Abstract
CD1d-restricted T cells are considered to play a host protective effect in tumor immunity, yet the evidence for a role of natural killer T (NKT) cells in tumor immune surveillance has been weak and data from several tumor models has suggested that some (type II) CD1d-restricted T cells may also suppress some types of antitumor immune response. To substantiate an important role for CD1d-restricted T cells in host response to cancer, we have evaluated tumor development in p53+/− mice lacking either type I NKT cells (TCR Jα18−/−) or all CD1d-restricted T cells (CD1d−/−). Our findings support a key role for type I NKT cells in suppressing the onset of sarcomas and hematopoietic cancers caused by p53 loss but do not suggest that other CD1d-restricted T cells are critical in regulating the same tumor development.
Introduction
Although numerically a minor T-cell subset, CD1d-restricted T cells can have a significant impact on immune responses in a wide variety of settings.1,2 The seemingly paradoxical nature of CD1d-restricted T-cell biology is well illustrated in tumor immunology where in some models activation of CD1d-restricted T cells results in potent tumor immunity, whereas in other cases, mice lacking CD1d-restricted T cells have enhanced antitumor responses. CD1d-restricted T cells can be divided into 2 subsets, type I natural killer T (NKT) cells with the Vα14-Jα18 TCR rearrangement, and type II NKT cells with semidiverse TCRs.3 Activation of type I NKT cells with the synthetic glycolipid alpha-galactosylceramide (α-GalCer) can generate potent antitumor immune responses against a wide variety of tumors, and the capacity of CD1d-restricted T cells to induce antitumor immune responses has been most studied in this context.1,4 In contrast, several reports have demonstrated that CD1d-restricted T cells have a detrimental effect on the antitumor immune response.5-10 CD1d−/− mice have been reported to be resistant to the growth of various experimental tumors.5-7,9-11 As there is no way to readily identify type II NKT cells, it has been difficult to discriminate between the contributions of each cell type to CD1d-dependent phenomena. At present, the most effective means is to compare tumor growth in CD1d-deficient versus TCRJα18 chain–deficient mice.
Type I NKT cells play a protective role in the surveillance of tumors, as is evident from the increased incidence of MCA-induced fibrosarcomas in Jα18−/− mice.12-15 It is currently unclear if this finding is specific for carcinogen-induced fibrosarcomas, or if it is reflective of a general role for CD1d-restricted T cells in tumor prevention. The role of CD1d-restricted T cells has not been evaluated in more clinically relevant mouse models of cancer, and therefore it remains to be established how generally important CD1d-restricted T cells are in the development of cancers. The p53 tumor suppressor gene is an important regulator of tumorigenesis in both humans16 and mice,17 and p53+/− mice develop a broad spectrum of tumors with age, in a strain-dependent fashion. Here, the p53+/− tumor model has been used to investigate the role of CD1d-restricted T cells in modulating tumor development.
Methods
Mouse strains
B6 p53+/− mice (n > 15 backcrosses to B6) were originally obtained from Dr Alan Harris (The Walter and Eliza Hall Institute of Medical Research [WEHI], Melbourne, Australia).17 B6 Jα18−/− mice (n = 12 backcrosses to B6) were obtained from Dr Masaru Taniguchi (Chiba, Japan). B6 CD1d−/− mice (n = 11 backcrosses to B6) were obtained from Dr Louis Schofield (WEHI). B6 p53+/−CD1d−/− mice were generated at the Peter MacCallum Cancer Centre by crossing C57BL/6 CD1d−/− and p53+/− mice. B6 p53+/−Jα18−/− mice were generated at the Peter MacCallum Cancer Centre by crossing C57BL/6 Jα18−/− and p53+/− mice. For aging experiments, mice were killed when tumor size reached 100 mm2 or mice were moribund and a full autopsy was performed. Mice were housed and experiments were conducted under specific pathogen-free conditions according to the Peter MacCallum Cancer Centre Animal Experimental Ethics Committee Guidelines.
Statistical analysis
Statistical significance was assessed using Prism 5.0 (Graphpad Software, San Diego, CA). For comparisons of means, a Mann-Whitney rank sum test (nonparametric data) was used, and multigroup comparisons were performed by one-way ANOVA (Kruskal-Wallis test) followed by a Dunn posttest. Incidence data were analyzed using a Fisher exact test, and survival curves were analyzed with a log-rank test. In all cases P values less than .05 were considered significant.
Results and discussion
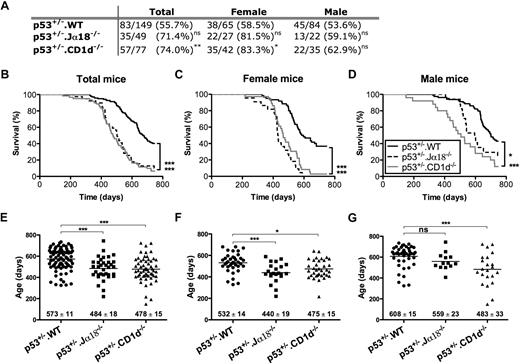
Cohorts of B6 p53+/−·WT, B6p53+/−·CD1d−/−, and B6 p53+/−·Jα18−/− mice were monitored long term for overall survival and tumor development (Figure 1). As indicated in Figure 1A, total tumor incidence (as a proportion of the starting cohort) was elevated in p53+/−.CD1d−/− mice compared with p53+/− controls (P = .009), and most prominent within the female cohort of mice. The trend for p53+/−.Jα18−/− mice was similar although not quite significantly different (P = .06). Analysis of survival times (excluding mice that died due to unknown causes) revealed even greater significance of the loss of Jα18 (P < .001) or CD1d (P ≤ .001) (Figure 1B) and again that sex influenced the survival times of mice that developed tumors (Figure 1C-D). A trend toward earlier tumor onset was noted for female mice (Figure 1C,F), compared with their genotype-matched male counterparts (Figure 1D,G). This latter finding was consistent with reports that both female mice18 and female humans19 carrying p53 mutations are more predisposed to cancer than their male counterparts. Notably, in all cases, mean survival times for p53+/−·CD1d−/− and p53+/−·Jα18−/− mice were significantly lower (P < .001) than that of their p53+/−.WT counterparts, the data further strongly supporting a role for CD1d-restricted T cells in inhibiting tumor development.
Tumor onset is accelerated in p53+/− mice lacking CD1d-restricted NKT cells. (A) Groups of p53+/− (C57BL/6 WT, CD1d−/−, and Jα18−/−) mice were aged, and the incidence of tumors (verified by histology as the proportion of the whole starting cohort with tumor) was determined after 750 days. (*P < .05; **P < .01; ns, not significant, using a Fisher exact test compared with B6 p53+/− control mice.) Survival curves depict the survival of p53+/− BL/6, p53+/−CD1d−/−, and p53+/−Jα18−/− mice (excluding the small number of those dying of unknown causes) monitored for 750 days (B). Panels (C: female) and (D: male) show survival curves of the same mice, divided on the basis of sex. Data are presented as percentage of survival over time. (*P < .05; ***P < .001, using a log-rank test compared with B6 p53+/− control mice.) The age at death of tumor burdened total (E), female (F), and male (G) mice is represented in scatterplots showing mean plus or minus SEM. Cohort sample sizes: n = 139 B6 p53+/− mice (n = 60 female and n = 79 male), n = 39 p53+/−Jα18−/− mice (n = 22 female and n = 17 male), n = 61 p53+/−CD1d−/− mice (n = 36 female and n = 25 male). (*P < .05; ***P < .001 using a Kruskal-Wallis test compared with B6 p53+/− control mice.)
Tumor onset is accelerated in p53+/− mice lacking CD1d-restricted NKT cells. (A) Groups of p53+/− (C57BL/6 WT, CD1d−/−, and Jα18−/−) mice were aged, and the incidence of tumors (verified by histology as the proportion of the whole starting cohort with tumor) was determined after 750 days. (*P < .05; **P < .01; ns, not significant, using a Fisher exact test compared with B6 p53+/− control mice.) Survival curves depict the survival of p53+/− BL/6, p53+/−CD1d−/−, and p53+/−Jα18−/− mice (excluding the small number of those dying of unknown causes) monitored for 750 days (B). Panels (C: female) and (D: male) show survival curves of the same mice, divided on the basis of sex. Data are presented as percentage of survival over time. (*P < .05; ***P < .001, using a log-rank test compared with B6 p53+/− control mice.) The age at death of tumor burdened total (E), female (F), and male (G) mice is represented in scatterplots showing mean plus or minus SEM. Cohort sample sizes: n = 139 B6 p53+/− mice (n = 60 female and n = 79 male), n = 39 p53+/−Jα18−/− mice (n = 22 female and n = 17 male), n = 61 p53+/−CD1d−/− mice (n = 36 female and n = 25 male). (*P < .05; ***P < .001 using a Kruskal-Wallis test compared with B6 p53+/− control mice.)
As can be seen from Figure 2, p53+/− mice develop a range of different tumor types classified into 5 categories (osteosarcomas; spindle cell tumors and other sarcomas; carcinomas and adenocarcinomas; other tumors; and hematopoietic tumors). Figure 2A depicts the overall tumor spectrum based on these classifications, and a breakdown of tumor types observed in male (Figure 2B) and female (Figure 2C) mice is included. There was no significant difference in the spectrum of tumors observed in the absence of TCRJα18 or CD1d. The observed tumor spectrum seems more reflective of p53 biology—osteosarcomas were the most commonly observed tumor type, consistent with a role for p53 in osteogenesis. Figures S1 through S3 (available on the Blood website; see the Supplemental Materials link at the top of the online article) show representative examples of H&E-stained tumor sections from osteosarcomas, spindle cell tumors, and other tumors such as large cell lymphoma, carcinoma/adenocarcinoma, and angiosarcomas. No distinct morphologic characteristics were noted between tumors of similar types that arose in the 3 different groups of mice (eg, osteosarcomas of diverse appearance and differing degrees of osteoid deposition were noted, but tumor appearance did not correlate with genotype, and was similarly diverse across all groups). To determine whether the tumors arising in CD1d-restricted T cell–deficient p53+/− mice were qualitatively different from tumors from p53+/−.WT mice, a limited series of lymphoid tumor lines was successfully derived from either the Jα18−/− or WT background, analyzed by flow cytometry, and tested for immunogenicity by transplant into WT and either Jα18−/− or CD1d−/− mice. Although all of the lymphoma lines derived from p53+/−.Jα18−/− mice displayed immunogenicity by comparative growth in Jα18−/− recipients compared with WT recipients (Figure S4), growth of the same lymphomas in CD1d−/− recipients was variable. By contrast, 2 lymphomas derived from p53+/−.WT mice were not overtly immunogenic. However, overall there were not sufficient numbers from each strain to determine whether p53+/− tumors were generally immunoedited by type I NKT cells. Osteosarcomas were also harvested with the intention of performing similar transplantation experiments, but they were not able to be reproducibly transplanted into secondary recipients (not shown).
Tumor spectrum in p53+/− mice lacking CD1d-restricted NKT cells. Tumor sections were analyzed by a trained pathologist in a blinded manner, and tumors were classified into 5 basic categories based on morphologic appearance: osteosarcomas, spindle cell tumors, and other sarcomas (SCT & other sarc), carcinomas and adenocarcinomas [(adeno)carcinoma], hematopoietic, and other malignancies are shown. Data are shown for all tumor burdened mice in various p53+/− strains, C57BL/6, Jα18−/−, and CD1d−/−, regardless of sex (A), and cohorts are further subdivided into both female (B) and male (C) cases.
Tumor spectrum in p53+/− mice lacking CD1d-restricted NKT cells. Tumor sections were analyzed by a trained pathologist in a blinded manner, and tumors were classified into 5 basic categories based on morphologic appearance: osteosarcomas, spindle cell tumors, and other sarcomas (SCT & other sarc), carcinomas and adenocarcinomas [(adeno)carcinoma], hematopoietic, and other malignancies are shown. Data are shown for all tumor burdened mice in various p53+/− strains, C57BL/6, Jα18−/−, and CD1d−/−, regardless of sex (A), and cohorts are further subdivided into both female (B) and male (C) cases.
Here, the role of CD1d-restricted T cells in regulating tumor development in p53+/− mice has been examined, and monitoring tumor development in these mice suggests that CD1d-restricted type I NKT cells may have a significant ability to suppress tumor onset regardless of tumor type. The remarkably similar survival time and overall incidence between p53+/−.Jα18−/− and p53+/−.CD1d−/− mice indicates that loss of type I NKT cells is dominant over the loss of type II NKT cells or the CD1d molecule itself. In contrast with several reports that describe the tumor-promoting activity of putative CD1d-restricted type II NKT cells in experimental tumor models,5-7,11,20 we found no evidence for the functional activity of these cells in this clinically relevant mouse model of cancer. In support of this conclusion, we have also found that CD1d−/− and Jα18−/− mice share very similar susceptibility to MCA-induced sarcoma development (Figure S5). Tumor development in p53+/− or p53−/− mice has previously been used to investigate the role of the immune system in controlling or preventing tumor growth. Previous reports demonstrated that α-GalCer, a type I NKT cell activating glycolipid, inhibited the development of sarcomas in p53−/− mice21 and that host interferon-γ, perforin, and TRAIL all significantly suppress the growth of tumors in p53 mutant mice.22-24 These results collectively indicate that the immune system can modulate tumorigenesis in mice deficient for p53. The establishment of an important role for type I NKT cells in this setting provides further impetus for further studying the tumor suppressive functions of these cells in humans, where thus far their function and number in tumors and appear to correlate with a more favorable prognosis in multiple myeloma25 and colorectal cancer.26
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Shannon Griffiths and Michelle Stirling for maintaining the mice at the Peter MacCallum Cancer Centre.
This work was supported by a National Health and Medical Research Council of Australia Program grant (454569; M.J.S., D.I.G.), Doherty Fellowship (A.P.U.) and Research Fellowships (M.J.S., D.I.G.), National Institutes of Health grant R01-CA106377, and an Australian Postgraduate Award (J.B.S.).
National Institutes of Health
Authorship
Contribution: J.B.S. performed research and analyzed data; A.P.U. performed research and prepared and analyzed the data; S.v.D., J.S., and W.K.M. performed research; D.I.G. wrote the paper; M.J.S. performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark J. Smyth, Cancer Immunology Program, Sir Donald and Lady Trescowthick Laboratories, Peter MacCallum Cancer Centre, Locked Bag 1, A'Beckett St, 8006, Victoria, Australia; e-mail: mark.smyth@petermac.org.

![Figure 2. Tumor spectrum in p53+/− mice lacking CD1d-restricted NKT cells. Tumor sections were analyzed by a trained pathologist in a blinded manner, and tumors were classified into 5 basic categories based on morphologic appearance: osteosarcomas, spindle cell tumors, and other sarcomas (SCT & other sarc), carcinomas and adenocarcinomas [(adeno)carcinoma], hematopoietic, and other malignancies are shown. Data are shown for all tumor burdened mice in various p53+/− strains, C57BL/6, Jα18−/−, and CD1d−/−, regardless of sex (A), and cohorts are further subdivided into both female (B) and male (C) cases.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/25/10.1182_blood-2009-01-198564/4/m_zh80200934920002.jpeg?Expires=1769110060&Signature=ACnQsNJWgvT~hADBhdpwisIyzHRzURRql4YpKzf84aVOMLjKOloAwNwAWCSnFG2J8n-X1cmXjOIr~zG~4WHJiwSdHzQDVjMsOYLYAvXZCwMgiiDJtNB12Itcj~gKK-0R6Yc6VHNQHN0MFa~q39pNUB1Hu2emjoPIk9-P8pFDn91Ci8gP-i64l0GPT2ozZj6ZtxlV4KTJVheNLjCbz3uyJbePYbIEMh51tPv5rESFFD4NgdBfkXVXlt4f92wMKpP6jx9aV-5Kd6KtikxZlooE7uKeDV689whJso06eTcZ3haaE6ROV3B7WnfiDbmYHnCEvsL63Vno8DpkfNs36MtutA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal