Abstract
Cerebrovascular and cardiovascular diseases are a major cause of morbidity and mortality. Soluble P-selectin (sP-selectin) is a biomarker for platelet/endothelial activation and is considered a risk factor for vascular disease. sP-selectin enhances procoagulant activity by inducing leukocyte-derived microparticle production and promotes activation of leukocyte integrins. However, it is not known whether it directly contributes to vascular complications. We investigated the effect of increased levels of sP-selectin on blood-brain barrier (BBB) function, stroke outcome, and atherosclerosis by comparing wild-type mice with P-selΔCT/ΔCT mice in which the endogenous P-selectin gene was replaced with a mutant that produces abnormally high plasma levels of sP-selectin. P-selΔCT/ΔCT mice presented several abnormalities, including (1) higher BBB permeability, with 25% of the animals showing differential permeability between the right and left hemispheres; (2) altered social behavior with increased aggression; (3) larger infarcts in the middle cerebral artery occlusion ischemic stroke model; and (4) increased susceptibility to atherosclerotic, macrophage-rich lesion development in both male and female mice on the apoE−/− genetic background. Thus, elevated sP-selectin is not only a biomarker for vascular disease, but also may contribute directly to atherosclerosis and cerebrovascular complications.
Introduction
P-selectin is a cell surface adhesion molecule stored in the membranes of platelets' α-granules and in the Weibel-Palade bodies of endothelial cells.1,2 It is expressed on the cell surface upon granule exocytosis and plays an essential role in the initial recruitment of leukocytes to the sites of injury during inflammation.3 P-selectin consists of an N-terminal lectin domain, followed by an epidermal growth factor–like motif, a series of short consensus repeats, a transmembrane domain, and a short cytoplasmic tail.4 Lectin and epidermal growth factor domains are required to bind P-selectin glycoprotein ligand-1 (PSGL-1) on leukocytes, an interaction critical for leukocyte rolling and capable of leukocyte activation.5 The cytoplasmic domain of P-selectin is essential for its storage in Weibel-Palade bodies and its internalization from the plasma membrane.6 The membrane form of P-selectin has been shown to be involved in both inflammation and thrombosis.7 P-selectin–deficient mice are defective in leukocyte rolling, extravasation, and hemostasis.3,8 P-selectin deficiency delays and reduces the formation of atherosclerotic plaques, suggesting that P-selectin is involved in atherosclerosis, a chronic inflammatory disease.9
A soluble form of P-selectin is derived either from alternative mRNA splicing that generates an isoform that lacks the transmembrane domain and/or from proteolytic cleavage of the membrane-bound form.10 Soluble P-selectin (sP-selectin) has been proposed as a useful biomarker in various pathologic states in which platelets and/or endothelial cells are activated. It is elevated in several conditions, including hyperlipidemia,11 hypertension,12 ischemic heart disease,13 atherosclerosis,14 and in patients with vascular disorders such as peripheral arterial occlusive disease15 and postangioplasty restenosis.16 Ridker et al17 showed that in apparently healthy women, elevated baseline levels of sP-selectin were associated with an increased risk of future myocardial infarction, stroke, and cardiovascular death.
Previously, Hartwell et al18 made genetically engineered knockin mice that express P-selectin without the cytoplasmic domain (P-selΔCT/ΔCT). The deletion of the cytoplasmic domain of P-selectin did not affect the level of P-selectin, neither on the endothelial cells' surface nor in the α-granules of platelets, but it severely compromised the storage and the regulated secretion of P-selectin from endothelial cells. The ΔCT P-selectin is proteolytically shed from the plasma membrane, which leads to several-fold increased levels of sP-selectin in the plasma.18 This increase in sP-selectin in P-selΔCT/ΔCT mice is associated with enhanced generation of tissue factor–expressing microparticles, resulting in shorter plasma-clotting time and increased fibrin deposition on platelet thrombi reflecting a procoagulant phenotype.19 Recently, the increase in fibrin deposition in the brain in a mouse model of Alzheimer disease was shown to be associated with increased blood-brain barrier (BBB) permeability.20
In this study, taking advantage of the genetic model P-selΔCT/ΔCT, we investigated the potential impact of elevated sP-selectin and the procoagulant phenotype of these mice on BBB permeability and infarct size in experimental stroke. We further investigated whether elevated sP-selectin levels influence inflammation/atherosclerosis when crossed onto atherosclerosis-susceptible apoE−/− background.
Methods
Animals
Except for the atherosclerosis study, the mice were siblings produced by crosses of P-selΔCT/WT mice on B6/129 background. Factor V Leiden (FVL) mice21 were obtained from The Jackson Laboratory (Bar Harbor, ME). The mice used for BBB and behavioral studies were 4 to 5 months old, both male and female. For atherosclerosis studies, P-selΔCT/ΔCT mice on C57BL/6J were crossed to apoE−/− mice on C57BL/6J to get P-selΔCT/ΔCT/apoE−/−. ApoE−/− controls were from The Jackson Laboratory. Mice were maintained on a 12-hour light/dark cycle and on chow diet for 4 months. Animals were bred and housed at the Immune Disease Institute, and all experimental procedures were approved by its Animal Care and Use Committee.
BBB permeability assay
The 2% Evans blue dye (EBD) in phosphate-buffered saline (PBS) was injected intraperitoneally (50 μg/g body weight). Three hours later, mice were killed; skin, spleen, and brain were harvested and placed in formamide for 72 hours (Sigma-Aldrich, St Louis, MO). EBD permeability was expressed as the optical density per gram of tissue (OD/g).
Localization of BBB permeability defects
To visualize the BBB damage, 2% EBD (50 μg/g body weight) was injected retroorbitally. After 6 hours, animals were anesthetized and perfused with 2000 kD fluorescein isothiocyanate (FITC)-dextran/PBS (Sigma-Aldrich) to outline the vasculature. Fluorescence in 50-μm frozen sections was visualized with a laser-scanning confocal microscope (Zeiss-Bio-Rad radiance 2000MP confocal/multiphoton system; Carl Zeiss, Gottingen, Germany). Four sagittal sections from each animal were used for the visualization of the EBD extravasation. Right and left hemispheres of the cerebrum were observed.
Middle cerebral artery occlusion model
We induced transient middle cerebral artery occlusion (MCAO) in males 10 to 12 weeks old. Mice were anesthetized with isoflurane in a mixture of 30% oxygen. A 7.0 silicon-precoated monofilament nylon suture was introduced into the internal carotid artery to occlude the right middle cerebral artery to produce transient focal cerebral ischemia. The filament was withdrawn after 90 minutes to allow reperfusion. A laser Doppler flowmeter (PERIMED System 5000, Stockholm, Sweden) was used to confirm induction of ischemia and reperfusion. Mouse body temperature (37° ± 1°C) was controlled throughout the experiment. Twenty-four hours after ischemia, the brains were removed, cut into 1-mm-thick coronal sections, and stained with 2% 2,3,5-triphenyl tetrazolium chloride (TTC). The infarct size in each section was determined with a computerized image analysis program (ImageJ software, National Institutes of Health, Bethesda, MD; http://rsbweb.nih.gov/ij/).
Social recognition test
Individual adult mice (4-5 months old, males and females) were tested in their home cage twice (I trial and II trial). Each trial began when a stimulus mouse (3-week-old juvenile mouse) was introduced into the home cage of 1 adult mouse. During the first exposure (I trial), a juvenile was exposed to the adult animal, and the duration of investigatory behavior of the adult toward the juvenile was recorded (Canon GL2 MiniDV digital camcorder; Canon, Kanagawa, Japan) on videotape for 2 minutes. The juvenile was then removed and returned to a holding cage. After 3 hours, the same juvenile was re-exposed to the same adult for 2 minutes (II trial). The duration of the investigatory behavior of the adult toward the juvenile was recorded and measured in both trials. Grooming, aggressive posturing, and sexual behaviors including mounting were not included in measures of investigation. Aggressive behavior such as chasing and attack was also analyzed. When an attack occurred, the juvenile was removed and the behavior of the adult mice was defined as aggressive.
Measurements of plasma levels of sP-selectin
Plasma levels of sP-selectin were measured by immunoassays (Quantikine; R&D Systems, Minneapolis, MN). Ethylenediaminetetraacetic acid (EDTA) was used as an anticoagulant, and plasma samples were diluted 1/50 before analysis.
Atherosclerotic lesion analysis
Mice were perfused with ice-cold PBS and 10% formalin. Hearts and aortas were carefully dissected, fixed in 10% formalin overnight, and infiltrated with 30% sucrose for 24 hours at 4°C. Hearts were embedded in optimal cooling temperature compound and sectioned (10 μm). Sections of the aortic sinus were stained with Oil Red-O and counterstained with hematoxylin. For each animal, 5 sections, 80 μm apart, were analyzed without knowledge of the genotype to determine atherosclerotic lesion size. Values represent the mean lesion area from 5 sections from each animal plus or minus SEM. The same sections were analyzed for the number of plaques, and the percentage of aortic sinus circumference covered by plaques was determined using a Zeiss Axioplan digital imaging microscope system with Zeiss AxioCam HRc camera and 10×/0.30 NA objective. AxioVision 4.6.3 software was used for image processing.
Quantification of plaque macrophage content
Macrophage staining in the aortic sinus was performed with a rat anti-mouse Mac-3 antibody (BD Biosciences, Franklin Lakes, NJ). Primary antibody was detected with a biotin-labeled anti-rat antibody (BD Biosciences). Sections were incubated with avidin-biotin complex reagent (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA), and color was developed using diaminobenzidine reagent (Vector Laboratories). Four sections per mouse were examined under high magnification field microscopy, and the number of macrophages with a visible nucleus was counted. Values represent mean plus or minus SEM.
Plasma lipids and lipoprotein measurements
Blood samples were collected to heparinized Eppendorf tubes by retro-orbital venous plexus puncture after a 4-hour fast. Plasma was separated by centrifugation for analysis. Total cholesterol, unesterified cholesterol, triglycerides, and phospholipid levels were determined by using enzymatic colorimetric assays (Wako Chemicals, Richmond, VA). Lipoproteins were isolated from plasma by fast protein liquid chromatography (FPLC), as previously described.22
Statistical analysis
The values are presented as mean plus or minus SEM. Differences between means were determined by using the Student 2-tailed t test when 2 groups were compared, and analysis of variance test followed by Bonferroni posttest. P values of .05 or less were regarded as statistically significant.
Results
BBB permeability is increased in P-selΔCT/ΔCT mice
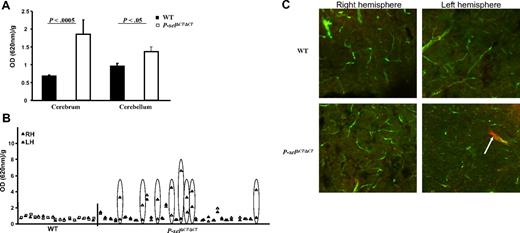
To determine the role of procoagulant state induced by increased levels of sP-selectin on the cerebrovasculature, we examined BBB permeability in the P-selΔCT/ΔCT mice. In this assay, EBD injected intraperitoneally enters the circulation, where it binds to albumin and therefore does not readily cross the BBB. Three hours after injection, the amounts of EBD in the blood, brain, spleen, and skin were measured. The amounts of EBD in the blood of wild-type (WT) and P-selΔCT/ΔCT mice were essentially identical (0.21 ± 0.04 and 0.23 ± 0.08 OD units/g, respectively, P = .6), suggesting that the mutant P-selectin did not alter EBD's transport to and gross clearance from the circulation. However, Figure 1A shows that EBD transfer into the cerebrum and cerebellum was significantly greater in P-selΔCT/ΔCT mice than in their WT littermate controls (2.7- and 1.4-fold, respectively). Additional analyses of the brains from these animals showed that all of the WT (17 of 17) and 19 of 31 of the P-selΔCT/ΔCT mice exhibited comparable, low levels of EBD leakage that were similar for the right and left cerebrum hemispheres (Figure 1B). Four P-selΔCT/ΔCT animals exhibited mild to substantial elevations of EBD leakage that were similar in both hemispheres. Strikingly, in 8 (25%) of the P-selΔCT/ΔCT animals, one hemisphere (left or right) exhibited substantially greater permeability than the other (indicated by ovals in Figure 1B). No statistical differences in EBD vascular permeability were observed in the skin (WT, 0.6 ± 0.04; P-selΔCT/ΔCT, 0.8 ± 0.1 OD/g, P = .1) or spleen (WT, 14 ± 0.5, P-selΔCT/ΔCT, 15 ± 0.7 OD/g, P = .1). These results suggest that in P-selΔCT/ΔCT mice there was an organ-specific increase in vascular permeability, and that it most likely resulted in an increased probability of events (breaks) in the BBB in some, but not all, animals at a particular point in time. Such events in brain vessels were not distributed evenly throughout, suggesting that they might be focal in nature.
Increased BBB permeability in P-selΔCT/ΔCT mice. (A) Quantification of EBD accumulation in the brain of P-selΔCT/ΔCT mice compared with WT. Data represent EBD extravasation calculated from A620 OD/g. n = 17 for WT and n = 31 for P-selΔCT/ΔCT mice. (B) Comparison of EBD accumulation between the right (RH, ▴) and left hemisphere (LH, ▵) in each animal. Ovals indicate the animals with a difference between RH and LH. (C) Representative images from confocal microscopy in the cortex of 4- to 5-month-old mice injected with EBD (red) 6 hours before perfusion with large dextran (green) at the time of sacrifice. Top panels show section of cerebrum from a WT; bottom panels are from P-selΔCT/ΔCT mice. Arrow points to the accumulation of EBD outside of the vessel in the left hemisphere of the P-selΔCT/ΔCT mice. Data represent mean ± SEM.
Increased BBB permeability in P-selΔCT/ΔCT mice. (A) Quantification of EBD accumulation in the brain of P-selΔCT/ΔCT mice compared with WT. Data represent EBD extravasation calculated from A620 OD/g. n = 17 for WT and n = 31 for P-selΔCT/ΔCT mice. (B) Comparison of EBD accumulation between the right (RH, ▴) and left hemisphere (LH, ▵) in each animal. Ovals indicate the animals with a difference between RH and LH. (C) Representative images from confocal microscopy in the cortex of 4- to 5-month-old mice injected with EBD (red) 6 hours before perfusion with large dextran (green) at the time of sacrifice. Top panels show section of cerebrum from a WT; bottom panels are from P-selΔCT/ΔCT mice. Arrow points to the accumulation of EBD outside of the vessel in the left hemisphere of the P-selΔCT/ΔCT mice. Data represent mean ± SEM.
To evaluate further the BBB leakage at the microscopic level, mice were injected with EBD, and 6 hours later were perfused with FITC-labeled large dextran (2000 kDa). The large dextran does not permeate the blood vessels and was used to outline the vessels to separate them from the extravascular space of the brain. Using confocal microscopy of brain sections, we observed little or no EBD accumulation outside the vasculature (Figure 1C top panels) of all 5 WT mice. In contrast, in 4 of 6 P-selΔCT/ΔCT mice, there were large, uneven foci of EBD outside the vasculature, and in 2 P-selΔCT/ΔCT mice 1 hemisphere showed more leakage than the other hemisphere in the same animal (Figure 1C bottom panels). The uneven distribution of the BBB leakage most likely explains the differences in EBD levels between hemispheres of the P-selΔCT/ΔCT mice (Figure 1B).
To understand whether increased BBB leakage in P-selΔCT/ΔCT mice was due to inflammation or procoagulant state, we also evaluated Factor V Leiden (FVL) mice, a procoagulant mouse model.21 We injected EBD in these mice and measured the EBD levels in the cerebrum, skin, and spleen 3 hours later. Overall, EBD leakage in the cerebrum was 1.7-fold higher than in WT (P < .001, n = 9). Similarly to P-selΔCT/ΔCT mice, we have found that in 2 of 9 FVL mice, 1 hemisphere showed several-fold greater EBD leakage than the other. No significant increase was observed in EBD vascular permeability of the skin or spleen (data not shown). The similar BBB phenotype of the FVL to P-selΔCT/ΔCT mice supports our hypothesis that elevated procoagulant activity can induce breaks in the BBB that are not observed in WT mice.
Our results suggest that increased levels of sP-selectin in P-selΔCT/ΔCT mice have a negative effect on brain vasculature and may be implicated in the disruption of BBB.
P-selΔCT/ΔCT mice showed larger infarcts after transient MCAO
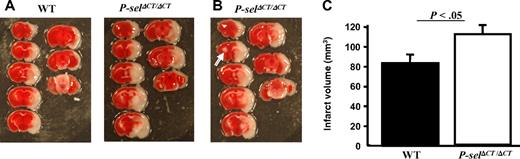
Because the results above might be a consequence of the propensity of the P-selΔCT/ΔCT mice to form small infarcts in the brain, we applied a stroke model in the P-selΔCT/ΔCT mice. Previously, P-selectin deficiency was shown to provide protection from stroke in the intraluminal MCAO model due to reduced neutrophil accumulation accompanied by increased postischemic cerebral reflow.23 In addition, high sP-selectin concentrations have been observed in patients with an acute ischemic stroke.24 To investigate the role of high sP-selectin in stroke, P-selΔCT/ΔCT and WT mice were subjected to MCAO for 90 minutes, followed by 22.5 hours of reperfusion. Doppler flowmetry was used in all mice to confirm ischemia and reperfusion. Only the mice that had blood flow less than 30% baseline of cerebral blood flow after MCAO and complete recovery after reperfusion were used. As shown in Figure 2A, infarction in the cortex and striatum was more severe in P-selΔCT/ΔCT mice than that observed in WT mice. The total infarct volume was significantly larger in the P-selΔCT/ΔCT (110 ± 9 mm3) than in WT (82 ± 9 mm3) mice (Figure 2C; P < .05). It is noteworthy that 1 of 9 P-selΔCT/ΔCT mice (Figure 2B arrow) had a spontaneous small infarct on the opposite side of the brain, the side that was not affected by the procedure. These results indicate that increased sP-selectin in P-selΔCT/ΔCT mice worsens the stroke outcome in the MCAO model of stroke.
Stroke infarct volume is increased in P-selΔCT/ΔCT mice. Intraluminal MCAO model of stroke on P-selΔCT/ΔCT mice and age-matched sibling WT controls. (A) Representative photographs of TTC staining of sections showing enhanced infarct volume in a P-selΔCT/ΔCT mouse brain, compared with a WT mouse brain. (B) White arrow indicates small spontaneous infarct present in the uninjured hemisphere of 1 P-selΔCT/ΔCT mouse. (C) Quantitative image analysis of the infarction area. n = 7 in each group. Error bars indicate SEM.
Stroke infarct volume is increased in P-selΔCT/ΔCT mice. Intraluminal MCAO model of stroke on P-selΔCT/ΔCT mice and age-matched sibling WT controls. (A) Representative photographs of TTC staining of sections showing enhanced infarct volume in a P-selΔCT/ΔCT mouse brain, compared with a WT mouse brain. (B) White arrow indicates small spontaneous infarct present in the uninjured hemisphere of 1 P-selΔCT/ΔCT mouse. (C) Quantitative image analysis of the infarction area. n = 7 in each group. Error bars indicate SEM.
Decreased social investigation and increased aggression in P-selΔCT/ΔCT mice
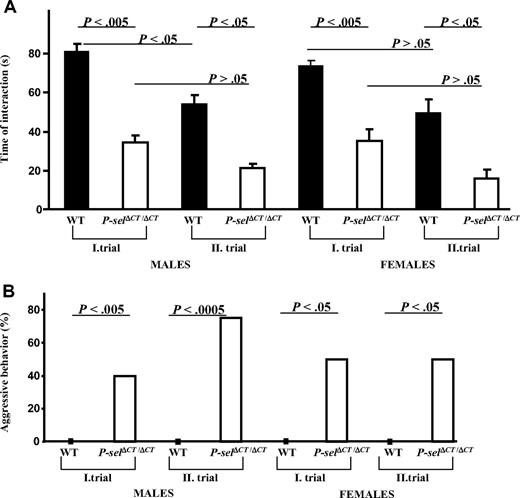
Increased BBB permeability and susceptibility to stroke in the P-selΔCT/ΔCT mice suggest that procoagulant state and elevated sP-selectin caused changes in the brain vessels, which over time could impact brain function and thus affect the behavior of the mice. To evaluate this, we examined social behavior in 4- to 5-month-old animals. Each single adult mouse was kept in a home cage, and behavior trials began when a juvenile mouse was introduced. Investigatory behavior was recorded on videotape for 2 minutes (I trial). Three hours later, the same juvenile was introduced again with the same adult mouse, and behavior was recorded (II trial). Investigation time was defined as direct, active, olfactory exploration of the stimulus (juvenile mouse) by the subject (adult mouse) and consisted of nosing and sniffing of the head and anogenital regions, as well as close following of the juvenile. As shown in Figure 3A, P-selΔCT/ΔCT mice had severely impaired social behavior (reduced time of interaction) in both trials compared with their respective WT littermates.
Behavior of P-selΔCT/ΔCT mice in the social recognition test is severely impaired. (A) Total time(s) spent by adult mice investigating a juvenile. Males and females were analyzed separately in 2 trials (I and II). Error bars indicate SEM. (B) Aggressive behavior (adult mouse attacking juvenile) is shown as the percentage of mice that showed aggressive behavior toward the juvenile. n = 7-21 males; n = 4-8 females.
Behavior of P-selΔCT/ΔCT mice in the social recognition test is severely impaired. (A) Total time(s) spent by adult mice investigating a juvenile. Males and females were analyzed separately in 2 trials (I and II). Error bars indicate SEM. (B) Aggressive behavior (adult mouse attacking juvenile) is shown as the percentage of mice that showed aggressive behavior toward the juvenile. n = 7-21 males; n = 4-8 females.
We also observed that adult P-selΔCT/ΔCT mice showed markedly increased aggressive behavior toward juveniles relative to their WT littermates. In P-selΔCT/ΔCT males, 40% of the animals showed aggressive behavior in the first trial and 75% in the second trial, whereas none of the WT mice showed aggression toward juveniles (Figure 3B). Similarly, in P-selΔCT/ΔCT females, aggressive behavior was observed in 50% of the animals in both trials compared with 0% in WT mice. These results suggest that increased levels of sP-selectin and procoagulant state in P-selΔCT/ΔCT mice can give rise to abnormal social behavior and increased aggression.
Enhanced atherosclerosis in P-selΔCT/ΔCT/apoE−/− mice
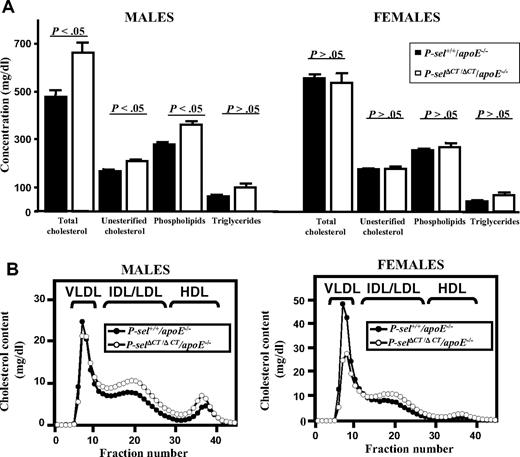
High levels of sP-selectin and hypercoagulability have been documented in a variety of cardiovascular disorders in humans, including atherosclerosis.25 Enhanced atherosclerosis has been also associated with procoagulant phenotype in FVL mice.21 To determine the effect of increased levels of sP-selectin in P-selΔCT/ΔCT mice on development of this inflammatory disease, we crossed P-selΔCT/ΔCT mice onto an apoE−/− background, known to be susceptible to atherosclerotic lesion development. To evaluate whether apoE deficiency influences sP-selectin plasma levels, we analyzed plasma samples from WT, P-selΔCT/ΔCT, P-sel+/+/apoE−/−, and P-selΔCT/ΔCT/apoE−/− mice (Table 1). Using ELISA, we found significantly increased sP-selectin levels in all groups compared with WT mice, and even observed slight elevation of sP-selectin in the apoE−/− genotype (Table 1). We then characterized levels of lipoproteins, known critical players in atherogenesis, in the plasma of P-sel+/+/apoE−/− and P-selΔCT/ΔCT/apoE−/− mice (Figure 4). We found that male, but not female, P-selΔCT/ΔCT/apoE−/− mice exhibited significantly greater plasma levels of total cholesterol, unesterified cholesterol, phospholipids, and triglycerides (Figure 4A). The lipoprotein cholesterol profiles suggest that much of the increase in cholesterol in male P-selΔCT/ΔCT/apoE−/− mice occurred in the intermediate density lipoprotein (IDL)/low density lipoprotein (LDL) and high density lipoprotein (HDL) size lipoprotein particles (Figure 4B). Body weights were not significantly different between the genotypes.
Comparison of sP-selectin levels in the plasma from WT, P-selΔCT/ΔCT, P-sel+/+/apoE−/−, and P-selΔCT/ΔCT/apoE−/− mice
| Genotype . | n . | sP-selectin, ng/mL . | P from WT . |
|---|---|---|---|
| WT | 12 | 127 ± 12 | |
| P-selΔCT/ΔCT | 9 | 1042 ± 68 | < .0005 |
| P-sel+/+/apoE−/− | 5 | 256 ± 37 | < .03 |
| P-selΔCT/ΔCT/apoE−/− | 13 | 1132 ± 83 | < .0005 |
| Genotype . | n . | sP-selectin, ng/mL . | P from WT . |
|---|---|---|---|
| WT | 12 | 127 ± 12 | |
| P-selΔCT/ΔCT | 9 | 1042 ± 68 | < .0005 |
| P-sel+/+/apoE−/− | 5 | 256 ± 37 | < .03 |
| P-selΔCT/ΔCT/apoE−/− | 13 | 1132 ± 83 | < .0005 |
Differences between groups were evaluated using analysis of variance test. Values are expressed as the mean ± SEM.
Plasma lipids and lipoprotein profiles in P-sel+/+/apoE−/− and P-selΔCT/ΔCT/apoE−/− mice. Plasma was collected from individual mice of both sexes, fasted for 4 hours. (A) Total cholesterol, unesterified cholesterol, phospholipid, and triglyceride levels in plasma from P-sel+/+/apoE−/− (■) and P-selΔCT/ΔCT/apoE−/− (□) mice. Data represent mean ± SEM (n = 6-9). Error bars indicate SEM. (B) Plasma samples from P-sel+/+/apoE−/− (●) and P-selΔCT/ΔCT/apoE−/− (○) individual animals were size fractionated by FPLC, and the total cholesterol content of each fraction was determined. The chromatograms are the average of multiple individually determined profiles (n = 4). Approximate elution positions of native very low density lipoprotein (VLDL), IDL/LDL, and HDL particles are indicated by brackets.
Plasma lipids and lipoprotein profiles in P-sel+/+/apoE−/− and P-selΔCT/ΔCT/apoE−/− mice. Plasma was collected from individual mice of both sexes, fasted for 4 hours. (A) Total cholesterol, unesterified cholesterol, phospholipid, and triglyceride levels in plasma from P-sel+/+/apoE−/− (■) and P-selΔCT/ΔCT/apoE−/− (□) mice. Data represent mean ± SEM (n = 6-9). Error bars indicate SEM. (B) Plasma samples from P-sel+/+/apoE−/− (●) and P-selΔCT/ΔCT/apoE−/− (○) individual animals were size fractionated by FPLC, and the total cholesterol content of each fraction was determined. The chromatograms are the average of multiple individually determined profiles (n = 4). Approximate elution positions of native very low density lipoprotein (VLDL), IDL/LDL, and HDL particles are indicated by brackets.
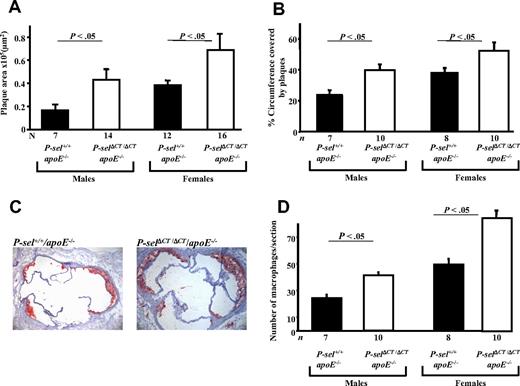
Next, we determined the size of atherosclerotic lesions spontaneously produced in male and female 4-month-old mice fed a standard chow diet. We measured the areas of neutral lipid accumulation in cross-sections of the aortic sinus stained with Oil Red-O (Figure 5A,C). Compared with P-sel+/+/apoE−/− controls, there was a 2.6-fold increase in total aortic sinus lesion area in P-selΔCT/ΔCT/apoE−/− males (P < .05; Figure 5A left) and a 1.8-fold increase in females (P < .05; Figure 5A right). There was no significant difference in the number of plaques (data not shown) in the aortic sinus among the P-selΔCT/ΔCT/apoE−/− mice. However, the plaques in P-selΔCT/ΔCT/apoE−/− mice covered larger areas of the aortic sinus in both male and female groups compared with controls (P < .05; Figure 5B). To address whether macrophages/monocytes contribute to the increased atherosclerotic lesion burden, we quantified the macrophages in the lesions separately in male and female mice by immunohistochemistry. We observed increased macrophage numbers in the lesions of P-selΔCT/ΔCT/apoE−/− compared with P-sel+/+/apoE−/− (P < .05; Figure 5D).
Atherosclerosis is accelerated in P-selΔCT/ΔCT/apoE−/− mice compared with P-sel+/+/apoE−/−. Animals were maintained on normal chow, and their aortic sinus lesions were evaluated at 16 weeks of age. (A) Quantitative image analysis of aortic sinus lesion area. Male group showed 2.6-fold increased size of the plaque area. Female mice had 1.8-fold larger lesions than control mice. (B) Measurement of percentage of the aortic sinus circumference covered by atherosclerotic plaques. (C) Representative images of Oil Red-O staining of the aortic sinus. Representative images, showing an increase in plaque area in P-selΔCT/ΔCT/apoE−/− mice compared with P-sel+/+/apoE−/− mice (female mice). (D) Quantitative image analysis of macrophage content in the aortic sinus atherosclerotic plaques. Data represent mean ± SEM.
Atherosclerosis is accelerated in P-selΔCT/ΔCT/apoE−/− mice compared with P-sel+/+/apoE−/−. Animals were maintained on normal chow, and their aortic sinus lesions were evaluated at 16 weeks of age. (A) Quantitative image analysis of aortic sinus lesion area. Male group showed 2.6-fold increased size of the plaque area. Female mice had 1.8-fold larger lesions than control mice. (B) Measurement of percentage of the aortic sinus circumference covered by atherosclerotic plaques. (C) Representative images of Oil Red-O staining of the aortic sinus. Representative images, showing an increase in plaque area in P-selΔCT/ΔCT/apoE−/− mice compared with P-sel+/+/apoE−/− mice (female mice). (D) Quantitative image analysis of macrophage content in the aortic sinus atherosclerotic plaques. Data represent mean ± SEM.
There are no previous reports indicating that sP-selectin influences plasma lipoprotein cholesterol levels. However, because both female and male P-selΔCT/ΔCT/apoE−/− mice exhibited greater atherosclerosis than P-sel+/+/apoE−/− controls and only males had elevated cholesterol (Figure 4A), it seems unlikely that altered levels of plasma lipoproteins were the only reason for the increased lesion size in P-selΔCT/ΔCT/apoE−/− mice. Thus, a procoagulant and proinflammatory state resulting from increased levels of sP-selectin may lead to increased monocyte recruitment and enhanced formation of early atherosclerotic lesions in both sexes.
Discussion
In vitro studies have shown that P-selectin, even in its soluble form, activates leukocytes to produce both procoagulant26-28 and proinflammatory activities.15,29-32 The central hypothesis of our study was that this might occur also in vivo, in animals that shed P-selectin at an increased rate, impacting their susceptibility to vascular disease.
Elevated plasma sP-selectin causes BBB permeability and affects murine behavior
Elevated levels of sP-selectin and hypercoagulability have been found in many vascular diseases,33 but their pathologic role is unclear. In our study, we found increased vessel permeability in the brains of P-selΔCT/ΔCT mice, but not in their skin or spleen, suggesting that this vessel damage is specific to the barrier function and specialized junctions of the brain endothelium. One possible mechanism could be fibrin deposition on the brain vessels of P-selΔCT/ΔCT mice. Previously, it has been shown that P-selΔCT/ΔCT mice have increased fibrin deposition on injured mesenteric blood vessels.19 Fibrin has been shown to disrupt vascular endothelial cell junctions in vitro.34 Furthermore, fibrin binds to vascular endothelial (VE)–cadherin, an adhesive molecule present at adherence junctions of endothelial cells, which mediates cell-cell contacts and regulates paracellular permeability.35 In the brain, the tight and adherence junctions of the cerebral capillary endothelium form the highly restrictive BBB. Recently, fibrin has been shown to provoke and accelerate neurovascular damage and neuroinflammation in an Alzheimer disease (AD) mouse model.20 These mouse models of AD have deficits in vascular integrity,36 as shown by areas with increased BBB permeability that colocalized with fibrin deposition.20 Spontaneous deposition of fibrin has been shown in the tissue of homozygous FVL mice.21 In our experiments, we have found increased BBB leakage in the FVL mice, thus supporting the hypothesis that elevated procoagulant activity and fibrin deposition might be responsible for the increased BBB leakage in the P-selΔCT/ΔCT mice.
We observed a difference in the BBB permeability between each of the 2 hemispheres of some individual P-selΔCT/ΔCT mice (Figure 1B) and FVL mice, suggesting local and uneven BBB disruption (also detected by microscopy) and/or small infarctions. Such cerebrovascular lesions, termed silent brain infarction (SBI), have been found in patients with obstructive sleep apnea,37 a disease associated with increased serum levels of sP-selectin,38 and increased cerebrovascular and cardiovascular morbidity and mortality.39 Occurrence of stroke in these patients is most likely preceded by SBI. Interestingly, the level of sP-selectin was higher in patients with obstructive sleep apnea who exhibited SBI compared with those without SBI.38
We also found differences in social behavior and a profound increase in aggression in P-selΔCT/ΔCT mice. P-selΔCT/ΔCT mice consistently showed impairment in social interaction with other mice. Changes in BBB permeability could have an effect on behavior. It is possible that impaired BBB function, caused by increased sP-selectin, resulted in the leakage of plasma proteins into the brain, causing injury and behavioral changes in P-selΔCT/ΔCT mice over time.
Previously, we have linked other risk factors for atherosclerosis in mice, such as increased homocysteine, apoE deficiency, and old age, with elevated BBB permeability.40-42 These risk factors also influence neurodegenerative diseases such as Alzheimer disease.43 Now we show that increased sP-selectin may play an important role in atherosclerosis and cerebrovascular function, and thus perhaps also in neurodegenerative diseases.
Increased sP-selectin intensifies the impact of experimental stroke
Plasma P-selectin has been shown to be elevated after stroke, and P-selectin deficiency and P-selectin antagonism protect from cerebral injury in models of MCAO.23,24,44 We found that increased levels of sP-selectin lead to an increased infarct volume in a mouse model of MCAO. The increase in infarct volume in P-selΔCT/ΔCT mice is most likely due to the increased procoagulant activity of these mice with elevated soluble tissue factor.19 This may result in excessive platelet activation, endothelial P-selectin expression, and fibrin formation. Pathologic coagulopathy and thrombus formation were shown to be involved in enhancement of ischemic cerebral injury.45-47 Our study supports the hypothesis that increased sP-selectin is not only a biomarker and predictor of stroke, but that it actually promotes stroke and worsens the stroke outcome.
Increased levels of sP-selectin accelerate atherosclerosis
A role for P-selectin in atherogenesis is supported by many studies.7,9,13,48,49 For example, P-selectin is up-regulated in the endothelium overlying the atherosclerotic plaque,48 P-selectin–deficient mice develop smaller, less complex atherosclerotic lesions,9 and anti-P-selectin antibodies inhibit monocyte rolling and attachment across the carotid endothelium.49 sP-selectin was shown to exhibit a consistent and independent association with atherosclerotic burden within a population of asymptomatic, nonsmoking, hypercholesterolemic men, suggesting that sP-selectin might be a useful biomarker of preclinical atherosclerosis.50 Our study is the first report demonstrating a causal role for increased levels of sP-selectin in the development of atherosclerosis. Adhesion and migration of inflammatory cells, in particular mononuclear cells, into the vascular wall are a hallmark of an atherosclerotic plaque. This process is fortified by the progressive deposition of lipids in the subendothelial layer.51 Presence of elevated sP-selectin in plasma of patients with peripheral arterial disease has been shown to induce the activation of leukocytes as detected by αMβ2 (Mac-1) activation, an integrin involved in leukocyte adhesion, and to enhance leukocyte adhesion on the fibrinogen or platelet monolayer in a shear-dependent manner.15 The signaling produced by sP-selectin binding to leukocyte PSGL-1 is similar to that produced by its membrane-bound form.15 Wang et al31 also found that sP-selectin partially restores impaired leukocyte adhesion and peritoneal accumulation in stimulated P-selectin–deficient mice. Therefore, sP-selectin can prime leukocyte integrin activation and leukocyte adhesion during inflammation,31 and this activity could explain the enhanced atherosclerotic lesion growth with elevated recruitment of monocytes to the lesion in the P-selΔCT/ΔCT/apoE−/− mice. In addition, elevated procoagulant activity was shown to promote atherosclerosis52,53 and may stimulate platelet activation that enhances lesion growth as well.54,55
Our animal results are also in agreement with clinical studies showing that increased sP-selectin is associated with increased plasma LDL in hypercholesterolemic individuals.56 It is possible that cholesterol synthesis or absorption is affected by high levels of sP-selectin or by inflammation resulting from sP-selectin elevation. The molecular and cellular mechanisms explaining how elevated sP-selectin leads to an altered lipid profile in P-selΔCT/ΔCT mice remain to be clarified.
In conclusion, our studies demonstrate that sP-selectin has a profound negative impact on mouse vasculature. This results in a defect in BBB function and greater susceptibility to atherosclerosis. Similar to P-selectin on activated platelets,1 sP-selectin appears to promote the vicious circle of inflammation leading to endothelial dysfunction. In addition, the procoagulant activity of sP-selectin can aggravate the consequences of an ischemic event such as stroke. Therefore, elevated sP-selectin should be considered not only as a biomarker of inflammation/thrombosis, but also as an active player in these conditions. Targeting sP-selectin in disease states where it is elevated could reduce future cerebrovascular and cardiovascular events.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Lesley Cowan for assistance in preparing the manuscript and Stephen Cifuni for technical assistance.
This work was supported by grants HL056949 (to D.D.W.) and HL066105 (to M.K. and D.D.W.) from the National Heart, Lung, and Blood Institute of the National Institutes of Health (Bethesda, MD).
National Institutes of Health
Authorship
Contribution: J.K., A.K.C., and D.D.W. designed the study. J.K., A.K.C., B.-Q.Z., I.S.P., and A.Y. obtained data. J.K. wrote the paper. All authors were involved in the interpretation of the results and read, commented on, and approved the final version of the manuscript.
Conflict-of-interest disclosure: D.D.W. has consulted for Hoffmann-LaRoche and Archemix, and M.K. has consulted for Roche. The remaining authors declare no competing financial interests.
The current address for J.K. is Department of Biology, Massachusetts Institute of Technology, Cambridge, MA. The current address for A.K.C. is Department of Internal Medicine, Division of Hematology/Oncology, University of Iowa, Iowa City, IA.
Correspondence: Dr Denisa D. Wagner, Immune Disease Institute, 3 Blackfan Circle, 3rd Floor, Boston, MA 02115; e-mail: wagner@idi.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal