (1 μg/mL) or diluent were added to 1 million freshly thawed cryopreserved PBMC, and cells were processed for flow cytometry according to published methods. For assays evaluating MIP-1β, TNF-α, and IFN-γ secretion as well as CD107a/b degranulation/mobilization, costimulatory antibodies to CD28 and CD49d (all antibodies were from Pharmingen, San Diego, CA) were added to the cultures to 1 μg/mL each at the same time as CMV pp65 PepMix or diluent. Flow-based degranulation was measured by incubation of PBMC with fluorescein isothiocyanate (FITC)–conjugated antibodies to CD107a and CD107b, as well as 1 μL monensin (GolgiStop; Pharmingen) and 1 μL brefeldin (GolgiPlug; Pharmingen) were also added to the medium before incubation.33
Flow cytometric analysis
For intracellular cytokine (ICC) assays evaluating only IFN-γ production, aliquots of cells were treated overnight (O/N) with pp65 PepMix, washed, and labeled for 20 minutes at 4°C with phycoerythrin (PE)–CD8 before permeabilization (Cytofix/Cytoperm; Pharmingen), followed by labeling with allophycocyanin (APC)–IFN-γ for 30 minutes. In the case of assays evaluating multiple cytokine secretion, cells were first stained with both anti–human PE-Cy5-CD8 and APC-Cy7-CD3 before permeabilization, followed by staining afterward with PE-Cy7–TNF-α, PE–MIP-1β, and APC–IFN-γ. Flow analysis was performed on a FACSCanto flow cytometer (Becton Dickinson). Flow gating strategies are described in the Results section. Events (100 000-200 000) were collected for each patient sample.
Statistical methods
Reactivation and antiviral use were analyzed by proportional hazards regression.39 Models were fit using the coxph function within the R statistical programming language, treating reactivation as time-to-event data, and antiviral use as recurrent events. The product-limit estimator was used to estimate survival functions from censored time-to-event data, and the cumulative distribution of recurrent antiviral use was estimated as the complement of the estimator of Kalbfleisch and Prentice.40 Both estimates were computed in R using the survfit function. These survival-time methods accommodate variation in time at risk and analysis of recurrent events. Wald tests within a multivariable model were used to evaluate the effect of donor CMV status while adjusting for baseline and time-dependent covariables. A propensity score41 was used to simultaneously adjust for baseline covariables, as discussed below. Simpler comparisons, such as Fisher exact and rank-sum tests, were also used where noted.
IFN-γ–CD8+ T-cell assays were performed on blood samples obtained for a subset of 135 R+ recipients. The longitudinal patterns of IFN-γ–CD8+ T-cell percentages were explored graphically and analyzed using linear models on a logarithmic scale, fit by the generalized estimating equation (gee) method using an independent working correlation.42 The generalized estimating equation and geepack packages for the R programming language were used for model fitting. This method accounts for the stochastic dependence of repeated measures on the same subject. Wald tests were calculated by dividing parameter estimates by their respective standard errors (SE), conservatively using the so-called naive SE if the GEE robust SE was smaller. The effect of donor CMV status was modeled simultaneously with the propensity score to accommodate pretreatment covariates, with additional adjustment for days posttransplant (DPT), prior reactivation, and GVHD. Interactions were modeled by the standard device of including product terms in the design matrix. Simultaneous adjustment for individual pretreatment covariates was used as an alternative to the propensity score, to check robustness.
Multifunctional CD8+ T-cell assays were evaluated on samples from 62 patients. Analysis of this subset addresses the effect of multifunctional T cells as a percentage of IFN-γ–CD8+ T cells, conditional on adequate numbers of the latter. GEE models were fit to accommodate the stochastic dependence of the longitudinal measurements. A square-root transformation was used to reduce skewness. Linear and logarithmic scales were also analyzed to assure robustness of findings. The effect of donor CMV status was adjusted for time-dependent covariables, including DPT, maximum steroid dose in the preceding 14 days, CMV reactivation, and the covariates established at HCT. The latter group of covariates was combined into a propensity score by fitting a logistic regression for donor CMV status41 using the subset of 62 patients.
Covariate adjustments in both proportional hazards and GEE models involved the use of propensity scores to adjust for potentially confounding factors associated with the patient or HCT procedure. For a variable to be confounding, it must be associated with the risk factor (ie, donor CMV status) and the outcome of interest. Statistical adjustment using a propensity score reduces confounding by addressing associations between covariates and risk factor. The propensity score is computed by fitting a logistic regression of donor CMV status on all of the potentially confounding covariates. This yields a score based on all covariates, which is then used for statistical adjustment via standard models. GVHD was treated as a time-dependent covariate.
Sampling considerations
Separate propensity scores were computed for each of the 3 subsamples (Table 1), as it is the actual imbalances in each sample that may require adjustment. The effect of subsampling on the distribution of covariates was subjected to statistical testing, as a screen for unanticipated selection effects. In addition to testing each covariate for association with subgroup membership, its association with donor CMV status and the joint association of covariate, donor CMV status, and subgroup were tested as a screen for possible systematic changes in covariate balance. Pearson χ2 was used for the categorical variables, and t tests were used for age. The full sample of 178 R+ patients, and the subsample of 135 R+ recipients with available blood samples, can both be regarded as unselected and unbiased cohorts of R+ HCT patients. The subset of 62 patients is a conditional sample, requiring minimal levels of IFN-γ–CD8+ T cells. Although all 135 recipients with blood samples might have been analyzed by assuming that low levels of IFN-γ–CD8+ T cells imply levels of multifunctional T cells below detection limits, such an approach would not distinguish effects on the numbers of IFN-γ–CD8+ T cells from effects on the fraction that are multifunctional. The subset of 62 patients addresses the latter question, which involves a distinct, conditional population.
Results
Pretransplant patient characteristics
HCT donors and recipients were enrolled in a longitudinal study investigating the role of adaptive immunity in limiting CMV sequelae.37 Table 1 summarizes pretransplant characteristics (upper half) and clinical outcomes (lower half) by donor type, within each of the 3 nested sets of transplants that are distinguished in this study. Group 1 (n = 178) includes the full set of R+ recipients who were followed for clinical outcome measures. Group 2 (n = 135) is the subset composed of all recipients who had blood samples available for monitoring IFN-γ–CD8+ T cells. Group 3 (n = 62) is a subset based solely on additional immunologic studies that were conducted and discussed later in this report. The most prominent pretransplant difference between D+/R+ and D−/R+ recipients in groups 1 to 3 was the higher percentage of unrelated D− donors, and the correspondingly higher number of sibling D+ donors. This difference is well beyond random variation (P < .001) and may reflect the similarity of CMV status among siblings. The relation of donor and recipient would confound the comparison of D+/R+ with D−/R+ transplants for any end point that is strongly associated with the relation, and we have observed that donor relation explains most of the difference between survival of D+/R+ and D−/R+ HCT recipients (data not shown). Donor relation is not, however, associated with any of the end points described in this report, and adjustment for donor relation did not alter any of our conclusions. None of the other pretransplant factors (Table 1) showed evidence of systematic differences between the D+/R+ and D−/R+ transplants in groups 1-3 (P > .2 generally, P = .09 for total body irradiation [TBI] in group 2 only). Neither did pretransplant factors show any statistical evidence of association with group membership, nor was subgroup definition associated with systematic variation in the balance of pretransplant factors across donor types. Although the validity of simple comparison of D+/R+ versus D−/R+ transplants in any of groups 1 to 3 is supported by the screening tests, and the lack of association of donor relation with the outcomes of interest, we also used statistical adjustment via propensity scores to adjust for the actual imbalances in all pretreatment covariables, regardless of statistical significance.
Clinical virology outcomes
The average length of follow-up was 10.4 months for D+/R+ recipients and 9.2 months for D−/R+ recipients, a small but statistically significant difference (P = .01, 2-sided rank-sum test). Consequently, statistical methods for censored event times were used, as these accommodate differences in time under study, as well as incorporating time-of-event information. However, most of the difference in follow-up developed late, after most of the events of interest had already occurred, so simple comparisons of proportions should have little bias. Component measures of CMV reactivation are shown in Table 1. All are more frequent in D−/R+ recipients, although most are not statistically significant when taken individually. Detection of viremia by PCR is one of the signals for treatment with GCV, and the hazard ratio for a first positive PCR was estimated between 0.85 and 1.9 (with 95% confidence, unadjusted). GCV was required by 56% of D−/R+ recipients, compared with 44% of D+R+ recipients (P = .05, by log-rank test). The estimated hazard ratio is 1.5, with 95% confidence limits of 0.99 and 2.5. Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) shows the Kaplan-Meier estimates of the probability of remaining GCV-free during the 1-year observation period for both donor groups. However, the statistical significance of this finding is not robust to adjustment for confounding variables via the propensity score (P = .18).
Association of donor CMV status with antiviral usage
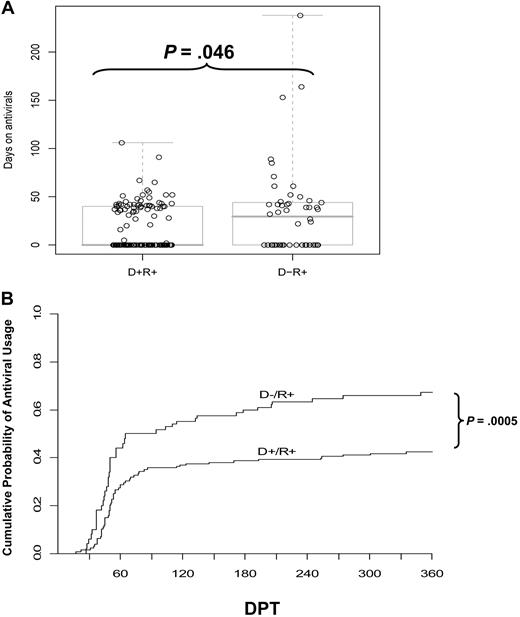
Treatment of HCT recipients in response to PCR detection of CMV reactivation often requires multiple rounds of antivirals. Prolonged or recurrent treatment reflects difficulty in resolving the infection and subjects a patient to an increased toxicity hazard. While the higher frequency of initiating GCV therapy in the D− transplants was of equivocal statistical significance, the average duration of therapy, once begun, was 20 days longer for D− than D+ recipients (Table 2). D− recipients were also twice as likely to require antivirals for more than 6 weeks and more than twice as likely to require more than 7 weeks of antiviral therapy (Table 2). The tendency of D− recipients toward more days on antivirals was statistically significant (P < .05, 2-sided rank-sum test). The total days on antiviral drugs are shown in Figure 1A, overlayed with a pair of boxplots. The D+ group includes more recipients who never received GCV (median at zero) and fewer outliers who required antivirals for extended periods.
Summary of antiviral use
| . | D+/R+ . | D−/R+ . |
|---|---|---|
| No. of HCT recipients | 128 | 50 |
| Antiviral therapy (n) | 44% (56) | 56% (28) |
| Mean duration of therapy, d | 41 | 61 |
| Mean days per subject | 18 | 34 |
| > 6 weeks on antivirals (n) | 14% (18) | 28% (14) |
| > 7 weeks on antivirals (n) | 8% (10) | 20% (10) |
| Recurrent treatment (n) | 0.7% (1) | 16% (8) |
| . | D+/R+ . | D−/R+ . |
|---|---|---|
| No. of HCT recipients | 128 | 50 |
| Antiviral therapy (n) | 44% (56) | 56% (28) |
| Mean duration of therapy, d | 41 | 61 |
| Mean days per subject | 18 | 34 |
| > 6 weeks on antivirals (n) | 14% (18) | 28% (14) |
| > 7 weeks on antivirals (n) | 8% (10) | 20% (10) |
| Recurrent treatment (n) | 0.7% (1) | 16% (8) |
Antiviral therapy administration is summarized. The numbers (n) initiating antiviral therapy (GCV or FOS), duration of therapy, and recurrent treatment (after a 14-day hiatus) are presented.
Impact of donor CMV status on antiviral use. (A) The number of days that each D+/R+ or D−/R+ recipient received antiviral drug therapy (GCV or foscarnet). The box plot overlays show the median and the central 50% of the data. The median is zero for the D+/R+ group, as the majority did not require antivirals. P value determined by rank-sum test. (B) The cumulative incidence of antiviral use, estimated by the method of Kalbfleisch and Prentice,40 incorporates recurrent events. Most events occur between 30 to 90 days posttransplant, but the separation of the curves continues to increase, as late events occur more frequently in the D− group. The excess risk in the D− group was statistically significant, adjusted for pre-HCT covariates (P < .001) and for GVHD (P = .009, Table 3). DPT, days posttransplant.
Impact of donor CMV status on antiviral use. (A) The number of days that each D+/R+ or D−/R+ recipient received antiviral drug therapy (GCV or foscarnet). The box plot overlays show the median and the central 50% of the data. The median is zero for the D+/R+ group, as the majority did not require antivirals. P value determined by rank-sum test. (B) The cumulative incidence of antiviral use, estimated by the method of Kalbfleisch and Prentice,40 incorporates recurrent events. Most events occur between 30 to 90 days posttransplant, but the separation of the curves continues to increase, as late events occur more frequently in the D− group. The excess risk in the D− group was statistically significant, adjusted for pre-HCT covariates (P < .001) and for GVHD (P = .009, Table 3). DPT, days posttransplant.
Association of donor CMV status with recurrent antiviral usage
We define recurrent antiviral usage as restarting antiviral drugs after a hiatus of more than 14 days. Subjects were regarded as at risk for a recurrent CMV event when they had gone 14 days without antivirals. The recurrent need for antivirals was almost entirely in recipients with D− donors (8 of 50 vs 1 of 128, Table 2). The difference in frequencies is highly significant (P < .001, Fisher exact test). Considering the time at risk for initial or recurrent antiviral use in a proportional hazards model leads to a similar conclusion (P < .001, likelihood ratio test), while allowing for variation in observed time at risk39 (Figure 1B). The plot of cumulative incidence shown in Figure 1B compares the D+ and D− donor groups with regard to prescribed antiviral treatments, estimated from recurrent CMV reactivation events. These estimates use the method of Kalbfleisch and Prentice and reflect differences between groups in both the need for a first course of GCV and in the recurrent need for antivirals, as defined in the legend for Table 2.40 Acute GVHD is associated with the hazard for requiring antivirals, but adjusting for GVHD has little effect (11%) on the estimated relative hazard associated with donor CMV status, which remains highly significant (Table 3). This indicates that the association of donor CMV status with antiviral usage by recipients is only minimally effected by incidence of GVHD. The excess hazard associated with a CMV-negative donor remained significant (P = .009) after adjusting for confounding factors via the propensity score (Table 3). These data are consistent with an effect of donor status on recurrent usage of antivirals, which is a critical risk factor that is known to effect survival outcomes in HCT.
Summary of estimated hazard ratios
| Model term . | P . | HR . | HR lower 95% . | HR upper 95% . |
|---|---|---|---|---|
| D−/R+ recipient | .009 | 1.8 | 1.2 | 2.9 |
| GVHD, grade II+ | .0015 | 2.1 | 1.3 | 3.3 |
| Propensity score, IQR units, for pre-HCT covariates | .6 | 1.1 | NA | NA |
| Model term . | P . | HR . | HR lower 95% . | HR upper 95% . |
|---|---|---|---|---|
| D−/R+ recipient | .009 | 1.8 | 1.2 | 2.9 |
| GVHD, grade II+ | .0015 | 2.1 | 1.3 | 3.3 |
| Propensity score, IQR units, for pre-HCT covariates | .6 | 1.1 | NA | NA |
Summary of results of a proportional hazards regression model for the hazard of requiring initial or recurrent antiviral therapy. Adjustment for baseline covariates is accomplished by inclusion of the propensity score, which is scaled to its interquartile range (IQR units) so that the estimated hazard ratio (HR) represents the effect of typical variation in baseline covariates. Methods for calculating P values can be found in “Statistical methods.”
NA indicates not applicable.
IFN-γ–CD8+ frequency in recipients with CMV-positive donors
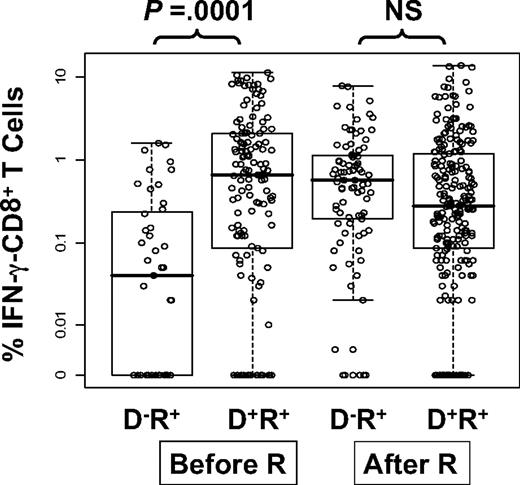
We measured immunity to CMV by flow detection of ex vivo CD8+ T cells producing IFN-γ (IFN-γ–CD8+) in response to stimulation with CMV-pp65 PepMix. Of 135 evaluated subjects, 98 received allografts from D+ and 37 from D− donors (Table 1). We modeled the level of IFN-γ–CD8+ as a function of DPT, donor CMV status, and whether CMV reactivation occurred before the sampling date. The data are displayed in Figure 2. Models were fit using the GEE method to accommodate the stochastic dependence of repeated measurements of the same subjects42 (Table 4). The major finding is that the average (geometric mean) level of IFN-γ–CD8+ T cells in D−/R+ patients without CMV reactivation is only 13% of mean levels in D+/R+ patients. However, levels of IFN-γ–CD8+ in postreactivation D−/R+ PBMC samples substantially rose and were at similar levels to PBMC samples from D+R+ patients. There was no detectable time trend (DPT row in Table 4), supporting the findings in Figure 2. All 4 groups displayed in Figure 2 were simultaneously evaluated in a model that tested for the effect of donor status on IFN-γ–CD8+ levels. Within the subset of measurements taken before reactivation, the D− donor group produced lower levels of IFN-γ–CD8+, on average, than the D+ donor group (Figure 2, P < .001, unadjusted for other factors). Of the pretransplant covariates (Table 1), only recipient age was associated with IFN-γ–CD8+ levels (P = .04) (data not shown). Furthermore, inclusion of acute GVHD in the model did not alter the significant difference of measurements made with prereactivation D−/R+ patients compared with all other groups. GVHD is not associated with IFN-γ–CD8+ levels and does not mediate the effect of CMV-negative donor status. In conclusion, taking into account pretransplant covariates and posttransplant outcomes, a substantially smaller level of IFN-γ–CD8+ is found in the D−/R+ prereactivation samples compared with all other groups (P < .001, adjusted for propensity score, DPT, GVHD in Table 4).
Levels of CMV pp65-specific IFN-γ–CD8+ in PBMC samples from R+ HCT recipients. The circles are average percentages of IFN-γ–CD8+ within PBMC samples from R+ HCT recipients in response to stimulation with CMV pp65 peptide library. All measurements from day 90 to day 360 were pooled together and divided into 4 groups according to donor CMV status and CMV reactivation post-HCT assessed by quantitative PCR (“Prospective study subjects”). The D−/R+ prereactivation group had significantly lower values compared with the other groups (P < .001, see Table 4). Within the prereactivation subset, D− has significantly lower levels than D+ (P = .002, adjusted as in Table 4, P < .001 unadjusted). The effect of donor status was significantly different in prereactivation versus postreactivation samples (P < .001 interaction test, adjusted as in Table 4). Each dot represents a single IFN-γ measurement. The lower and upper horizontal border of the box drawn for each group marks the 25th and 75th percentiles, with a solid bar at the median and whiskers covering the range of the data. Horizontal scatter within boxes is introduced to avoid overlap. Pre-R and post-R refers to CMV reactivation.
Levels of CMV pp65-specific IFN-γ–CD8+ in PBMC samples from R+ HCT recipients. The circles are average percentages of IFN-γ–CD8+ within PBMC samples from R+ HCT recipients in response to stimulation with CMV pp65 peptide library. All measurements from day 90 to day 360 were pooled together and divided into 4 groups according to donor CMV status and CMV reactivation post-HCT assessed by quantitative PCR (“Prospective study subjects”). The D−/R+ prereactivation group had significantly lower values compared with the other groups (P < .001, see Table 4). Within the prereactivation subset, D− has significantly lower levels than D+ (P = .002, adjusted as in Table 4, P < .001 unadjusted). The effect of donor status was significantly different in prereactivation versus postreactivation samples (P < .001 interaction test, adjusted as in Table 4). Each dot represents a single IFN-γ measurement. The lower and upper horizontal border of the box drawn for each group marks the 25th and 75th percentiles, with a solid bar at the median and whiskers covering the range of the data. Horizontal scatter within boxes is introduced to avoid overlap. Pre-R and post-R refers to CMV reactivation.
Model of IFN-γ–CD8 levels
| . | Coeff . | Estimate . | Effect ratio . | Robust z score . | P . |
|---|---|---|---|---|---|
| D−/R+, Pre-reactivation, N = 43 | b1 | −2.01 | 0.13 | −3.43 | .0006 |
| D+/R+, Pre-reactivation, N = 137 | b2 | 0.35 | 1.42 | 0.80 | .43 |
| D−/R+, Post-reactivation, N = 85 | b3 | 0.50 | 1.65 | 1.21 | .23 |
| DPT | b4 | 0.001 | 1.00 | 1.00 | .32 |
| Propensity score, IQR units | b5 | 0.014 | 1.01 | 0.08 | .94 |
| Acute GVHD, grade > 1 | b6 | −0.12 | 0.88 | −0.34 | .74 |
| Intercept | a | −1.54 | NA | NA | NA |
| . | Coeff . | Estimate . | Effect ratio . | Robust z score . | P . |
|---|---|---|---|---|---|
| D−/R+, Pre-reactivation, N = 43 | b1 | −2.01 | 0.13 | −3.43 | .0006 |
| D+/R+, Pre-reactivation, N = 137 | b2 | 0.35 | 1.42 | 0.80 | .43 |
| D−/R+, Post-reactivation, N = 85 | b3 | 0.50 | 1.65 | 1.21 | .23 |
| DPT | b4 | 0.001 | 1.00 | 1.00 | .32 |
| Propensity score, IQR units | b5 | 0.014 | 1.01 | 0.08 | .94 |
| Acute GVHD, grade > 1 | b6 | −0.12 | 0.88 | −0.34 | .74 |
| Intercept | a | −1.54 | NA | NA | NA |
Coefficients (Coeff) are additive terms in the model for the natural logarithm of T-cell levels, fit by gee.42 The model for the expected log measurement is of the form L = a + b1x1 + … +b6x6, where x1, x2, and x3, each take the value 1 or 0 indicating whether or not the observation has the respective combination of donor status and reactivation status listed in the first three lines of the table; where x4 is the day posttransplantation, x5 is the propensity score, adjusting for baseline covariates, and x6 is 1 if acute GVHD has occurred and 0 otherwise. The estimated coefficients b1…b6 are shown in the third column. The first three coefficients (D−/R+, Pre-reactivation; D+R+, Pre-reactivation; D−R+, Post-reactivation) are comparisons to the 225 D+/R+ post-reactivation measurements of IFN-γ–CD8, which were taken as the reference group (see Figure 2). The coefficient “Acute GVHD, grade > 1” is the adjusted average difference of those with and without acute GVHD. The coefficients “DPT” and “Propensity Score” multiply those variables in the model, and the intercept serves to calibrate the overall predicted level of IFN-γ–CD8 T cells. Effect ratios are the antilog of the estimated coefficients (eg, the antilog of b1 is 0.13, meaning that “D−R+, Pre-reactivation” samples averaged 13% of the mean for the reference group). Robust z score is the coefficient estimate divided by its robust SE. An absolute (positive or negative) z score greater than 2 is statistically significant. The P value is obtained by referring the robust z score to a normal distribution and multiplying the tail area by 2, yielding the 2-sided Wald-test significance probability.
DPT indicates days posttransplantation.
Analysis and gating scheme for CD8+ T cells expressing multiple cytokines and CD107
We observed IFN-γ levels to be similar, on average, in D+/R+ and D−/R+ recipients who experienced CMV reactivation (Figure 2). However, the D+/R+ group had less frequent need for GCV (Figures 1A,B). We speculated that this difference based on donor status might be explained by functional characteristics other than IFN-γ levels of CMV-specific T cells. Therefore, we investigated functional properties of CD8+ T cells in a subset of 62 HCT recipients (group 3, Table 1) who had available lymphocyte samples at day 90, day 180, or day 360 post-HCT with measurable IFN-γ–CD8+ greater than 0.2% of CD8+ T cells. Eligibility criteria was solely conditional on a measurable quantity of IFN-γ–CD8+. The immunologic analysis consisted of an expanded set of T-cell functional parameters (IFN-γ, TNF-α, CD107a/b, and MIP-1β) measured simultaneously using 6-color flow cytometry.34,43 Figure S2 shows the gating scheme. A primary gate was placed on lymphocytes by forward and side scatter, and a second gate on CD3+/CD8+ T cells (Figure S2A). These cells were then analyzed for coexpression of IFN-γ, TNF-α, CD107a/b, and MIP-1β by Boolean gating. Representative examples of D+/R+ (Figure S2B) and D−/R+ subjects (Figure S2C) are shown. Mock stimulated samples sometimes contained low levels of CD8+ T cells that were positive for only IFN-γ, MIP-1β, or CD107 (Figure S2B and C, bottom rows of plots). These single-positive populations were thus considered nonantigen-specific, and their levels were subtracted from the observed levels of pp65-stimulated T cells.
D+/R+ recipients maintain higher levels of CMV-pp65 CD8+ T-cell subsets with multiple functions than D−/R+
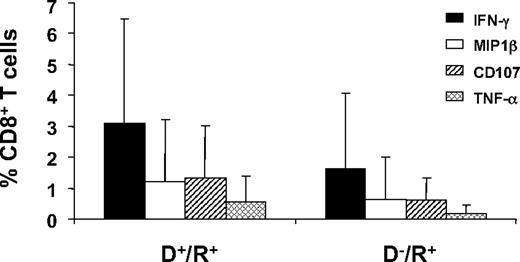
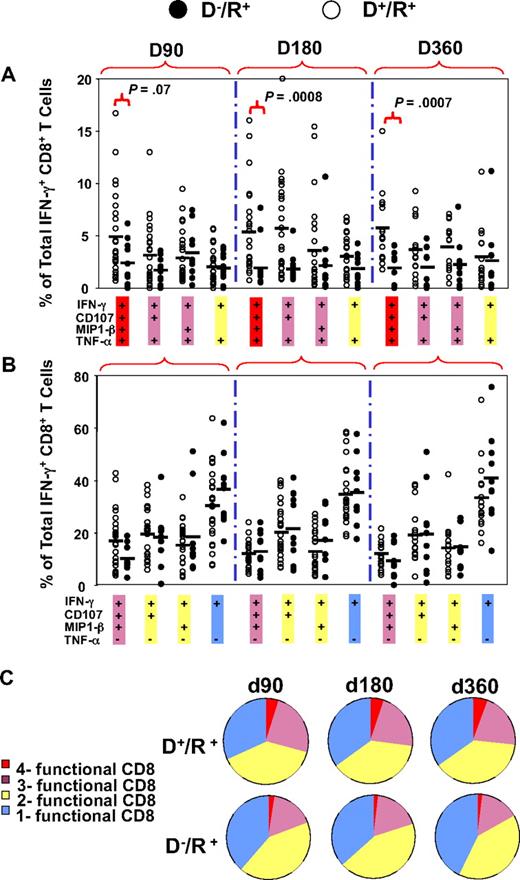
An initial evaluation of the expression of 4 markers that were followed in the subset of 62 patients (group 3, Table 1) was done as individual staining of each marker on PBMC and grouping all longitudinal measurements (Figure 3). In all 62 subjects tested, CD8+ T-cell subsets producing TNF-α, MIP-1β, or CD107a/b, were present at a lower frequency than CD8+ T-cell subsets producing IFN-γ (Figure 3). Among the 4 markers evaluated, only IFN-γ levels were significantly different based on donor CMV status, and only when all 3 observation times were combined (in contrast, see Figure 4B). Therefore, we examined another approach to comparing T-cell phenotypes by measuring subsets of IFN-γ–CD8+ composed of 8 different combinations of all 4 markers, while graphically comparing D−/R+ and D+/R+ patients for differences in frequencies of all subsets (Figure 4). We refer to the left-most points for each longitudinal comparison shown in Figure 4A as “4-functional” T cells. T cells positive for TNF-α were not common, with medians in the 1% to 5% range for each combination of markers that includes TNF-α (Figure 4A). D+/R+ subjects reconstituted significantly higher levels of 4-functional CD8+ T cells at day 180 (P < .001) and day 360 (P < .001), with weaker evidence (P = .07) at day 90 (Figure 4A). In contrast, higher levels of subsets lacking TNF-α were not correlated with CMV-positive donor status (Figure 4B). In summary, CMV-pp65 CD8+ T-cell populations from D+/R+ recipients contained higher levels of 4-functional and 3-functional subsets than D−/R+ recipients, whose CD8+ T cells expressed fewer functions, analogous to HIV progressors34 (Figure 4C). Levels of IFN-γ+-CD8+ were comparable, albeit highly variable, in D−/R+ and D+/R+ subjects who reactivated CMV (Figure 2).
CD8+ T cells in D+/R+ and D−/R+ HCT recipients expressing each of 4 functional markers. The average percentage of CD8+ T cells in samples from D+/R+ (n = 41) and D−/R+ (n = 21) HCT recipients (Table 1) that were positive for each of the markers IFN-γ, MIP-1β, CD107, or TNF-α are shown as separate bars. The data bars represent an average of measurements from all 3 time points in all HCT recipients in group 3 (Table 1). Error bars illustrate the range of measurements for each marker.
CD8+ T cells in D+/R+ and D−/R+ HCT recipients expressing each of 4 functional markers. The average percentage of CD8+ T cells in samples from D+/R+ (n = 41) and D−/R+ (n = 21) HCT recipients (Table 1) that were positive for each of the markers IFN-γ, MIP-1β, CD107, or TNF-α are shown as separate bars. The data bars represent an average of measurements from all 3 time points in all HCT recipients in group 3 (Table 1). Error bars illustrate the range of measurements for each marker.
Comparison of levels of multifunctional CD8+ T cells in D+/R+ and D−/R+ HCT recipients. The percentages of 8 combinations of single-, double-, triple-, and quadruple-functional subsets within the total population of IFN-γ–CD8+ were compared between D+/R+ and D−/R+ recipients at 3 time points after HCT. Each dot represents an individual measurement. Average percentages are shown as horizontal black bars. Four combinations of TNF-α+ subsets are shown in (A) and 4 combinations of TNF-α− subsets are shown in panel B. (C) All 8 possible combinations of response categories are summarized and shown in the pie chart, in which each slice of the pie represents the average response across all individual samples. The fraction of the total IFN-γ–CD8+ response for 8 different functional marker combinations are shown sequentially in different colors from single-functional cells (blue) through double-functional (yellow), triple-functional (pink), and quadruple-functional (red).
Comparison of levels of multifunctional CD8+ T cells in D+/R+ and D−/R+ HCT recipients. The percentages of 8 combinations of single-, double-, triple-, and quadruple-functional subsets within the total population of IFN-γ–CD8+ were compared between D+/R+ and D−/R+ recipients at 3 time points after HCT. Each dot represents an individual measurement. Average percentages are shown as horizontal black bars. Four combinations of TNF-α+ subsets are shown in (A) and 4 combinations of TNF-α− subsets are shown in panel B. (C) All 8 possible combinations of response categories are summarized and shown in the pie chart, in which each slice of the pie represents the average response across all individual samples. The fraction of the total IFN-γ–CD8+ response for 8 different functional marker combinations are shown sequentially in different colors from single-functional cells (blue) through double-functional (yellow), triple-functional (pink), and quadruple-functional (red).
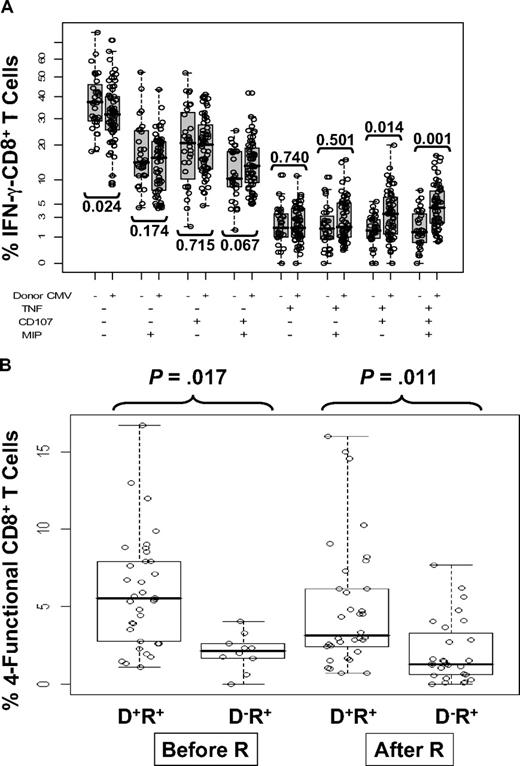
We used a more statistical approach of highlighting the differences in phenotypic subsets of IFN-γ–CD8+ by testing association of donor status with all 8 marker combinations in a GEE model on a transformed scale (Figure 5A). This test was statistically significant (P < .001, on 8 degrees of freedom), justifying further analysis. Examination of the 8 immune marker combinations, averaged over time, showed that D− recipients had, on average, a substantially higher fraction of IFN-γ–CD8+ that were negative for all other markers (40.6% in D− vs 32% in D+) and relatively fewer IFN-γ–CD8+ that were positive for at least 2 of the other markers (Figure 5A). The difference in T cells positive for all markers was striking (mean 1.8% in D− vs 4.7% in D+, rank-sum test P < .001). Individual comparisons shown in Figure 5A also showed striking differences exemplified by comparing triple-positive CD8 (IFN-γ, TNF-α, and CD107a/b) T cells in recipients with D− and D+ donors (P = .014) as well as 4-functional T cells (P = .001). Because CMV reactivation influenced IFN-γ–CD8+ levels differently depending on donor status, we analyzed 4-functional results similarly to Figure 2 as shown in Figure 5B. In addition, the effects of steroids were added to the model as described in the next section. The unadjusted P values shown in Figure 5B show a clear difference based on CMV status that affects levels of 4-functional T cells, either prior or postreactivation.
Relationship between multifunctional CMV-specific CD8+ T cell levels, CMV reactivation, and donor CMV status. (A) The 8 combinations of T cells positive or negative for TNF-α, CD107, and MIP are shown as a percentage of IFN-γ–CD8+ T cells and subdivided according to donor CMV status. Each circle represents a single measurement, and samples from all 3 time points are pooled for display. The vertical scale uses square-root spacing, labeled with percentages. Boxes cover the central 50% of the observations, with a central bar at the median. Significance probabilities (P), shown for each subset are from a 2-sided rank-sum test after reducing the data to a single mean per subject. (B) The percentages of 4-functional CD8+ T cells from D+/R+ and D−/R+ recipients were further divided according to CMV reactivation status as defined by PCR. There was no statistically significant time trend, so samples from 3 time points were pooled for display from day 90, day 180, and day 360 observations. Each dot represents an individual measurement. Boxes cover the central 50% of the observations, with a central bar at the median. Nomenclature for patients and CMV reactivation defined in Figure 2 legend. The P values are from rank-sum tests on the pre- or postreactivation subsets, using one mean value for each subject, and are not adjusted. Using all data and adjusting for covariates, recipients with D− donors have significantly lower average 4-function T cell levels (P = .01, Table 5).
Relationship between multifunctional CMV-specific CD8+ T cell levels, CMV reactivation, and donor CMV status. (A) The 8 combinations of T cells positive or negative for TNF-α, CD107, and MIP are shown as a percentage of IFN-γ–CD8+ T cells and subdivided according to donor CMV status. Each circle represents a single measurement, and samples from all 3 time points are pooled for display. The vertical scale uses square-root spacing, labeled with percentages. Boxes cover the central 50% of the observations, with a central bar at the median. Significance probabilities (P), shown for each subset are from a 2-sided rank-sum test after reducing the data to a single mean per subject. (B) The percentages of 4-functional CD8+ T cells from D+/R+ and D−/R+ recipients were further divided according to CMV reactivation status as defined by PCR. There was no statistically significant time trend, so samples from 3 time points were pooled for display from day 90, day 180, and day 360 observations. Each dot represents an individual measurement. Boxes cover the central 50% of the observations, with a central bar at the median. Nomenclature for patients and CMV reactivation defined in Figure 2 legend. The P values are from rank-sum tests on the pre- or postreactivation subsets, using one mean value for each subject, and are not adjusted. Using all data and adjusting for covariates, recipients with D− donors have significantly lower average 4-function T cell levels (P = .01, Table 5).
Multivariate analysis of hazards that influence immunologic reconstitution
Steroids are given in response to a diagnosis of acute or chronic GVHD, and their use at levels greater than 1.0 mg/kg patient weight is known to suppress immune responses.44 We calculated the dose level of administered steroids by dividing the amount taken by the weight of the patient, and recording the maximum, in mg/kg, in a 14-day window before each blood sampling time point (Table 1). Most steroid doses in our sample were below 1.0 mg/kg. Nonetheless, we fit a model to test for the effect of donor CMV status on the levels of 4-function T cells while adjusting for steroid dose, DPT, reactivation status, and pretransplant covariates (ie, propensity score). To accommodate the stochastic dependence of repeated measurements of the same subjects, we fit the model using the GEE approach.42 Our initial analysis used a square root transformation to remove skewness, but the findings with regard to donor CMV status were robust to the scale used. The fitted model estimates and test statistics are shown in Table 5, using the original scale for ease of interpretation. Donor CMV status was significantly associated with 4-function CD8+ T-cell levels (P < .003 on all scales, GEE Wald test), adjusting for all pretransplant covariates via a propensity score,41 and simultaneously adjusting for prior CMV reactivation, DPT, and steroid dose. The model-adjusted T-cell deficit associated with D− donors is 2.08, which corresponds to 62% lower levels of 4-function T cells in the D−/R+ than D+/R+ recipients. Omitting the adjustment for steroid dose, which was not significantly associated with T-cell levels (P > .34, all scales, Wald test), allowed the recovery of 11 observations that had missing steroid data, which gave stronger evidence of association of donor status and T-cell levels (P < .001, square-root scale, Wald test). Substituting GVHD (grades 2-4) for steroid usage in the model did not statistically alter the results or conclusions reached (data not shown). The association of donor status with 4-function CD8 T-cell levels was robust to the method of covariate adjustment, remaining statistically significant when the adjustment for the pretransplant covariates was also made via simultaneous inclusion of covariates in a large multiple regression model (P < .02, all scales, Wald test). Most of the variables included in the large model had no evidence of any relationship to multifunctional T-cell levels. There was some evidence that reactivation and donor sex may be weakly associated with multifunctional T-cell levels, but these findings were not robust to adjustments for other variables. Only donor CMV status was consistently associated with multifunction T-cell levels.
Model of D− donor effect on levels of 4-functional T cells, adjusted for pre- and posttransplantation factors
| . | Estimate . | Robust SE . | Robust z . | P . |
|---|---|---|---|---|
| Intercept | 4.61 | 0.88 | NA | NA |
| Propensity | −0.51 | 0.33 | −1.54 | .12 |
| CMV reactivation | −1.31 | 0.85 | −1.55 | .12 |
| Steroids (> 1.0 mg/kg) | 1.08 | 1.25 | 0.87 | .38 |
| DPT | 0.003 | 0.003 | 0.94 | .35 |
| D− donor | −2.08 | 0.82 | −2.53 | .01 |
| . | Estimate . | Robust SE . | Robust z . | P . |
|---|---|---|---|---|
| Intercept | 4.61 | 0.88 | NA | NA |
| Propensity | −0.51 | 0.33 | −1.54 | .12 |
| CMV reactivation | −1.31 | 0.85 | −1.55 | .12 |
| Steroids (> 1.0 mg/kg) | 1.08 | 1.25 | 0.87 | .38 |
| DPT | 0.003 | 0.003 | 0.94 | .35 |
| D− donor | −2.08 | 0.82 | −2.53 | .01 |
The estimates are additive terms in a linear model predicting the percentage (untransformed) of 4-functional T cells. The estimate column gives the estimated regression coefficient for each term in the model. CMV reactivation, steroids and D− donor were coded as binary (0 or 1) indicator variables, so each of these estimates is a comparison of two groups. The estimated deficit associated with CMV-negative donors is 2.08, which is conservative compared to the crude difference of means (5.44-2.06) for the two donor CMV-status groups. The Robust z column is the estimate divided by the robust SE, a z-score greater than 2 in absolute value being statistically significant. The robust z is referred to a normal distribution, and the tail area multiplied by 2 to yield the 2-sided Wald-test P value. Only the D− donor effect was statistically significant, but other terms were included to adjust for potential confounding. The D− donor effect was stronger and more significant in all models in which non-significant terms were dropped.
NA indicates not applicable.
Discussion
Reconstitution of CMV-specific cellular immunity post-HCT is a critical determinant of the control of CMV infection. We hypothesized that donor CMV status plays a major role in the extent and quality of CMV-specific immunity, which will have profound effects on patient outcomes. The major clinical outcome that was influenced by donor CMV status was control of CMV infection as indicated by treatment using antivirals. Interestingly, initial CMV reactivation as measured by PCR showed no strong differences that were dependent on donor CMV status, after accounting for confounders. This indicates that donor immunity is important for control of CMV infection, but is insufficient or uninvolved in control of CMV reactivation. In this regard, initial GCV usage, an event tied to CMV reactivation, was weakly but not robustly associated with donor CMV status. However, the major clinical finding was the strong association of duration of initial GCV therapy and recurrent GCV use tied to donor CMV status. This association was evident both in simple comparisons and in multivariable statistical models. Neither GVHD incidence nor pretransplant covariates could account for the large influence of donor CMV status. These results suggest that the initial barrier to CMV reactivation is not strongly influenced by the quality of CMV-specific adaptive immunity transferred to the recipient. However, duration of CMV complications treatable by GCV and recurrence of CMV infection may require more mature CMV-specific immunity provided to the recipient by a CMV-positive donor (see last paragraph on this page).
We focused our immunologic analysis on the CMV-pp65 antigen, given its well-characterized role as an immunodominant target of the CD8+ T-cell response to CMV. We acknowledge that other CMV antigens, including the immediate early 1 (IE1) protein, may also serve as important targets of the CMV-specific immune response.45 However, in an earlier study of HCT patients, we measured reduced immunologic function of IE1-specific T cells, that was significantly discordant in comparative measurements of pp65 trifunctional T-cell populations at day 180.33 In addition, low levels of IE1-specific IFN-γ–CD8+ T cells until day 180 suggested to us that we should focus on the pp65-specific T-cell response in a longitudinal study in which immunologic measurements started at day 90. In our study, D+/R+ recipients were generally able to reconstitute anti-CMV T-cell immunity even in the absence of detectable CMV reactivation. The typically high levels of functional CD8+ T cells in D+/R+ patients at day 90 post-HCT corresponds to the reduced CMV reactivation in that group. In D−/R+ patients, reconstitution of CMV-specific cellular immunity was dependent on CMV antigen exposure during reactivation, and CD8+ T cells generated after acute infection showed lower functionality through day 360. In contrast, both D−/R+ and D+/R+ recipients produced comparable levels of IFN-γ–producing T cells in response to reactivation, which occurred as early as day 40. However, before reactivation, D−/R+ recipients had lower levels of IFN-γ–producing T cells. This is consistent with other investigations.44
We have gone beyond single cytokine monitoring of T-cell function by simultaneously assessing IFN-γ, TNF-α, MIP-1β, and CD107a/b in the same cell, thereby revealing new associations of donor CMV status and quality of T-cell immunity. Our results indicate that D+/R+ recipients reconstitute multifunctional CD8+ T cells rapidly post-HCT. Very few CMV-specific CD8+ T cells from D−/R+ recipients simultaneously expressed 4 functional markers, and the majority of them only expressed 1 or 2 cytokines or only had CD107 function. This dramatic difference compared with D+/R+ recipients lasted until at least day 360, regardless of CMV reactivation status. Early reconstitution of highly differentiated CD8+ T cells in D+/R+ recipients post-HCT could result from direct expansion of CMV-specific memory CD8+ T cells from D+ donors. These observations are restricted to T cell–replete HCT, as T-cell depletion (TCD) physically removes mature effectors, which impacts the tempo of T-cell reconstitution post-HCT.8,46,47 Lack of functional activity by CD8+ T cells early post-HCT in D−/R+ patients could be due to lengthier time required for differentiation from naive CD8+ T cells to effector cytotoxic T lymphocytes (CTL). However, it was unexpected that D−/R+ patients would poorly reconstitute 4-functional CTL through day 360. One explanation is the lack of cognate pp65-specific CD4+ helper T cell (TH) in these recipients due to persistent immunosuppression.48,49 In support of that contention, CD4+ T-cell responses against pp65 were consistently lower in D−/R+ recipients through day 360, compared with D+/R+ recipients (Figure S3). We did not conduct a formal study of these differences in functional levels beyond IFN-γ single cytokine measurements, although it would be instructive to do so in the future. Similar observations were made by others.50
Betts and colleagues have reported that HIV nonprogressors preferentially maintain multifunctional HIV-specific CD8+ T cells, whose frequency is inversely correlated with VL in patients with uncontrolled infection, termed progressors.34 This promising association of T-cell quality and protective function in HIV patients has been confirmed by other groups, although large-scale studies are still needed.32,35,36,51 If D−/R+ recipients are immunologically analogous to HIV progressors, this is consistent with our finding that D−/R+ recipients tend to generate or maintain fewer 4-functional T cells and require more frequent GCV usage. In contrast, levels of IFN-γ–CD8+ were comparable in all D+/R+ recipients at all time points post-HCT. Furthermore, levels of 4-functional CD8+ T cells were significantly higher at day 90 in D+/R+ patients without measurable CMV reactivation. Thus, the frequency of pp65-specific T cells producing a single cytokine may not associate with viral control; instead, the size and presence of specific functional subpopulations may be associated with protection, as is the case in several infectious disease models43,52-55 and clinically for CMV in the solid organ transplant setting.56,57 An important finding was the association of recurrent CMV infection and GCV usage in greater numbers of recipients with D− donors. Of the 9 patients (Table 2) who had recurrent GCV usage, only 5 of them had parallel, but incomplete immunologic analyses in which we could assess a connection with depressed levels of 4 functional T cells. Consequently, no statistical treatment is possible. In all 5 cases we observed low levels of 4 functional T cells, below the mean for others in comparable groups (data not shown).
Among the 4 evaluated markers, TNF-α was least frequently detected in pp65-specific CD8+ T cells. In healthy CMV-positive subjects, concomitant production of TNF-α and IFN-γ dominated the effector subset of CD8+ T cells (∼ 70% of total cytokine-producing T cells, W.Z., S.F.L., D.J.D., unpublished observations, 2008). Ozdemir et al found that CMV antigenemia following allo-HCT was associated with the presence of dysfunctional antigen-specific CD8+ T cells that were unable to produce TNF-α.60 It has also been reported that in subjects infected with HIV-1, impaired TNF-α production by CMV-specific CD4+ T cells was associated with susceptibility to CMV end-organ disease, including retinitis.61 These results agree with our observations and suggest that the inability to produce TNF-α could reflect impaired T-cell function. Thus, to more accurately monitor immune responses, secretion of IFN-γ and TNF-α should be tested simultaneously to evaluate the size of the bifunctional antigen-specific CD8+ T-cell pool.32
What is the clinical impact of multifunctional CD8+ T cells in D+/R+ recipients? CMV is known to be immunosuppressive and to increase the risk of bacterial and fungal infections in HCT recipients.6,10 However, we (data not shown) and others did not find a statistically significant improvement in D+/R+ survival compared with D−/R+, with the major risk factors being the donor type and recipient disease status at HCT.1,60 The European Group for Blood and Marrow Transplantation (EBMT) working group established a pattern of association of donor CMV status with improved survival in the context of a URD transplant.10 Smaller, more recent studies uncovered a benefit of the D+ donor for limiting CMV reactivation.12,29,50 However, superior CMV-specific immunity in D+/R+ patients did not translate into improved overall survival, which suggests that other risk factors including donor type and disease status should be given priority when making treatment decisions (data not shown).4 Our conclusions are consistent with a recent review that also acknowledges that more studies are needed to resolve the differences between the National Marrow Donor Program (NMDP) and EBMT analyses.4
The finding that CMV reactivation results in a significant boost in cellular immunity in D−/R+ recipients, supports development of CMV vaccine strategies for these patients. The advantage of a D+ donor for increased frequencies of CMV-specific multifunctional CD8+ T cells may be further enhanced by vaccination as a means to boost CMV-specific T-cell levels. We are testing this hypothesis in a clinical trial of a CMV-pp65 peptide vaccine and a viral vector in development.61,62
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Public Health Service (PHS) grants CA030206 (D.J.D., S.J.F.), CA077544, and CA114889 (D.J.D.) from the National Cancer Institute (NCI), AI062496 (D.J.D.) and AI058148 (J.A.Z.) from the National Institute of Allergy and Infectious Diseases (NIAID), DK077374 (S.F.L.) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and Leukemia & Lymphoma Society (LLS) award R6145-05 (S.F.L.). The General Clinical Research Center (GCRC) is supported by M01-RR00043-39 (PHS) and the City of Hope Comprehensive Cancer Center is supported by CA033572 (PHS).
National Institutes of Health
Authorship
Contribution: W.Z. and L.T. conducted flow cytometry studies and PCR measurements; W.Z. and J.L. compiled patient data for statistical analysis; J.L., S.F.L., W.Z., and D.J.D. wrote the paper and analyzed the data; J.L. and J.M.P. conducted statistical analysis; J.L. edited the manuscript for statistical accuracy; and J.A.Z., R.S., G.G.-H., R.N., and S.J.F. wrote and supported the clinical protocol or edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Don J. Diamond, Rm 1001B, Fox South Bldg, Laboratory of Vaccine Research, Beckman Research Institute of the City of Hope, 1500 East Duarte Rd, Duarte, CA 91010-3000; e-mail: ddiamond@coh.org.
References
Author notes
*W.Z. and J.L. contributed equally to this work and are co-first authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal