In this issue, Swee and colleagues show that supraphysiologic levels of Flt3L indirectly lead to proliferation of peripheral naturally occurring CD4+ T regulatory cells, and correlate these findings to prevention of death in a graft-versus-host disease model.
Dendritic cells (DCs) are a numerically small population of cells that control T-cell responses via efficient antigen presentation on major histocompatibility complex molecules in combination with finely tuned costimulatory signals.1 Fms-like tyrosine kinase 3 ligand (Flt3L) is a nonredundant cytokine required for DC homeostasis in lymphoid tissues, with its absence leading to reduction and supraphysiologic levels leading to massive expansion of DCs.2 Flt3, the receptor for Flt3L, is broadly expressed on hematopoietic progenitor cells. However, the only differentiated hematopoietic cells expressing detectable levels of Flt3 in steady state are DCs.3
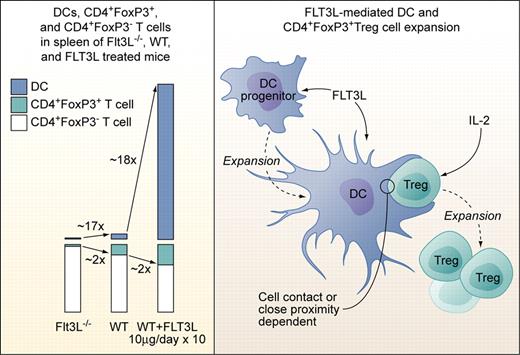
Left panel: Proportions of DCs, CD4+FoxP3+Treg cells, and CD4+FoxP3− T cells in spleens of Flt3L-deficient (Flt3L−/−), wild-type, and Flt3L-injected mice (10 μg/day i.p. or s.c. over 10 days). Right panel: Factors involved in Flt3L-mediated DC and subsequent CD4+FoxP3+Treg cell expansion. Flt3L expands DCs from DC progenitors but does not act directly on CD4+FoxP3+Treg cells. Treg expansion is dependent on exogenous IL-2 not produced by DCs, and on direct cell contact or close proximity to DCs. The factors provided by DCs to Tregs need to be defined, but Treg expansion in experimental settings used by Swee et al is not dependent on TCR-MHCII complex interaction. Professional illustration by Kenneth X. Probst.
Left panel: Proportions of DCs, CD4+FoxP3+Treg cells, and CD4+FoxP3− T cells in spleens of Flt3L-deficient (Flt3L−/−), wild-type, and Flt3L-injected mice (10 μg/day i.p. or s.c. over 10 days). Right panel: Factors involved in Flt3L-mediated DC and subsequent CD4+FoxP3+Treg cell expansion. Flt3L expands DCs from DC progenitors but does not act directly on CD4+FoxP3+Treg cells. Treg expansion is dependent on exogenous IL-2 not produced by DCs, and on direct cell contact or close proximity to DCs. The factors provided by DCs to Tregs need to be defined, but Treg expansion in experimental settings used by Swee et al is not dependent on TCR-MHCII complex interaction. Professional illustration by Kenneth X. Probst.
Naturally occurring CD4+ regulatory T cells (NTregs) are critical for suppression of autoimmunity, and the control of potentially harmful immune responses induced by non-self antigens via thus far not fully understood mechanisms. NTregs are generated from thymocytes carrying T-cell receptors (TCRs) with high affinity for self-peptide major histocompatibility class II (MHCII) complexes, express high levels of the IL-2 receptor, and specifically express the regulatory T cells (Tregs) master-regulator transcription factor FoxP3 (CD4+CD25+FoxP3+NTreg).4 NTregs, together with a smaller fraction of Tregs generated in the periphery from CD4+ T cells, account for about 10% of the total CD4+ T-cell pool. They are homeostatically maintained in steady state by some TCR-MHCII and, perhaps more importantly, costimulatory molecule interactions occurring preferentially with DCs, by IL-2, produced mostly by nonregulatory T cells and by TGFβ.4-6
In the current study, Swee et al elegantly link Flt3L, DCs, and Treg numbers and point to a potential clinical application of their findings.7 They first demonstrate that absence of Flt3L leads to a 2-fold reduction, while injection of Flt3L leads to a 2-fold increase of Tregs in mice (see figure). Total CD4+ cell numbers were not significantly affected. Flt3L-mediated Treg expansion occurred in the periphery, as thymic Treg numbers were not altered and comparable Treg expansion occurred in thymectomized mice. Transfer of non-Tregs and subsequent Flt3L injection as well as in vivo labeling of DNA in replicating cells revealed that Flt3L-induced Treg expansion was achieved by a fraction of preexisting NTregs and not by conversion of CD4+FoxP3− T cells to Tregs. Since T cells do not express functional Flt3, Flt3L-induced NTreg expansion must be indirect. NK cells, which are the second (after DCs) major fold-expanded cell population in Flt3L-injected mice, were ruled out as contributors to NTreg expansion, pointing to DCs as the likely critical cell population. Indeed, coculture experiments confirmed that DCs, in combination with non-DC–produced IL-2, induced proliferation of a fraction of NTregs. Of note, NTreg proliferation occurred only upon cell-to-cell contact or close proximity of DCs and NTregs, and was possible even in absence of MHCII expression on DCs and thus, Treg-TCR stimulation. Previous studies demonstrated a role of DCs and Tregs in modulating graft-versus-host disease (GVHD), with recipient Flt3L pretreatment before transplantation or donor Treg infusions after transplantation reducing death rates from GVHD in different model systems.8,9 Swee and colleagues tested effects of Flt3L injection in 2 parent to F1 (C57BL6- > C57BL6xDBA/2) GVHD models. Flt3L pretreatment with lymphocyte only transplantation and continued Flt3L treatment with sublethal irradiation followed by combined bone marrow and lymphocyte transplantation led to full and partial protection from GVHD, respectively. The latter was correlated with higher relative host DCs, and host and donor Treg numbers after transplantation.
This study thus reveals a view to a so far nonappreciated Flt3L-driven, numeric DC-Treg cell dependency and starts to unravel the mechanisms behind it. However, as pointed out by the authors, the findings also raise new questions: What are the cell-contact or close proximity signals exchanged between DCs and Tregs? Are low-level “costimulatory” signals without TCR signaling sufficient, given appropriate Treg IL-2R stimulation? Is restricted in vivo availability of IL-2 the limiting factor for “only” 2-fold expansion of Tregs compared with the approximately 18-fold expansion of DCs upon Flt3L administration? Does Treg expansion in a chimeric MHC-mismatched setting in combination with irradiation or chemotherapy-induced lymphopenia follow the same rules as in the syngeneic, that is, fully MHC-matched setting? Is the observed amelioration of GVHD associated with a general state of immunosuppression, equally limiting graft-versus-leukemia and possibly reactions to invading pathogens? Or could strong DC-activating agents as TLR agonists from infectious agents turn Flt3L-expanded DCs into strong stimulators of alloresponses, enhancing GVHD? And finally, how do the data on combined DC and NTreg expansion correlate with the findings that selective Treg ablation in mice leads to increase DC numbers?10 Continuing research along the lines of Swee and colleagues will likely broaden future therapeutic options in transplantation and other clinical settings requiring immune-system modulation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal