In this issue of Blood, Lundqvist and colleagues report enhanced antitumor effects of NK cells when adoptively infused into mice subsequent to treatment with bor-tezomib and depletion of regulatory T cells.
Natural killer (NK) cells were initially identified as lymphocytes capable of lysing tumor cells without prior sensitization. Meanwhile, numerous studies have established that NK cells play an important role in the immunosurveillance of tumors.1,2 With regard to their antitumor activity, NK cells abide by the law of “missing-self,” implying the preferential elimination of cells with a deficient expression of major histocompatibility complex (MHC) class I molecules. This recognition mode constitutes a fail-safe mechanism for MHC class I–restricted tumor recognition by cytotoxic T cells. In addition, triggering of NK-cell cytotoxicity requires stimulation via activating NK receptors that engage their cognate tumor-associated ligands. Apart from the intricate balance of activating and inhibitory signals provided by receptors recognizing ligands on tumor cells, additional factors, such as cytokines and interactions with other immune cells, determine NK-cell responsiveness. Ultimately, killing of tumor targets is achieved by secretion of granules containing both lytic perforin and granzymes, or by triggering death receptors (eg, by TNF-related apoptosis-inducing ligand [TRAIL]), respectively. Currently, the therapeutic potential of NK cells is exploited in the treatment of leukemia. Upon haploidentical stem cell transplantation, disparity of NK-relevant MHC class I molecules between donor and recipient is associated with an improved clinical outcome due to the failure of the recipient's leukemia cells to inhibit subsets of donor NK cells via their MHC class I–specific killer immunoglobulin-like receptors (KIRs).1 However, available data indicate that solid tumors are largely resistant to therapeutic means employing NK cells.1
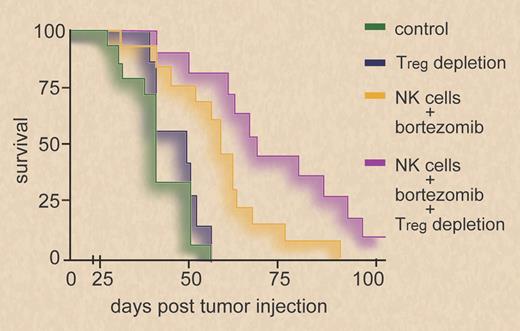
Survival of BALB/c mice inoculated with RENCA tumor cells is enhanced when mice are treated with bortezomib before adoptive infusion of syngeneic NK cells. Survival is further improved by depletion of regulatory T cells (Tregs) before bortezomib treatment and NK-cell infusion. Professional illustration by Debra T. Dartez.
Survival of BALB/c mice inoculated with RENCA tumor cells is enhanced when mice are treated with bortezomib before adoptive infusion of syngeneic NK cells. Survival is further improved by depletion of regulatory T cells (Tregs) before bortezomib treatment and NK-cell infusion. Professional illustration by Debra T. Dartez.
In this issue of Blood, Lundqvist et al provide experimental evidence (see figure) that antitumor efficacy of adoptively infused NK cells in mouse models of solid tumors can be enhanced by administration of bortezomib and depletion of regulatory T cells (Tregs).3 The proteasome inhibitor bortezomib is well established in the treatment of multiple myeloma and other malignant lymphomas due to its ability to induce apoptosis in transformed cells. However, accumulating evidence indicates that bortezomib may also bolster NK-mediated antitumor immunity. In preceding studies, Lundqvist et al observed enhanced NK-cell cytotoxicity against bortezomib-treated human tumor cell lines.4 This was attributed to increased susceptibility to TRAIL-mediated apoptosis, and sensitization to TRAIL was also reported to underlie the NK-cell stimulatory effect of bortezomib in another study employing murine NK cells.4,5 Now, the authors report that augmented NK-cell killing of bortezomib-treated mouse tumor cells was not dependent on TRAIL but mediated through the perforin/granzyme pathway.3 Could an increased susceptibility to perforin/granzyme-induced apoptosis be the central mechanism by which bortezomib augments NK-cell cytotoxicity against these mouse tumor cells, as the authors seem to suggest? Other studies indicate that bortezomib also promotes susceptibility of tumor cells toward NK-mediated cytolysis by a second, distinct mechanism: proteasome inhibition up-regulates the expression of ligands of NKG2D (NKG2DLs), thereby enhancing NK-cell cytotoxicity.6,7 NKG2D is an activating immunoreceptor on cytotoxic lymphocytes known to engage MHC I–related surface molecules induced by various forms of cell stress, including genotoxic stress, and it contributes significantly to tumor immunosurveillance.2 Of note, up-regulation of mouse NKG2DLs H60 and Mult-1 on bortezomib-treated cells was also observed in the present study. However, circumstantial evidence suggests that bortezomib may also impair cellular antitumor immunity: high doses of bortezomib have been shown to inhibit NK-cell reactivity.6 In the present study, NK-cell transfer was performed 1 day after bortezomib treatment, and thus bortezomib may not have directly affected NK-cell functionality.
Considering the variety of mechanisms by which bortezomib affects both immune and tumor cells, it will be challenging to elucidate in greater detail how bortezomib impacts tumor cell-NK cell interaction in vivo. Nevertheless, independently of the underlying mechanism(s), Lundqvist and coworkers convincingly demonstrate that in vivo application of bortezomib can serve to enhance antitumor reactivity of adoptively infused NK cells. Additional depletion of Tregs further enhanced NK-cell antitumor reactivity. This is in line with previous studies, which demonstrated that Tregs suppress NK cell–mediated antitumor activity, particularly against NKG2DL-positive tumors.8 It remains to be determined whether a combination of these treatment modalities can also boost NK reactivity in other tumor models and ultimately may be beneficial in a human setting. The present work constitutes one step forward in the attempt to exploit NK cells in the treatment of cancer by combining strategies that relieve NK-cell suppression and enhance tumor cell susceptibility to NK cytolysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal