To the editor:
We read with interest the elegant studies of Koyama and colleagues1 in which they demonstrate the ability of recipient conventional dendritic cells (cDCs) and plasmacytoid DC (pDCs) to prime allogeneic T-cell responses and initiate graft-versus-host disease (GVHD) when adoptively transferred into irradiated MHC class II−/− recipients. Intriguingly, they report that the ability of recipient pDCs to prime allogeneic donor T-cell responses is dependent on their activation by the inflammatory environment generated by total body irradiation (TBI).
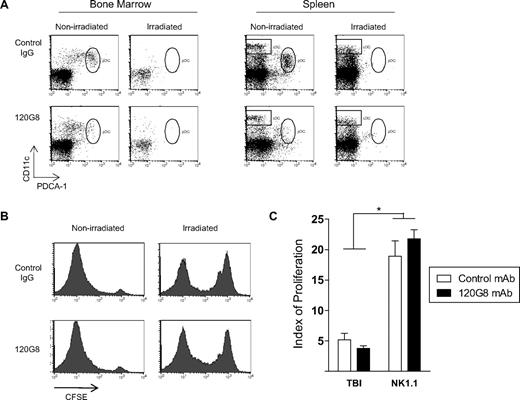
We recently investigated the role of pDCs in GVHD, and in the course of this study we were surprised to observe that recipient pDCs were exquisitely sensitive to myeloablative doses of TBI.2 As such, we were intrigued by the paradox presented by Koyama et al's report, that is, that pDCs have the capacity to present alloantigen only when activated by TBI; but in response to TBI, we have observed their complete elimination within 24 hours (Figure 1A).
Effects of recipient pDCs on donor T-cell priming. (A) B6D2F1 mice were irradiated with 1300 cGy in 2 split doses of 650 cGy, 3 hours apart. At the time of the second irradiation, mice were injected with 1 mg of the pDC-depleting mAb 120G8 or isotype control antibody MAC49. Twenty-four hours later (the usual time of transplant), bone marrow and spleen were examined for pDC and cDC content. Anti–PDCA-1 (Miltenyi Biotec, Bergisch Gladbach, Germany) and CD11c (Biolegend, San Diego, CA) mAbs were used for flow cytometric analysis. DCs were enriched using density-gradient centrifugation before examination.5 Representative plots shown. (B) B6D2F1 mice received TBI or 1 mg anti-NK1.1 as pretransplant conditioning, followed by 120G8 or control mAb. Purified carboxyfluorescein succinimidyl ester (CFSE)–labeled B6 CD45.1+CD4+ T cells were injected and proliferation of splenic T cells analyzed by CFSE dilution 3 days later. Cells were stained with CD45.1, CD4, and the vital dye 7-AAD. Histograms shown are gated on live CD45.1+CD4+ cells. No difference in alloresponse was observed in the absence of pDC in either the irradiated or nonirradiated setting. (C) Modfit software (Verity Software House, Topsham, ME) was used to quantify the extent of donor T-cell proliferation. Calculated proliferation index = Σ all cells/computed number of parent cells. Data shown representative of 3 experiments, with n = 12 and n = 7 in respective TBI and NK1.1 groups. P = .37 irradiated control mAb versus irradiated 120G8. P = .31 nonirradiated control mAb versus nonirradiated 120G8. *P < .001 irradiated versus nonirradiated control and 120G8 mAb treated.
Effects of recipient pDCs on donor T-cell priming. (A) B6D2F1 mice were irradiated with 1300 cGy in 2 split doses of 650 cGy, 3 hours apart. At the time of the second irradiation, mice were injected with 1 mg of the pDC-depleting mAb 120G8 or isotype control antibody MAC49. Twenty-four hours later (the usual time of transplant), bone marrow and spleen were examined for pDC and cDC content. Anti–PDCA-1 (Miltenyi Biotec, Bergisch Gladbach, Germany) and CD11c (Biolegend, San Diego, CA) mAbs were used for flow cytometric analysis. DCs were enriched using density-gradient centrifugation before examination.5 Representative plots shown. (B) B6D2F1 mice received TBI or 1 mg anti-NK1.1 as pretransplant conditioning, followed by 120G8 or control mAb. Purified carboxyfluorescein succinimidyl ester (CFSE)–labeled B6 CD45.1+CD4+ T cells were injected and proliferation of splenic T cells analyzed by CFSE dilution 3 days later. Cells were stained with CD45.1, CD4, and the vital dye 7-AAD. Histograms shown are gated on live CD45.1+CD4+ cells. No difference in alloresponse was observed in the absence of pDC in either the irradiated or nonirradiated setting. (C) Modfit software (Verity Software House, Topsham, ME) was used to quantify the extent of donor T-cell proliferation. Calculated proliferation index = Σ all cells/computed number of parent cells. Data shown representative of 3 experiments, with n = 12 and n = 7 in respective TBI and NK1.1 groups. P = .37 irradiated control mAb versus irradiated 120G8. P = .31 nonirradiated control mAb versus nonirradiated 120G8. *P < .001 irradiated versus nonirradiated control and 120G8 mAb treated.
To eliminate the possibility that the observed sensitivity of lymphoid organ pDCs to TBI simply reflects their trafficking into additional sites, we administered the pDC-depleting 120G8 mAb to irradiated B6D2F1 mice before the transplantation of allogeneic T cells. Equivalent donor T-cell responses were observed when mice treated with TBI were administered pDC-depleting or control antibody (Figure 1B,C). These data confirm that pDCs are systemically depleted by TBI (or alternatively, that residual pDCs remain in nonlymphoid tissue, but make no contribution to alloreactivity), and therefore strongly argue against the assertion that host pDCs play a significant role in the presentation of host alloantigen to donor T cells after TBI conditioning.
It is nonetheless possible that pDCs may play a role in priming allogeneic donor T-cell responses after non–TBI-based transplantation. We therefore also examined the effect of pDC depletion on the priming of allogeneic parental T cells when transferred into nonirradiated F1 recipients treated with NK1.1 to eliminate antidonor hybrid resistance. Consistent with the requirement for pDC activation by TBI demonstrated in the Koyama paper, no difference in donor T-cell responses were seen in the presence or absence of recipient pDCs (Figure 1B,C). As an intriguing aside, the only statistically significant finding was that donor cells injected into TBI-treated mice underwent significantly less proliferation than in the nonirradiated setting. This likely reflects the 10-fold greater numbers of host antigen-presenting cells (APCs) remaining at the time of bone marrow transplantation (BMT) in nonirradiated recipients.3,4 Because host pDCs fail to prime donor T cells in the absence of TBI conditioning1 and TBI itself rapidly depletes host pDCs, we suggest that this APC subset is unlikely to be an important population in the initiation of GVHD.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Geoffrey R. Hill, Bone Marrow Transplantation Laboratory, Queensland Institute of Medical Research, 300 Herston Rd, Herston, QLD, Australia, 4006; e-mail: geoff.hill@qimr.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal