Abstract
IL-12 activates STAT4, which is a critical regulator of inflammation and T helper type I (Th1) lineage development in murine systems. The requirement for STAT4 in the generation of human Th1 cells has not been examined thoroughly. Compared with control Th1 cultures, expression of the Th1 genes IFNγ, IL-12Rβ2, and TNFα is greatly reduced in Th1 cultures of CD4 T cells isolated from lymphoma patients after autologous stem cell transplantation who have acquired STAT4 deficiency. Moreover, IL-4 and IL-5 production is increased in patient Th1 cultures though there are no defects in the development of Th2 cells. Reconstitution of STAT4 in patient T cells allowed recovery of IFNγ and IL-12Rβ2 expression, whereas ectopic expression of IL-12Rβ2 did not rescue STAT4 expression, and increased IFNγ production only to levels intermediate between control and patient samples. These results demonstrate that, as in murine systems, STAT4 is required for optimal human Th1 lineage development.
Introduction
After cytokine exposure, CD4+ T lymphocytes differentiate into distinct subsets of T helper (Th) cells, which regulate the immune response in resistance to pathogens. IL-12 promotes the differentiation of T helper type I (Th1) cells that produce the proinflammatory cytokine IFNγ, whereas IL-4 promotes the development of Th2 cells that secrete IL-4, IL-5, and IL-13. Signal transducer and activator of transcription 4 (STAT4) is activated upon IL-12 binding to the receptor chains IL-12Rβ1 and IL-12Rβ2,1,2 and subsequently translocates to the nucleus where it binds target genes to activate transcription.3 The requirement for IL-12 receptors in the development of Th1 immunity is demonstrated in human patients genetically deficient for IL-12Rβ1.4 Experiments with STAT4-deficient mice have demonstrated the requirement for STAT4 in IL-12–mediated biologic functions including Th1 development and IFNγ production.5,6 However, the role of STAT4 in human Th1 differentiation has not been directly demonstrated
We have previously described a profound deficiency in STAT4 expression by peripheral blood mononuclear cells (PBMCs) obtained from lymphoma patients after chemotherapy and autologous stem cell transplantation.7 Posttransplantation STAT4 deficiency is associated with markedly defective production of IFNγ in response to IL-12 both in vitro and in vivo.7,8 In this report, we have used STAT4-deficient posttransplantation patient lymphocytes to define the role of STAT4 in the development of human Th1 cells.
Methods
Human PBMC samples
PBMCs were obtained as previously described from patients with relapsed or refractory lymphoma who had undergone high-dose chemotherapy or chemoradiotherapy followed by autologous peripheral blood stem cell transplantation.7 Control PBMCs were obtained from healthy volunteer donors. Patient blood samples were collected on a study approved by the Institutional Review Board at Indiana University Medical Center and written informed consent was obtained from each study subject in accordance with the Declaration of Helsinki.
T helper cell differentiation
CD4+ T cells isolation and differentiation are described in figure legends. Because patient samples used in this study were still CD4 T lymphopenic, it was required to pool isolated CD4 T cells from 2 to 5 patients to obtain sufficient number of cells for in vitro differentiation. Immunoblot was used to confirm STAT4 deficiency in cultured Th1 cells. Quantitative polymerase chain reaction (qPCR), Western blot, and flow cytometry were performed as described.7,9 Cytokine was measured by enzyme-linked immunosorbent assay (ELISA) or Luminex (Millipore, Billerica, MA).
Reconstitution of human STAT4 and IL-12Rβ2 expression
Th1 cells differentiated from control and posttransplantation patient PBMCs were transfected with plasmids encoding human STAT4,10 IL-12Rβ2,11 or vector alone using a Human T cell Nucleofector Kit (Amaxa, Gaithersburg, MD) following the manufacturer's instructions. Transduction of a bicistronic retroviral vector encoding murine STAT4 and EGFP or EGFP alone12 (provided by Dr John O'Shea, National Institutes of Health [NIH], Bethesda, MD) into differentiated Th1 cells was performed as described previously.9 The amphotropic packaging cell line (SD3443) was provided by Dr Janice Blum (Indiana University). At the end of the second round of differentiation, EGFP-positive cells were sorted by flow cytometry for qPCR, and intracellular cytokine staining.9
Results and discussion
Although mice are widely used models of the immune system, there are significant differences between murine and human immune systems.13-15 Thus although STAT4 is an important component of Th1 differentiation in the murine system, the requirement for STAT4 in human cells has not been directly demonstrated. To determine the function of STAT4 in human T cells, we took advantage of posttransplantation patient PBMCs, which are deficient in expression of STAT4.7 STAT4 protein expression was decreased in several purified lymphocyte populations including CD4 T cells obtained from posttransplantation patients, compared with control PBMCs (not shown).
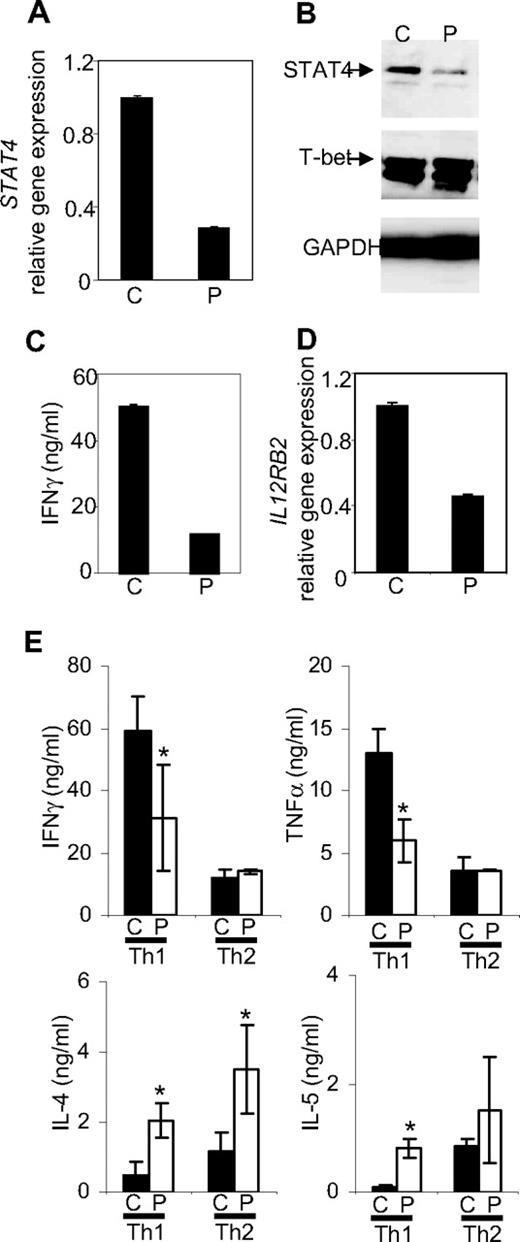
STAT4-deficient CD4+ T lymphocytes purified from posttransplantation patient PBMCs were then used for assays of in vitro T helper differentiation. STAT4 deficiency persisted in patient T cells cultured under Th1-promoting conditions for a week, with little effect on expression of T-bet, another important regulator of Th1 development (Figure 1A,B).16 An impaired Th1 phenotype in patient samples was evident from reduced IFNγ production, compared with Th1 cells differentiated from healthy subjects (Figure 1C). IL-12Rβ2, selectively expressed on human Th1 but not Th2 clones,17 was reduced in STAT4-deficient human Th1 cultures (Figure 1D), similar to other reports where STAT4 expression was diminished using siRNA oligonucleotides.18 STAT4 deficiency was apparent in cultures even after 3 weeks of differentiation (data not shown) and was associated with significantly decreased IFNγ and TNFα19 production, and increased IL-4 and IL-5 production in patient Th1 cultures, compared with control cells (Figure 1E). In contrast, long-term Th2 development was not impaired, and patient cultures had increased IL-4 production (Figure 1E). These results suggest an important role for STAT4 in human Th1 differentiation and that human STAT4 may negatively regulate Th2 cytokines, as was observed in murine cells.5
Th differentiation from control (C) or posttransplantation patients (P) cells. CD4+ T cells were purified from PBMCs using CD4 magnetic beads (Miltenyi Biotech, Auburn, CA). CD4+ T cells (5 × 105/mL) were cultured with the Dynabeads CD3/CD28 T Cell Expander (Invitrogen, Carlsbad, CA) under Th1 (20 ng/mL hIL-12 (PeproTech, Rocky Hill, NJ) + 2.5 μg/mL anti–IL-4 mAb (R&D Systems, Minneapolis, MN)) or Th2 (20 ng/mL hIL-4 (PeproTech) + 2.5 μg/mL anti-IFNγ mAb (R&D Systems) differentiation conditions. After 4 days of culture, cells were expanded with human IL-2 (100 unit/mL). After a total of 7 days of culture, differentiated Th cells were analyzed or used for extended culture. Cells were analyzed for STAT4 expression (A,B) and IL12RB2 expression (D) using real-time PCR (A,D) or immunoblot (B). Cells differentiated in panel A were washed and stimulated with anti-CD3 for 1 day before IFNγ levels were measured using ELISA (C). (E) For long-term cultures, cells were resuspended at 106/mL, stimulated with plate-bound anti-CD3 (4 μg/mL) and soluble anti-CD28 (1 μg/mL) in the presence of Th1 or Th2 conditions at each weekly interval. After 3 weeks, cultures were washed and stimulated with anti-CD3 for 1 day. Levels of IFNγ, TNFα, IL-4, and IL-5 in the supernatant were determined using either ELISA or multiplex Fluorokine MultiAnalyte Profiling Kit in the Luminex 200 analyzer (Millipore). Data are presented as mean ± SD from a total of 4 healthy control and 9 patient samples (2-5 pooled/experiment) across 3 independent experiments. Statistical significance was evaluated with an independent Student t test using SPSS 16.0 program (SPSS, Chicago IL), and P < .05 was considered significant. *Significantly different from healthy controls (P < .05).
Th differentiation from control (C) or posttransplantation patients (P) cells. CD4+ T cells were purified from PBMCs using CD4 magnetic beads (Miltenyi Biotech, Auburn, CA). CD4+ T cells (5 × 105/mL) were cultured with the Dynabeads CD3/CD28 T Cell Expander (Invitrogen, Carlsbad, CA) under Th1 (20 ng/mL hIL-12 (PeproTech, Rocky Hill, NJ) + 2.5 μg/mL anti–IL-4 mAb (R&D Systems, Minneapolis, MN)) or Th2 (20 ng/mL hIL-4 (PeproTech) + 2.5 μg/mL anti-IFNγ mAb (R&D Systems) differentiation conditions. After 4 days of culture, cells were expanded with human IL-2 (100 unit/mL). After a total of 7 days of culture, differentiated Th cells were analyzed or used for extended culture. Cells were analyzed for STAT4 expression (A,B) and IL12RB2 expression (D) using real-time PCR (A,D) or immunoblot (B). Cells differentiated in panel A were washed and stimulated with anti-CD3 for 1 day before IFNγ levels were measured using ELISA (C). (E) For long-term cultures, cells were resuspended at 106/mL, stimulated with plate-bound anti-CD3 (4 μg/mL) and soluble anti-CD28 (1 μg/mL) in the presence of Th1 or Th2 conditions at each weekly interval. After 3 weeks, cultures were washed and stimulated with anti-CD3 for 1 day. Levels of IFNγ, TNFα, IL-4, and IL-5 in the supernatant were determined using either ELISA or multiplex Fluorokine MultiAnalyte Profiling Kit in the Luminex 200 analyzer (Millipore). Data are presented as mean ± SD from a total of 4 healthy control and 9 patient samples (2-5 pooled/experiment) across 3 independent experiments. Statistical significance was evaluated with an independent Student t test using SPSS 16.0 program (SPSS, Chicago IL), and P < .05 was considered significant. *Significantly different from healthy controls (P < .05).
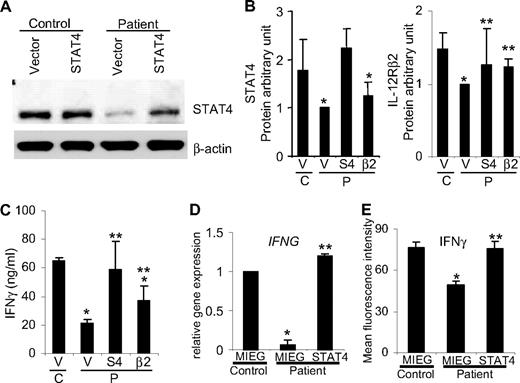
Because posttransplantation patient PBMCs could be deficient in other proteins in addition to STAT4, we next tested the ability of ectopic STAT4 expression to recover the Th1 phenotype in these cultures using 2 methods. Using transient transfection, patient Th1 cells were successfully reconstituted with STAT4 expression (Figure 2A) and subsequently demonstrated significantly increased IFNγ production (Figure 2C). In contrast, ectopic expression of IL-12Rβ2 in patient Th1 cells did not rescue STAT4 expression (Figure 2B), though it did increase IFNγ production to levels intermediate between control and patient samples (Figure 2C). This result suggests that STAT4 has a dominant role in human Th1 development and IFNγ production. Because STAT4 levels are not completely absent in posttransplantation patient lymphocytes, the partial recovery of IFNγ production in cells transduced with IL-12Rβ2 is likely due to the enhanced IL-12 signaling. IL-12Rβ2 expression enhanced IL-12–induced IFNγ production in wild-type but not STAT4-deficient cells, supporting the requirement for STAT4 in this response.20
Reconstitution of STAT4 and IL-12Rβ2 expression in differentiated Th1 cells. Th1 cells after 1 week of differentiation from control and posttransplantation patients were transiently transfected with plasmids encoding human STAT410 (S4), IL-12Rβ211 (β2), or vector alone using a Human T cell Nucleofector Kit (Amaxa). Protein expression was analyzed by Western blot 3 days after transfection from total cell populations using anti-STAT4 (C-20; Santa Cruz Biotechnology) and antihuman IL12Rβ2 (R&D Systems) antibodies. (A) Representative immunoblot of STAT4 protein expression. (B) Immunoblot band intensity was quantified by densitometry, normalized to GAPDH (Cell Signaling, Beverly, MA), and expressed as the ratios of STAT4 or IL-12Rβ2 to GAPDH. The protein levels are expressed in arbitrary units and presented as mean plus or minus SD of 4 independent experiments. (C) One day after transient transfection, as in panels A and B, cells were washed and stimulated with anti-CD3 plus IL-12 (2 ng/mL). After 2 days of stimulation, IFNγ levels in supernatants were measured using ELISA and presented as mean ± SD of 4 independent experiments. In panels B and C, there was a total of 5 healthy controls and 12 patient samples (2-5 pooled/experiment) across 4 independent experiments. (D) Th1 cells cultured for 1 week were transduced with retroviruses encoding STAT4 and EGFP or EGFP alone. At the end of the second week of differentiation, EGFP-positive cells were sorted and total RNA was extracted to analyze IFNG and IL12RB2 gene expression by real-time PCR.9 Results shown are mean ± SD from a total of 2 healthy control and 7 patient samples (2-5 pooled/experiment) across 2 independent experiments. (E) Intracellular cytokine staining was performed on transduced cells in panel D with anti-CD3 (4 μg/mL) plus IL-12 (2 ng/mL) for 5 hours of stimulation in the presence of monensin at the last 2 hours of incubation. Expression of IFNγ was evaluated on 5000 events of GFP-positive cells by flow cytometry and presented as mean fluorescence intensity (MFI). Values are presented as mean MFI ± SD from a total of 4 healthy control and 6 patient samples (2 pooled/experiment) across 3 independent experiments. For all panels: *Significantly different from healthy controls (P < .05); **significantly different from patients (P < .05).
Reconstitution of STAT4 and IL-12Rβ2 expression in differentiated Th1 cells. Th1 cells after 1 week of differentiation from control and posttransplantation patients were transiently transfected with plasmids encoding human STAT410 (S4), IL-12Rβ211 (β2), or vector alone using a Human T cell Nucleofector Kit (Amaxa). Protein expression was analyzed by Western blot 3 days after transfection from total cell populations using anti-STAT4 (C-20; Santa Cruz Biotechnology) and antihuman IL12Rβ2 (R&D Systems) antibodies. (A) Representative immunoblot of STAT4 protein expression. (B) Immunoblot band intensity was quantified by densitometry, normalized to GAPDH (Cell Signaling, Beverly, MA), and expressed as the ratios of STAT4 or IL-12Rβ2 to GAPDH. The protein levels are expressed in arbitrary units and presented as mean plus or minus SD of 4 independent experiments. (C) One day after transient transfection, as in panels A and B, cells were washed and stimulated with anti-CD3 plus IL-12 (2 ng/mL). After 2 days of stimulation, IFNγ levels in supernatants were measured using ELISA and presented as mean ± SD of 4 independent experiments. In panels B and C, there was a total of 5 healthy controls and 12 patient samples (2-5 pooled/experiment) across 4 independent experiments. (D) Th1 cells cultured for 1 week were transduced with retroviruses encoding STAT4 and EGFP or EGFP alone. At the end of the second week of differentiation, EGFP-positive cells were sorted and total RNA was extracted to analyze IFNG and IL12RB2 gene expression by real-time PCR.9 Results shown are mean ± SD from a total of 2 healthy control and 7 patient samples (2-5 pooled/experiment) across 2 independent experiments. (E) Intracellular cytokine staining was performed on transduced cells in panel D with anti-CD3 (4 μg/mL) plus IL-12 (2 ng/mL) for 5 hours of stimulation in the presence of monensin at the last 2 hours of incubation. Expression of IFNγ was evaluated on 5000 events of GFP-positive cells by flow cytometry and presented as mean fluorescence intensity (MFI). Values are presented as mean MFI ± SD from a total of 4 healthy control and 6 patient samples (2 pooled/experiment) across 3 independent experiments. For all panels: *Significantly different from healthy controls (P < .05); **significantly different from patients (P < .05).
Long-term reconstitution of STAT4 in patient Th1 cells was achieved using a bicistronic retroviral vector encoding STAT4 and EGFP or EGFP alone.12 Patient Th1 cells transduced with STAT4 retroviruses exhibited IFNγ (Figure 2D,E) and IL-12Rβ2 (data not shown) expression levels similar to those of vector-transduced control Th1 cells. Thus, rescuing STAT4 expression restores Th1 differentiation in patient samples.
These results demonstrate that a deficiency of STAT4 in CD4 T cells isolated from posttransplantation patient PBMCs results in defective Th1 development and validates a large body of work examining STAT4 function in the murine system.21 These data further suggest that after certain chemotherapy regimens, a deficiency in STAT4 may impact attempts at immunotherapy that rely upon Th1 immunity and IFNγ production, or type I IFN signaling, which may also depend upon STAT4 in some conditions.22 In contrast to STAT4 deficiency, expression and activation of STAT1 (not shown), STAT3,7 or STAT67 are normal in posttransplantation patient PBMCs, and expression of T-bet is comparable in patient and control Th1 cultures (Figure 1B). Thus, acquired, restricted deficiency of STAT4 is intriguing and therapeutic attempts to modify STAT4 gene expression in human disease are warranted.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the nursing staff in the General Clinical Research Center and Adult Bone Marrow Transplant Clinic at Indiana University Medial Center for collection of blood samples. We also thank Dr J. O'Shea and Dr J. Blum for reagents, and Dr W. Yao, Dr V. Crotzer, and J. Walker for technical assistance.
This work was supported in part by the Indiana University Pilot Funding for Research Use of Core Facilities (H.-C.C.), Showalter Trust Funds (H.-C.C.), Indiana University Biomedical Research Pilot Grant through ITRACK (H.-C.C.), and the Oncological Sciences Center in Discovery Park at Purdue (H.-C.C.), and by NIH grants AI45515 (M.H.K.), 3M01 RR00750-27S3 (M.J.R.), MO1 RR750 (M.J.R.), and CA118118 (M.J.R.), and by an Immunology and Hematopoiesis Program grant (M.J.R.) from the Indiana University Simon Cancer Center (P30CA82709).
National Institutes of Health
Authorship
Contribution: H.-C.C. designed and performed research, analyzed data, and wrote the paper; L.H., R.G., D.P., and E.T.N. performed research and analyzed data; and M.J.R and M.H.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hua-Chen Chang, Wells Center for Pediatric Research, Department of Pediatrics, Indiana University School of Medicine, 702 Barnhill Dr, RI Room 2632, Indianapolis, IN 46202; e-mail: huchang@iupui.edu; or Mark H. Kaplan, Wells Center for Pediatric Research, Indiana University School of Medicine, Departments of Pediatrics and Microbiology and Immunology, 702 Barnhill Dr, RI Room 2600, Indianapolis, IN 46202; e-mail: mkaplan2@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal