Abstract
The development, homeostasis, and function of B lymphocytes involve multiple rounds of B-cell receptor (BCR)–controlled proliferation and prolonged maintenance. We analyzed the role of transcription factor Zfx, a recently identified regulator of hematopoietic stem cell maintenance, in B-cell development and homeostasis. Panhematopoietic or B cell–specific deletion of Zfx in the bone marrow blocked B-cell development at the pre-BCR selection checkpoint. Zfx deficiency in peripheral B cells caused accelerated B-cell turnover, depletion of mature recirculating B cells, and delayed T-dependent antibody responses. In addition, the numbers and function of B-1 cell lineage were reduced. Zfx-deficient B cells showed normal proximal BCR signaling, but impaired BCR-induced proliferation and survival in vitro. This was accompanied by aberrantly enhanced and prolonged integrated stress response and by delayed induction of cyclin D2 and Bcl-xL proteins. Thus, Zfx restrains the stress response and couples antigen receptor signaling to cell expansion and maintenance during B-cell development and peripheral homeostasis. These results identify a novel transcriptional regulator of the B-cell lineage and highlight the common genetic control of stem cell maintenance and lymphocyte homeostasis.
Introduction
As the key cell type mediating humoral adaptive immunity, B lymphocytes undergo highly regulated development, peripheral homeostasis, and antigen-dependent responses.1,2 In the adult murine bone marrow (BM), the initial commitment to the B-cell lineage in late pro-B cells (also called Hardy fraction B/C, Fr. B/C) is followed by the rearrangement of the immunoglobulin (Ig) μ heavy chain gene and formation of the pre–B-cell receptor (pre-BCR) complex. Signaling from the pre-BCR complex at the so-called pre-BCR selection checkpoint allows pro-B to pre–B-cell transition and initiates a wave of proliferation in large pre-B cells (Fr. C′). These subsequently differentiate into small pre-B cells (Fr. D), in which the rearrangement of the κ and λ light chain genes occurs. Pairing of the Ig heavy and light chains results in the formation of the B-cell receptor (BCR) at the surface of immature B cells (Fr. E). After the selection process that eliminates cells with self-reactive or nonfunctional BCR, immature B cells migrate into the spleen as transitional B cells (T1 and T2) and subsequently differentiate into follicular (FO) or marginal zone (MZ) splenic B cells. FO cells reside in the splenic follicles and recirculate in the blood, the lymph nodes (LNs), and the BM. In the BM, mature recirculating B cells represent a distinct population (Fr. F) residing in discrete areas around the vascular sinusoids.3 FO B cells are essential for T cell–dependent immune responses, in which BCR-activated B cells enter the germinal center (GC) reaction that involves massive expansion and Ig class switching. MZ B cells, however, are noncirculating and mediate rapid T-cell–independent immune responses against bloodborne pathogens. Finally, a distinct B-1 cell subset emerges from unique progenitors4 and can be maintained by self-renewal; these cells reside mainly in the peritoneal and pleural cavities and produce low-affinity “natural” IgM against common microbial determinants.5
The development and homeostasis of B cells involves multiple stages of rapid proliferative expansion (eg, large pre-B cells, GC reaction) followed by long-term maintenance with infrequent cell division (eg, recirculating B cells, memory B cells, B-1 cells). Notably, the signals from BCR (or pre-BCR in the case of pre-B cells) are critical for both proliferation and maintenance of B cells at all developmental stages.1,6 These signals are further relayed by transcription factors to achieve stage- and subset-specific outcomes. For instance, Ca2+/calcineurin pathway is essential for BCR-induced B-cell proliferation7 and activates transcription factor Mef2c to induce cyclin D2 and Bcl-xL proteins required for B-cell proliferation and survival, respectively.8 However, Ca2+/calcineurin-regulated NFAT transcription factors collectively restrict B-cell proliferation and effector function.9 Furthermore, transcription factors IRF4 and IRF8 facilitate cell cycle exit of large pre-B cells,10 c-Myb promotes both pro-B to pre–B-cell transition and cytokine BAFF-mediated peripheral B-cell survival,11 and Aiolos restricts BCR-induced B-cell proliferation.12 Thus, transcriptional control mechanisms are critical for translating BCR signals into B-cell proliferation or survival or both.
The survival and proliferation of a cell are tightly linked to nutrient availability and protein synthesis capacity through a conserved mechanism called the integrated stress response (ISR).13-15 Nutrient deprivation or endoplasmic reticulum (ER) stress trigger ISR by the phosphorylation of translation initiation factor eIF2α, which reduces global protein synthesis but facilitates the translation of transcription factor ATF4. Although ATF4 activity leads to increased nutrient uptake and protein synthesis capacity, prolonged or dysregulated ISR may cause apoptosis through ATF4 target Ddit3/CHOP.16 A parallel pathway induced by ER stress, the unfolded protein response (UPR), activates transcription factor XBP-1 through alternative splicing to increase the cell's secretory capacity and to promote survival.17 The physiologic ISR or UPR or both are important for optimal protein secretion in lymphocytes, including effector T cells18 and plasma cells.19 In contrast, B cells undergo only low-level, transient UPR after BCR stimulation,20 suggesting that ISR/UPR restriction is important for their proliferation or survival or both. However, the mechanisms regulating the ISR in the developing and mature B cells remain unclear.
Zfx is a zinc finger transcription factor encoded on the mammalian X chromosome that is highly conserved among vertebrates. With the use of conditional gene targeting, our laboratory has identified Zfx as an essential transcriptional regulator of hematopoietic stem cell (HSC) function.21 Constitutive or inducible Zfx deletion in HSCs (using Tie2-Cre and Mx1-Cre deleter strains, respectively) abolished the self-renewal and decreased survival of HSCs. This was accompanied by a severe block of B-cell development but relatively normal erythromyeloid development, suggesting that Zfx may have additional cell-intrinsic functions in lymphoid development and homeostasis. To test this notion, we have analyzed the consequences of Zfx deletion in developing or mature B lymphocytes. We report that Zfx is required for pro-B to pre–B-cell transition, maintenance of mature recirculating B cells and B-1 cells, and optimal GC response. Furthermore, Zfx loss caused aberrant activation of the ISR in BCR-stimulated B cells and led to their impaired proliferation and survival.
Methods
Mice
Mice with the LoxP-flanked Zfx allele21 were crossed to Tie2-Cre,22 Mb1-Cre,23 or CD19-Cre24 strains. The resulting conditional knockout (CKO) mice (Zfxflox/y, Cre+) were on 129SvEv (CD19-Cre) or mixed 129/B6 (Tie2-Cre, Mb1-Cre) background. Control mice included Cre+ wild-type and Cre−Zfxflox/y animals, which were indistinguishable by all assays. Only Zfx wild-type, Cre+ animals were used for in vitro BCR stimulation as controls for CD19-Cre+ CKO mice. All animal studies were performed according to the investigator's protocol approved by the Institutional Animal Care and Use Committee of Columbia University.
Ex vivo cell analysis
Single-cell suspensions were stained for 4- or 5-color analysis with the fluorochrome-conjugated antibodies (BD PharMingen, San Diego, CA; or eBioscience, San Diego, CA) or peanut agglutinin (PNA; Vector Laboratories, Burlingame, CA). For intracellular μ staining, BM cells were stained for the indicated surface markers, fixed, permeabilized with 4% paraformaldehyde/0.1% saponin, and stained with an antibody against IgM. The samples were acquired using the LSR II flow cytometer or sorted on FACSAria flow sorter (BD Biosciences, San Jose, CA), and analyzed using FlowJo software (TreeStar, Ashland, OR).
For cell-cycle analysis, mice were injected intraperitoneally with 1 mg bromodeoxyuridine (BrdU; Sigma-Aldrich, St Louis, MO) and killed after 50 minutes. For pulse-chase experiment, mice were fed 0.8 mg/mL BrdU in drinking water for 2 weeks. Single-cell suspensions were stained for surface markers, fixed, permeabilized, and stained with an antibody against BrdU according to the BD PharMingen BrdU Flow Kit protocol.
Immunizations and enzyme-linked immunosorbent assays
Age-matched mice (8-12 weeks old) were immunized by intraperitoneal injection of 50 μg PC(8)–keyhole limpet hemocyanin (KLH) mixed with complete Freund adjuvant, 25 μg NP(67)–Ficoll, or 50 μg NP(28)–KLH mixed with alum (Biosearch Technologies, Novato, CA). For memory response, mice were boosted with NP(28)–KLH in PBS on day 42 after initial immunization. Serum antibody titers were determined by enzyme-linked immunosorbent assay (ELISA) as described previously.25 Anti-Ig isotype antibodies, anti-T15 idiotype antibody AB1-2 Ab,26 PC, or NP conjugated to bovine serum albumin were used as capture reagents for total or antigen-specific serum antibodies, respectively.
In vitro B-cell stimulation assays
Splenic B cells were enriched by negative selection against CD43/TCRβ/Ter119/CD11b/CD11c/Gr1/DX5-expressing cells using magnetic-activated cell sorting (MACS; Miltenyi Biotec, Auburn, CA). Cells (2.5 × 106/mL for apoptosis, 106/mL for proliferation) were cultured with 10 μg/mL anti-IgM F(ab)2 (Jackson ImmunoResearch Laboratories, West Grove, PA), 2 ng/mL IL-4, 1 μg/mL anti-CD40 (BD PharMingen), or 1 μg/mL lipopolysaccharide (LPS). To measure apoptosis, cells were stained with fluorescein isothiocyanate (FITC)–conjugated annexin V (BD PharMingen) and 7-aminoactinomycin D (7-AAD; Invitrogen, Carlsbad, CA) 24 hours later. For proliferation assay, B cells were labeled with 5- (and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) before stimulation and analyzed 3 days later. For calcium influx, splenic B cells were stained with anti-B220, incubated in Fluo-4 and Fura-Red (Invitrogen), and stimulated with 10 μg/mL anti-IgM.
Protein and gene expression analysis
Whole-cell extracts (WCEs) of in vitro–stimulated B cells were probed with antibodies to ATF4, cyclin D2 (Santa Cruz Biotechnology, Santa Cruz, CA), Bcl-xL (Cell Signaling Technology, Danvers, MA), total phosphotyrosine (4G10; Upstate Biotechnology, Charlottesville, VA), and β-actin or tubulin (Sigma-Aldrich). Total cell RNA was reverse-transcribed and assayed by quantitative real-time polymerase chain reaction (qPCR) as described.21
For global gene expression analysis, sorted follicular B cells (B220+AA4.1−CD23hiCD21int) were cultured with 10 μg/mL anti-IgM for 0, 2, and 12 hours. Reverse-transcribed RNA was amplified, labeled, and hybridized in duplicates to Mouse Genome 430 2.0 arrays (Affymetrix, Santa Clara, CA) as described.21 Hierarchical clustering and principal component analyses were done using the NIA Array software.27 Microarray data were deposited in the GEO database28 under accession number GSE13547.
Immunochemistry
Frozen spleen sections were stained with AF568-conjugated IgM and biotin-conjugated PNA followed by AF488-conjugated streptavidin, mounted using Vectashield medium (Vector Laboratories and analyzed on an Olympus IX71 fluorescent microscope equipped with 10×/0.3 NA and 20×/0.5 NA objectives (Olympus, Center Valley, PA). Images were acquired by a model C8484 monochrome camera (Hamamatsu, Bridgewater, NJ) and processed using Microsuite Five software (Olympus).
Statistical analysis
Statistical significance was estimated using the unpaired, 2-tailed Student t test.
Results
Zfx is required for early B-cell development at the pre-BCR selection checkpoint
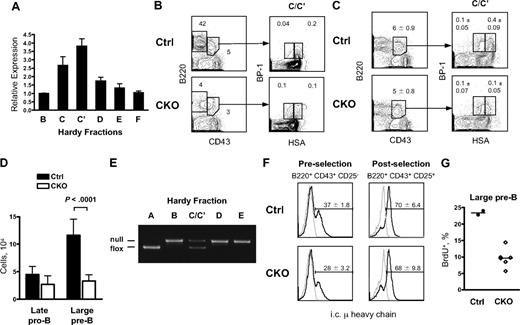
We measured Zfx levels in developing and mature B cells from the bone marrow (BM) and spleen by qPCR. As shown in Figure 1A, Zfx was expressed in all BM B-cell subsets, with the highest level in the Fr. C′ pre-B cells that have passed the pre-BCR selection checkpoint. To test whether Zfx is functionally required at this stage, we analyzed B-cell development in the BM of Tie2-Cre+Zfxflox/y CKO mice. The Tie2-Cre transgene mediates constitutive Cre recombination in endothelium and in all hematopoietic cells starting from fetal HSCs.22 Because Zfx deletion in Tie2-Cre CKO precedes lymphopoiesis, this system should show the role of Zfx at the earliest stages of B-cell development. Young adult Tie2-Cre CKO mice showed impaired HSC activity and a moderate reduction of mature T lymphocytes; in contrast, B cells were nearly absent from the BM and spleen.21 The BM of Tie2-Cre+ CKO mice showed an accumulation of late pro-B cells (Fr. C) at the expense of pre-B cells (Fr. C′; Figure 1B), suggesting a specific block at the pre-BCR selection checkpoint. Furthermore, CKO embryos and newborns showed a profound reduction of fetal liver B220+CD43+ pro/pre-B cells (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), whereas fetal liver HSCs were normal,21 and T-cell development was only slightly delayed (data not shown). Thus, Zfx appears to regulate early B-cell development independently of its role in HSC maintenance or general lymphopoiesis.
Zfx deletion results in a block in early B-cell development. (A) The expression of Zfx in sorted B-cell populations from the BM of wild-type mice. Shown are normalized Zfx expression levels relative to the Hardy fraction B sample, as determined by qPCR (mean ± SD of triplicate reactions). (B) The analysis of Hardy fractions in the BM of control and Tie2-Cre+Zfxflox/y CKO mice at 4 to 5 weeks. The percentages of fractions A-C′ (B220+ CD43+), D-F (B220hi CD43−), C (late pro-B, HSAint BP-1+), and C′ (large pre-B, HSAhigh BP-1+) in the total BM are shown for a representative staining. The ratio of Fr. C′/Fr. C populations was 4 in control and 1 in CKO BM (P = .003, average of 4 mice per group). (C) The analysis of Hardy fractions in the BM of control and Mb1-Cre+Zfxflox/y CKO mice at 4 to 6 weeks. The percentages of fractions A-C′, C, and C′ are indicated (mean ± SD of 6-7 mice per group). (D) Absolute numbers of fractions C and C′ in control and Mb1-Cre+Zfxflox/y CKO mice (mean ± SD of 6-7 mice per group). (E) Excision efficiency of Zfx in sorted Hardy fractions of Mb1-Cre+Zfxflox/y CKO mice as determined by genomic PCR. (F) The expression of intracellular μ heavy chain in control and Mb1-Cre+Zfxflox/y CKO mice. Intracellular IgM histogram profiles of B220+ CD43+ CD25− late pro-B cells and B220+ CD43+ CD25+ large pre-B cells are shown; the IgM+ fraction is indicated (mean percentage ± SD of 3 mice per group). Gray histograms represent isotype control. (G) Proliferation of large pre-B cells in control and Mb1-Cre+Zfxflox/y CKO mice. Mice were injected intraperitoneally with BrdU 50 minutes before analysis. The fraction of BrdU+ cells in the B220+ CD43+ CD25+ large pre-B cells of individual mice is shown.
Zfx deletion results in a block in early B-cell development. (A) The expression of Zfx in sorted B-cell populations from the BM of wild-type mice. Shown are normalized Zfx expression levels relative to the Hardy fraction B sample, as determined by qPCR (mean ± SD of triplicate reactions). (B) The analysis of Hardy fractions in the BM of control and Tie2-Cre+Zfxflox/y CKO mice at 4 to 5 weeks. The percentages of fractions A-C′ (B220+ CD43+), D-F (B220hi CD43−), C (late pro-B, HSAint BP-1+), and C′ (large pre-B, HSAhigh BP-1+) in the total BM are shown for a representative staining. The ratio of Fr. C′/Fr. C populations was 4 in control and 1 in CKO BM (P = .003, average of 4 mice per group). (C) The analysis of Hardy fractions in the BM of control and Mb1-Cre+Zfxflox/y CKO mice at 4 to 6 weeks. The percentages of fractions A-C′, C, and C′ are indicated (mean ± SD of 6-7 mice per group). (D) Absolute numbers of fractions C and C′ in control and Mb1-Cre+Zfxflox/y CKO mice (mean ± SD of 6-7 mice per group). (E) Excision efficiency of Zfx in sorted Hardy fractions of Mb1-Cre+Zfxflox/y CKO mice as determined by genomic PCR. (F) The expression of intracellular μ heavy chain in control and Mb1-Cre+Zfxflox/y CKO mice. Intracellular IgM histogram profiles of B220+ CD43+ CD25− late pro-B cells and B220+ CD43+ CD25+ large pre-B cells are shown; the IgM+ fraction is indicated (mean percentage ± SD of 3 mice per group). Gray histograms represent isotype control. (G) Proliferation of large pre-B cells in control and Mb1-Cre+Zfxflox/y CKO mice. Mice were injected intraperitoneally with BrdU 50 minutes before analysis. The fraction of BrdU+ cells in the B220+ CD43+ CD25+ large pre-B cells of individual mice is shown.
To ensure that the block in early B-cell development is intrinsic to B cells, we used the Mb1-Cre knockin strain that mediates B cell–specific Cre recombination commencing at the late pro–B-cell stage (Fr. B).23 Analysis of Mb1-Cre+ CKO mice showed a significant reduction of large pre-B cells (Fr. C′) (Figure 1C,D). The analysis of Zfx recombination efficiency in CKO B cells showed efficient deletion starting from Fr. B; however, the Fr. C/Fr. C′ population contained a large proportion of nonrecombined Zfx-proficient cells (Figure 1E). Subsequent fractions contained a fully recombined Zfx allele, probably because of the ongoing activity of Cre recombinase in developing B cells. This result suggests that Zfxnull pro-B cells are disadvantaged at the transition to pre-B cells, leading to the expansion of residual nondeleted cells in the Fr. C/C′ population. The proportion of late pro-B cells expressing the μ heavy chain was only marginally reduced in CKO mice (Figure 1F), suggesting that pre-BCR formation is largely intact in the absence of Zfx. However, the proliferation of large pre-B cells from CKO mice was impaired, as shown by their reduced BrdU incorporation rate (Figure 1G). Altogether, these data show that Zfx is required for the proliferative response to pre-BCR signaling during early B-cell development.
Zfx regulates the homeostasis of peripheral B cells
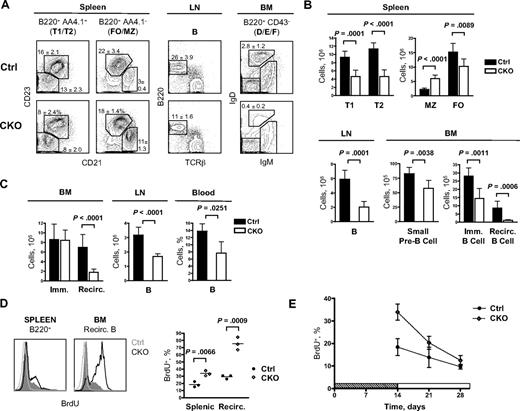
Consistent with a partial developmental block largely bypassed by nondeleted cells, Mb1-Cre+ CKO mice showed only a modest reduction in peripheral B-cell numbers. Indeed, BM small pre-B cells (Fr. D), immature B cells (Fr. E), splenic transitional type 1 (T1) and type 2 (T2) immature B cells, and splenic FO B cells were reduced approximately 1.5- to 2-fold (Figure 2A,B). Notably, MZ B-cell numbers were increased in young (4-6 weeks) CKO mice (Figure 2B). Although the increase was not significant in older (8-10 weeks) CKO mice (Figure S2), the MZ/FO ratio was increased in all cases, suggesting that Zfx may normally oppose MZ subset development. In contrast, B cells in the LNs were reduced 3-fold, and mature recirculating B cells in the BM (Fr. F) were reduced 8-fold. Thus, Zfx deletion severely impaired the maintenance of mature recirculating B cells.
Defective maintenance of peripheral B cells in the absence of Zfx. (A) The analysis of B-cell populations in the BM, spleen, and LNs of control and Mb1-Cre+Zfxflox/y CKO mice at 4 to 6 weeks. Representative staining profiles are shown, with the percentages of select B-cell subsets indicated (mean ± SD of 5-7 mice per group). (Left) Splenic transitional type T1 (CD21lowCD23low) and T2 (CD21+CD23+), follicular (FO; CD21int CD23high), and marginal zone (MZ; CD21highCD23low); (middle) lymph node B cells; (Right) BM Fr. F recirculating B cells (IgMintIgDhigh). (B) The absolute number of B-cell subsets in control and Mb1-Cre+Zfxflox/y CKO mice (mean ± SD of 5-7 mice per group). (C) The analysis of B-cell populations in the BM, LNs, and blood of control and CD19-Cre+Zfxflox/y CKO mice at 8 to 12 weeks. The graphs show the absolute number of immature (Fr. E) and mature recirculating (Fr. F) BM B cells (mean ± SD of 10-11 mice), the absolute number of LN B220+ B cells (mean ± SD of 6 mice), and the fraction of blood B220+ B cells (mean ± SD of 3-5 mice). (D) B-cell turnover in control and CD19-Cre+Zfxflox/y CKO mice. Mice were left untreated (open gray histograms) or fed BrdU for 2 weeks. Representative BrdU staining of splenic B220+ B cells and B220hi IgDhi recirculating BM B cells is shown. The graph indicates average percentage of BrdU+ B cells in individual control and CKO mice. Immature B cells in both control and CKO BM were 70% to 85% BrdU+ (not shown). (E) B-cell lifespan in control and CD19-Cre+Zfxflox/y CKO mice pulsed with BrdU for 2 weeks. The graph shows the fraction of BrdU+ splenic B cells at the indicated time points after BrdU withdrawal (mean ± SD of 3 mice).
Defective maintenance of peripheral B cells in the absence of Zfx. (A) The analysis of B-cell populations in the BM, spleen, and LNs of control and Mb1-Cre+Zfxflox/y CKO mice at 4 to 6 weeks. Representative staining profiles are shown, with the percentages of select B-cell subsets indicated (mean ± SD of 5-7 mice per group). (Left) Splenic transitional type T1 (CD21lowCD23low) and T2 (CD21+CD23+), follicular (FO; CD21int CD23high), and marginal zone (MZ; CD21highCD23low); (middle) lymph node B cells; (Right) BM Fr. F recirculating B cells (IgMintIgDhigh). (B) The absolute number of B-cell subsets in control and Mb1-Cre+Zfxflox/y CKO mice (mean ± SD of 5-7 mice per group). (C) The analysis of B-cell populations in the BM, LNs, and blood of control and CD19-Cre+Zfxflox/y CKO mice at 8 to 12 weeks. The graphs show the absolute number of immature (Fr. E) and mature recirculating (Fr. F) BM B cells (mean ± SD of 10-11 mice), the absolute number of LN B220+ B cells (mean ± SD of 6 mice), and the fraction of blood B220+ B cells (mean ± SD of 3-5 mice). (D) B-cell turnover in control and CD19-Cre+Zfxflox/y CKO mice. Mice were left untreated (open gray histograms) or fed BrdU for 2 weeks. Representative BrdU staining of splenic B220+ B cells and B220hi IgDhi recirculating BM B cells is shown. The graph indicates average percentage of BrdU+ B cells in individual control and CKO mice. Immature B cells in both control and CKO BM were 70% to 85% BrdU+ (not shown). (E) B-cell lifespan in control and CD19-Cre+Zfxflox/y CKO mice pulsed with BrdU for 2 weeks. The graph shows the fraction of BrdU+ splenic B cells at the indicated time points after BrdU withdrawal (mean ± SD of 3 mice).
To study the role of Zfx in peripheral B-cell homeostasis without affecting early B-cell development, we used the B-cell–specific CD19-Cre knockin deleter strain.24 In this strain, Zfx deletion was initiated in small pre-B cells and became prominent only in immature BM B cells and T1/T2 splenic B cells (Figure S3). Nuclear Zfx staining was absent from IgD+ B cells in the spleen, and Zfx protein was undetectable in purified B cells from CD19-Cre+ CKO mice (Figure S3). B-cell development in the BM and spleen appeared largely normal in CD19-Cre+ CKO mice, including normal absolute numbers of MZ and FO B cells and normal splenic architecture (Figure S4). Importantly, CD19-Cre+ CKO mice showed reduced B-cell numbers in blood and LNs and a prominent depletion of recirculating B cells in the BM (Figure 2C; Figure S4). These and similar observations in Mb1-Cre+ CKO mice suggest that Zfx controls the homeostasis of peripheral B cells, particularly of the recirculating mature B-cell population.
To confirm this notion, we measured the rate of BrdU incorporation into peripheral B cells in CD19-Cre+ CKO and control mice. After 2 weeks of continuous BrdU exposure, the fraction of BrdU+ total splenic B cells was increased in CKO mice, showing their accelerated turnover (Figure 2D). Moreover, most recirculating BM B cells were BrdU− in controls but BrdU+ in CKO mice, suggesting that preexisting BrdU− cells failed to survive beyond 2 weeks in the latter. After BrdU withdrawal, the proportion of BrdU+ B cells declined to approximately 50% of the initially labeled fraction in control mice (Figure 2E). Although CD19-Cre+ CKO mice started with a greater proportion of BrdU+ B cells, these labeled B cells declined rapidly to only 35% of the pulsed fraction after 2 weeks. The proliferation rate of Zfx-deficient splenic and mature BM B cells was not increased (Figure S5), ruling out hyperproliferation as a cause of accelerated BrdU loss. Therefore, the increased turnover of peripheral B cells and especially of recirculating BM B cells reflects their impaired survival without Zfx.
Impaired B-1 cell development and function in the absence of Zfx
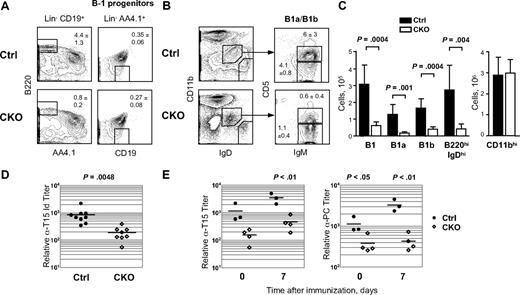
In view of the impaired homeostasis of conventional B cells, we analyzed the role of Zfx in the B-1 lineage. A normal fraction of AA4.1+CD19+B220− B-1 cell progenitors were present in the BM of CD19-Cre+ CKO mice (Figure 3A). In contrast, IgM+ CD11bint B-1 cells, including the CD5− B-1b and especially the CD5+ B-1a subtypes, were severely reduced in the peritoneal cavity (Figure 3B,C). Similar B-1 cell depletion was observed in Mb1-Cre+ CKO mice (data not shown). Accordingly, sera of CD19-Cre+ CKO mice contained significantly lower IgM levels of the T15 idiotype,26 which is predominantly secreted by B-1 cells29 (Figure 3D). Next, we immunized mice with phosphorylcholine (PC) hapten conjugated to keyhole limpet hemocyanin (PC-KLH), the antigen that mainly triggers a B-1–mediated immune response.30 In CKO mice, the secretion of T15 idiotype and especially of PC-specific IgM was severely reduced in response to PC-KLH, showing a defective B-1 cell–mediated immune response in vivo (Figure 3E). These results show that Zfx is required for the maintenance and function of B-1 lineage.
Impaired B1 cell–mediated responses in the absence of Zfx. (A) B-1 cell progenitors in the BM of control and CD19-Cre+Zfxflox/y CKO mice at 8 to 12 weeks. The fraction of lineage (Lin; CD11b/Gr-1/Ly-6C/IgM/Ter119/NK1.1/CD8/TCR)− AA4.1+ CD19+ B220− B-1 progenitors in the total BM is indicated (mean ± SD of 4 mice); (left) mature recirculating B cells are shown for comparison. (B) B-1 B cells in the peritoneal cavity of control and CD19-Cre+Zfxflox/y CKO mice at 8 to 12 weeks. Representative staining profiles show CD11b− IgDhi conventional B cells and CD11bint IgDint IgMhi CD5+ (B-1a) and CD5− (B-1b) B1 cells, with the percentage among total peritoneal cavity cells indicated (mean ± SD of 3-4 mice). (C) Absolute numbers of B-1 cells, conventional B cells (B220+ IgDhi) and macrophages (CD11bhi) in the peritoneal cavity of control and CKO mice (mean ± SD of 6 mice). (D) B-1 cell–derived T15 idiotype IgM titers in the sera of naive control and CD19-Cre+Zfxflox/y CKO mice. Symbols represent individual mice and horizontal lines represent the mean values. (E) T15 idiotype and anti-PC IgM titers in control and CD19-Cre+Zfxflox/y CKO mice immunized with PC-KLH. Symbols represent individual mice and horizontal lines represent the mean values.
Impaired B1 cell–mediated responses in the absence of Zfx. (A) B-1 cell progenitors in the BM of control and CD19-Cre+Zfxflox/y CKO mice at 8 to 12 weeks. The fraction of lineage (Lin; CD11b/Gr-1/Ly-6C/IgM/Ter119/NK1.1/CD8/TCR)− AA4.1+ CD19+ B220− B-1 progenitors in the total BM is indicated (mean ± SD of 4 mice); (left) mature recirculating B cells are shown for comparison. (B) B-1 B cells in the peritoneal cavity of control and CD19-Cre+Zfxflox/y CKO mice at 8 to 12 weeks. Representative staining profiles show CD11b− IgDhi conventional B cells and CD11bint IgDint IgMhi CD5+ (B-1a) and CD5− (B-1b) B1 cells, with the percentage among total peritoneal cavity cells indicated (mean ± SD of 3-4 mice). (C) Absolute numbers of B-1 cells, conventional B cells (B220+ IgDhi) and macrophages (CD11bhi) in the peritoneal cavity of control and CKO mice (mean ± SD of 6 mice). (D) B-1 cell–derived T15 idiotype IgM titers in the sera of naive control and CD19-Cre+Zfxflox/y CKO mice. Symbols represent individual mice and horizontal lines represent the mean values. (E) T15 idiotype and anti-PC IgM titers in control and CD19-Cre+Zfxflox/y CKO mice immunized with PC-KLH. Symbols represent individual mice and horizontal lines represent the mean values.
Delayed T cell–dependent immune response in the absence of Zfx
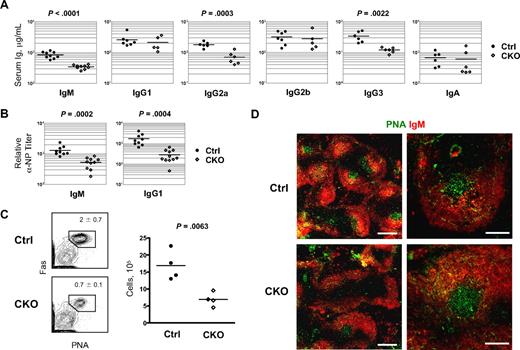
Next, we analyzed the antibody-secreting capacity of Zfx-deficient B cells. As shown in Figure 4A, sera from naive CD19-Cre+ CKO mice contained lower total IgM, which are mainly secreted by B-1 cells in naive mice. In addition, serum IgG2a and IgG3 levels were moderately reduced, whereas IgG1, IgG2b, and IgA levels were normal (Figure 4A). After immunization with T-independent antigen 4-hydroxy-3-nitrophenylacetyl (NP) conjugated to Ficoll (NP-Ficoll), NP-specific IgM secretion in CD19-Cre+ CKO mice was slightly reduced at 7 days but normal at 14 days (Figure S6). Secretion of NP-specific IgG was normal at both time points, indicating that Zfx is generally dispensable for T-independent immune responses.
Delayed antibody response to T-dependent antigen after B cell–specific Zfx deletion. (A) Serum Ig levels in naive control and CD19-Cre+Zfxflox/y CKO mice at 8 to 12 weeks. Significant differences between the groups are indicated. (B) Antibody response to T-dependent antigen in control and CD19-Cre+Zfxflox/y CKO mice. Mice were immunized with NP-KLH, and relative anti-NP IgM and IgG1 titers were determined 7 days later. (C) The analysis of GC B cells in control and CKO mice immunized as in panel C. Shown are staining profiles of splenic B220+ IgDlo B cells, with the fraction of PNA+ Fas+ GC B cells indicated (mean ± SD of 4 mice). The graph shows absolute GC B-cell numbers per spleen. (D) Immunochemical analysis of germinal center (GC) response to T-dependent antigen. Control and CD19-Cre+Zfxflox/y CKO spleens 7 days after immunization with NP-KLH were stained for GC B cells with PNA in combination with anti-IgM. Bars represent 0.5 mm (left) and 0.2 mm (right).
Delayed antibody response to T-dependent antigen after B cell–specific Zfx deletion. (A) Serum Ig levels in naive control and CD19-Cre+Zfxflox/y CKO mice at 8 to 12 weeks. Significant differences between the groups are indicated. (B) Antibody response to T-dependent antigen in control and CD19-Cre+Zfxflox/y CKO mice. Mice were immunized with NP-KLH, and relative anti-NP IgM and IgG1 titers were determined 7 days later. (C) The analysis of GC B cells in control and CKO mice immunized as in panel C. Shown are staining profiles of splenic B220+ IgDlo B cells, with the fraction of PNA+ Fas+ GC B cells indicated (mean ± SD of 4 mice). The graph shows absolute GC B-cell numbers per spleen. (D) Immunochemical analysis of germinal center (GC) response to T-dependent antigen. Control and CD19-Cre+Zfxflox/y CKO spleens 7 days after immunization with NP-KLH were stained for GC B cells with PNA in combination with anti-IgM. Bars represent 0.5 mm (left) and 0.2 mm (right).
When immunized with a T-dependent antigen NP-KLH, CD19-Cre+ CKO mice mounted lower titers of NP-specific IgM, IgG1, IgG2a, and IgG2b 7 days after immunization (Figure S7). However, NP-specific Ig reached normal levels after 21 days and showed normal induction after boost immunization. The delayed response in CKO mice was further evident in decreased NP-specific IgG1 titers (Figure 4B) and in reduced numbers of GC B cells 7 days after immunization (Figure 4C). Histologic analysis showed a lower frequency but relatively normal size of PNA+ GC in CKO spleens (Figure 4D), suggesting impaired survival of activated Zfx-deficient B cells before the initiation of GC formation, or their higher activation threshold, or both. Consistent with the latter, we observed normal GC response to a stronger stimulus, sheep red blood cells (data not shown). Thus, Zfx expression is required for the optimal GC reaction and antibody response to T-dependent antigens.
Zfx promotes BCR-induced survival and proliferation
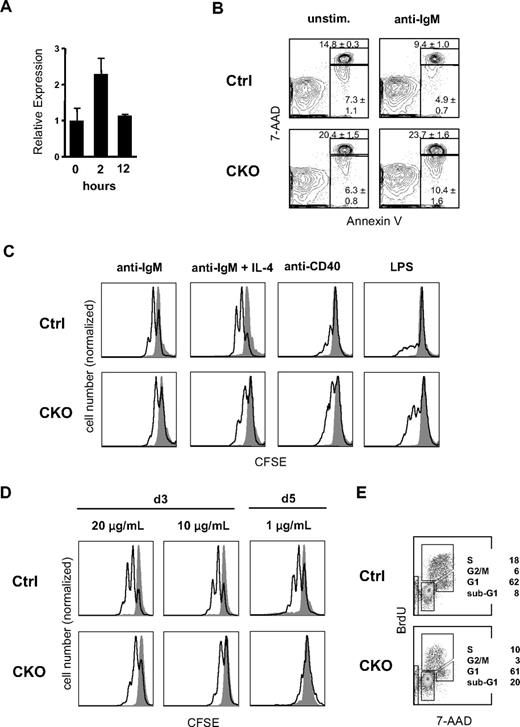
Because mature B-cell homeostasis and GC reaction are governed by the BCR, we examined the role of Zfx in BCR signaling. As shown in Figure 5A, the expression of Zfx was induced approximately 2-fold in mature B cells 2 hours after BCR crosslinking with anti-IgM in vitro. The analysis of splenic B cells from CD19-Cre+ CKO mice showed a significant increase in apoptotic (annexin V+7-AAD−) and dead (annexin V+7-AAD+) cell fractions after stimulation with anti-IgM (Figure 5B), as well as with LPS or anti-CD40 (Figure S8). However, CKO B cells (including sorted T1 subset) showed the expected increase in survival after incubation with B-cell survival factor BAFF (Figure S9). The analysis of CFSE dye dilution showed that Zfx-deficient B-cell proliferation was impaired in response to anti-IgM stimulation alone or with IL-4 (Figure 5C) but not to anti-CD40 or LPS. The proliferation defect was most pronounced at low concentrations of anti-IgM (Figure 5D). Furthermore, anti–IgM-stimulated Zfx-deficient B cells showed a reduction in the S and G2/M phases of the cell cycle and an increase of sub-G0/G1 apoptotic cells (Figure 5E). Altogether, these results show the role of Zfx in BCR-induced cell expansion and survival.
Impaired BCR-induced survival and proliferation in Zfx-deficient B cells. (A) The expression of Zfx in wild-type FO B cells after BCR stimulation. Normalized Zfx expression levels were determined by qPCR relative to the nonstimulated sample (mean ± SD of triplicate reactions). (B) BCR-induced apoptosis of B cells from control and CD19-Cre+Zfxflox/y CKO mice. Representative staining profiles of annexin V and DNA content (7-AAD) are shown for restingB cells cultured in vitro with or without anti-IgM for 24 hours. The fractions of annexin V+7-AAD− apoptotic cells and annexin V+7-AAD+ dead cells are indicated (mean ± SD of 3 mice per group). (C) Proliferation of IgM-stimulated B cells from control and CD19-Cre+Zfxflox/y CKO mice. Shown is CFSE dilution in B220+ B cells cultured for 3 days after anti-IgM stimulation (open histograms) or in medium alone (gray histograms). Note that the histograms are normalized by the height of the CFSEbright quiescent cell peak, which is decreased in CKO B cells because of apoptosis; hence, the apparent increase in CKO B-cell response to anti-CD40 and LPS. Representative of 4 independent experiments. (D) Dose response of BCR-induced B-cell proliferation. B-cell proliferation was measured as above 3 or 5 days after stimulation with the indicated concentrations of anti-IgM. (E) Cell-cycle analysis of BCR-stimulated B cells from control and CD19-Cre+Zfxflox/y CKO mice. Splenic B cells were cultured in vitro with anti-IgM for 48 hours and pulsed with BrdU 45 minutes before analysis. Representative staining profiles of BrdU and DNA content (7-AAD) with the percentage of cells in the indicated cell-cycle fractions are shown.
Impaired BCR-induced survival and proliferation in Zfx-deficient B cells. (A) The expression of Zfx in wild-type FO B cells after BCR stimulation. Normalized Zfx expression levels were determined by qPCR relative to the nonstimulated sample (mean ± SD of triplicate reactions). (B) BCR-induced apoptosis of B cells from control and CD19-Cre+Zfxflox/y CKO mice. Representative staining profiles of annexin V and DNA content (7-AAD) are shown for restingB cells cultured in vitro with or without anti-IgM for 24 hours. The fractions of annexin V+7-AAD− apoptotic cells and annexin V+7-AAD+ dead cells are indicated (mean ± SD of 3 mice per group). (C) Proliferation of IgM-stimulated B cells from control and CD19-Cre+Zfxflox/y CKO mice. Shown is CFSE dilution in B220+ B cells cultured for 3 days after anti-IgM stimulation (open histograms) or in medium alone (gray histograms). Note that the histograms are normalized by the height of the CFSEbright quiescent cell peak, which is decreased in CKO B cells because of apoptosis; hence, the apparent increase in CKO B-cell response to anti-CD40 and LPS. Representative of 4 independent experiments. (D) Dose response of BCR-induced B-cell proliferation. B-cell proliferation was measured as above 3 or 5 days after stimulation with the indicated concentrations of anti-IgM. (E) Cell-cycle analysis of BCR-stimulated B cells from control and CD19-Cre+Zfxflox/y CKO mice. Splenic B cells were cultured in vitro with anti-IgM for 48 hours and pulsed with BrdU 45 minutes before analysis. Representative staining profiles of BrdU and DNA content (7-AAD) with the percentage of cells in the indicated cell-cycle fractions are shown.
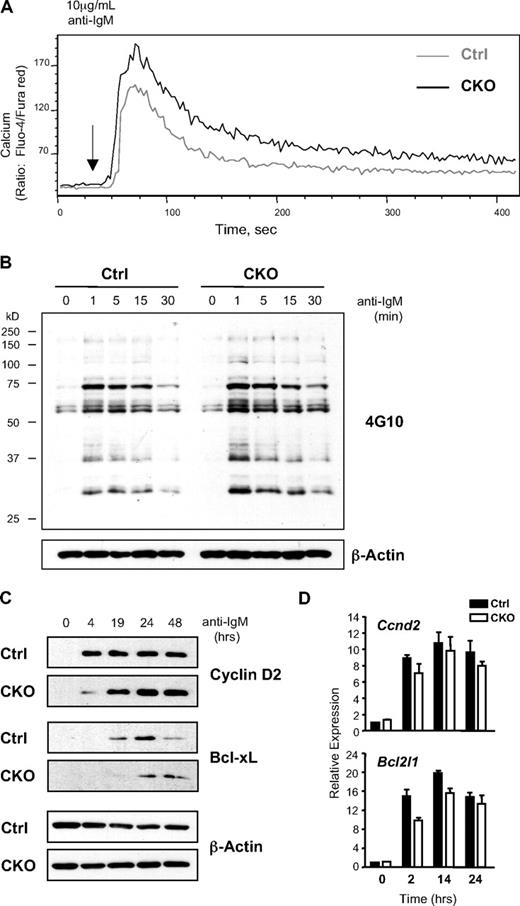
Despite the observed impairment of BCR-induced survival and proliferation, Ca2+ flux in response to anti-IgM stimulation was intact and even slightly elevated in Zfx-deficient B cells (Figure 6A). Similarly, the induction of total tyrosine phosphorylation was normal in BCR-stimulated CKO B cells (Figure 6B), and the activation of major signaling pathways, including ERK, JNK, p38, NF-κB, and Akt, appeared relatively normal (Figure S10). In contrast, the subsequent induction of critical proliferation and survival proteins cyclin D2 and Bcl-xL31,32 was delayed in Zfx-deficient B cells (Figure 6C). Notably, the induction of both transcripts was comparable in CKO and control cells at all time points (Figure 6D), pointing to a defect at the posttranscriptional level. These data suggest that Zfx is required for optimal synthesis of key effector proteins such as cyclin D2 and Bcl-xL in BCR-stimulated B cells.
Impaired response to BCR stimulation in Zfx-deficient B cells. (A) The analysis of BCR-induced Ca2+ mobilization in B cells from control and CD19-Cre+Zfxflox/y CKO mice. Histogram profiles show intracellular Ca2+ flux of splenic B220+ B cells on the addition of anti-IgM. Data are representative of 3 independent experiments. (B) The analysis of proximal signaling events in BCR-stimulated B cells from control and CKO mice. Splenic B cells at the indicated time points after anti-IgM stimulation were analyzed for total tyrosine phosphorylation by Western blotting. Data are representative of 4 independent experiments. (C) The analysis of late signaling events in BCR-stimulated B cells from control and CKO mice. Splenic B cells at the indicated time points after anti-IgM stimulation were probed for cyclin D2 and Bcl-XL protein expression. Data are representative of 3 independent experiments. (D) The expression of Ccnd2 (cyclin D2) and Bcl2l1 (Bcl-XL) transcripts in BCR-stimulated B cells. Shown are normalized expression levels relative to the nonstimulated sample as determined by qPCR (mean ± SD of triplicate reactions).
Impaired response to BCR stimulation in Zfx-deficient B cells. (A) The analysis of BCR-induced Ca2+ mobilization in B cells from control and CD19-Cre+Zfxflox/y CKO mice. Histogram profiles show intracellular Ca2+ flux of splenic B220+ B cells on the addition of anti-IgM. Data are representative of 3 independent experiments. (B) The analysis of proximal signaling events in BCR-stimulated B cells from control and CKO mice. Splenic B cells at the indicated time points after anti-IgM stimulation were analyzed for total tyrosine phosphorylation by Western blotting. Data are representative of 4 independent experiments. (C) The analysis of late signaling events in BCR-stimulated B cells from control and CKO mice. Splenic B cells at the indicated time points after anti-IgM stimulation were probed for cyclin D2 and Bcl-XL protein expression. Data are representative of 3 independent experiments. (D) The expression of Ccnd2 (cyclin D2) and Bcl2l1 (Bcl-XL) transcripts in BCR-stimulated B cells. Shown are normalized expression levels relative to the nonstimulated sample as determined by qPCR (mean ± SD of triplicate reactions).
Zfx restrains integrated stress response after BCR stimulation
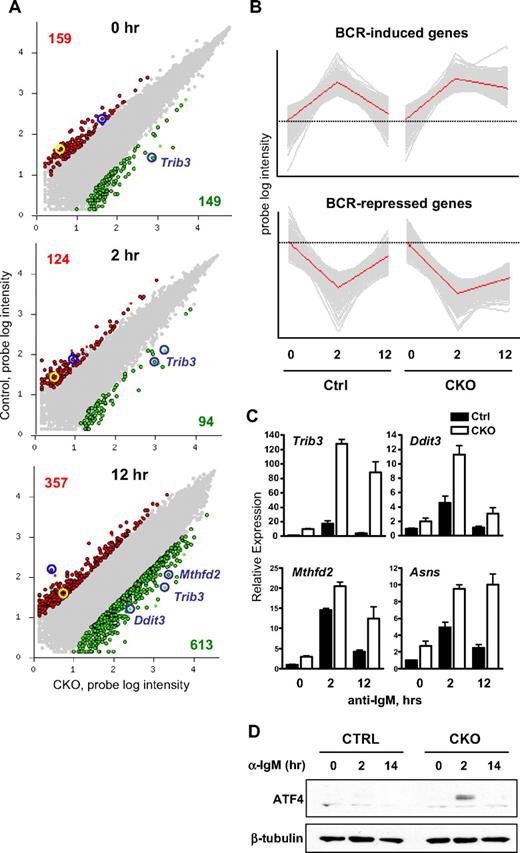
To gain insight into the molecular basis of Zfx activity in B cells, we compared global expression profiles of sorted FO B cells from control and CD19-Cre+ CKO mice without stimulation or stimulated with anti-IgM for 2 and 12 hours. Previously identified Zfx target genes 6720467C03Rik and Dis3l (formerly annotated as AV340375) were among the genes down-regulated in Zfx-deficient B cells at all times (Figure 7A), validating the expression data. Comparison of the genes differentially expressed at least 5-fold between control and Zfx-deficient B cells indicated that 308 genes were differentially expressed at the 0 hour time point, 218 genes at 2 hours, and 970 genes at 12 hours (Figure 7A). The major increase in differentially expressed genes at 12 hours suggests that Zfx-deficient B cells are impaired at late time points of BCR stimulation. Moreover, principal component analysis (PCA) of the data showed a large fraction of genes (n = 744) that were transiently up-regulated at 2 hours in both control and CKO B cells but failed to return to the basal level at 12 hours only in the CKO (Figure 7B). Similarly, many genes reduced at 2 hours (n = 526) failed to reach their original levels only in Zfx-deficient cells. Therefore, CKO B cells exhibit correct global expression changes in response to BCR stimulation, whereas their subsequent recovery and progression appears impaired.
Zfx loss induces integrated stress response in BCR-activated B cells. (A) Differential gene expression in BCR-stimulated B cells from control and CD19-Cre+Zfxflox/y CKO mice. Sorted FO B cells were stimulated with anti-IgM for 0, 2, or 12 hours and analyzed by microarray expression profiling. Shown is pair-wise comparison of control (Ctrl) and CKO cells at each time point; the scatter plots represent normalized log intensities of individual microarray probe signals. The probes increased or decreased greater than 5-fold in CKO cells are indicated in green and red, respectively, with the number of each probe set indicated. Probes for previously identified Zfx targets 6720467C03Rik and Dis3l are highlighted in yellow and blue circles, respectively. The probes for ISR target genes induced in CKO cells are indicated. (B) Principal component analysis (PCA) of global gene expression in BCR-stimulated FO B cells. Shown are the top principal components in the dataset, with gray lines representing individual probes and red lines representing the average probe intensity in the probe set. The average intensities at the zero time point are indicated by dashed lines for each principal component. (C) The expression of ISR genes in BCR-stimulated FO B cells from control and CD19-Cre+Zfxflox/y CKO mice. FO B cells were stimulated as in Figure 6A. Shown are normalized expression levels of indicated genes relative to the unstimulated control sample, as determined by qPCR (mean ± SD of triplicate reactions). (D) Expression of ATF4 protein in BCR-stimulated B cells from control and CD19-Cre+Zfxflox/y CKO mice. Resting B cells were stimulated with anti-IgM for the indicated time points. Whole cell lysates were analyzed by Western blotting for ATF4 and β-tubulin. Data are representative of 3 independent experiments.
Zfx loss induces integrated stress response in BCR-activated B cells. (A) Differential gene expression in BCR-stimulated B cells from control and CD19-Cre+Zfxflox/y CKO mice. Sorted FO B cells were stimulated with anti-IgM for 0, 2, or 12 hours and analyzed by microarray expression profiling. Shown is pair-wise comparison of control (Ctrl) and CKO cells at each time point; the scatter plots represent normalized log intensities of individual microarray probe signals. The probes increased or decreased greater than 5-fold in CKO cells are indicated in green and red, respectively, with the number of each probe set indicated. Probes for previously identified Zfx targets 6720467C03Rik and Dis3l are highlighted in yellow and blue circles, respectively. The probes for ISR target genes induced in CKO cells are indicated. (B) Principal component analysis (PCA) of global gene expression in BCR-stimulated FO B cells. Shown are the top principal components in the dataset, with gray lines representing individual probes and red lines representing the average probe intensity in the probe set. The average intensities at the zero time point are indicated by dashed lines for each principal component. (C) The expression of ISR genes in BCR-stimulated FO B cells from control and CD19-Cre+Zfxflox/y CKO mice. FO B cells were stimulated as in Figure 6A. Shown are normalized expression levels of indicated genes relative to the unstimulated control sample, as determined by qPCR (mean ± SD of triplicate reactions). (D) Expression of ATF4 protein in BCR-stimulated B cells from control and CD19-Cre+Zfxflox/y CKO mice. Resting B cells were stimulated with anti-IgM for the indicated time points. Whole cell lysates were analyzed by Western blotting for ATF4 and β-tubulin. Data are representative of 3 independent experiments.
One of the most significant genes up-regulated in Zfx-deficient B cells at all time points was Trib3, a sensitive and specific target of the integrated stress response (ISR).33,34 At 12 hours, the up-regulation of additional canonical ISR target genes such as Mthfd2 and Ddit3 (CHOP) became evident, suggesting that Zfx-deficient B cells undergo ISR after BCR stimulation (Figure 7A). Indeed, ISR targets Trib3, Mthfd2, Asns, and Ddit314,34 showed only transient induction in control B cells but a strong and sustained increase in Zfx-deficient B cells (Figure 7C). Furthermore, the key ISR mediator protein ATF4 was induced in Zfx-deficient B cells 2 hours after stimulation, but it was undetectable in control B cells (Figure 7D). In contrast, no difference in the splicing of XBP-1 transcript was detected in the absence of Zfx, suggesting the lack of UPR pathway activation (Figure S11). Thus, BCR stimulation of Zfx-deficient B cells leads to unrestrained ISR, which probably contributes to the delayed cyclin D2 and Bcl-xL synthesis and impaired proliferation and survival.
Discussion
In this work, conditional gene targeting was used to analyze the role of transcription factor Zfx in the B-cell lineage. We found that Zfx is required for the pro-B to pre-B-cell transition, as evidenced by a profound block after early pan-hematopoietic Zfx deletion in Tie2-Cre CKO mice. Furthermore, B cell–specific deletion in Mb1-Cre CKO mice caused partial block with strong counter-selection at that stage. Although cytokine IL-7 is critical for B-cell development, the absence of IL-7 or of its receptor causes an earlier block at the pro–B-cell stage.35 However, the observed developmental block is consistent with defective pre-BCR function, which may include pre-BCR assembly or signaling, or impaired cellular response to pre-BCR. Although the expression of pre-BCR complex appeared relatively normal, reduced BrdU incorporation by Zfx-deficient pre-B cells suggests an impaired pre–B-cell expansion as a cause of the phenotype.
In the periphery, the loss of Zfx was compatible with normal splenic B-cell development but caused the depletion of mature recirculating B cells in the blood, LN, and particularly the BM. Although B-cell homing or recirculation defects cannot be excluded, microarray analysis showed normal expression of key adhesion molecules, including integrins (LFA-1), selectins (CD62L) and chemokine receptors (CCR7, CXCR4). Furthermore, migration of Zfx-deficient B cells in response to CXCR4 ligand CXCL12 was only marginally impaired in a transwell assay (not shown). However, elevated B-cell turnover suggests the impaired survival as the most likely cause of peripheral B-cell loss. In addition, a defect in the generation or maintenance or both of the B-1 subset has been observed. Cytokine BAFF is essential for the survival of peripheral B cells36 ; however, relatively normal splenic B-cell numbers in vivo and a clear response to BAFF in vitro do not support a major role of Zfx downstream of BAFF. Another critical input into peripheral B-cell survival is provided by BCR signaling, because its blockade in mature B cells rapidly abolishes their maintenance.37 Stronger BCR signals were proposed to favor FO over MZ subset development38 ; hence, the decreased FO/MZ ratio in Mb1-Cre CKO animals may reflect reduced BCR signaling. Furthermore, B-1 cells do not require BAFF but depend on strong BCR signals for their development.1,6 Indeed, the deletion of specific downstream components of the BCR pathway predominantly affects the B-1 subset.39,40 The regulation of long-lived recirculating B cells appears particularly complex, with both decreased40,41 and increased12,42 BCR signaling leading to recirculating B-cell depletion. Thus, the results of Zfx deletion in mature B cells are consistent with aberrant cellular response to BCR signals, which would particularly affect long-lived recirculating and B-1 subsets.
Because pre-BCR and mature BCR are thought to signal in a generally similar way, the overall phenotype points to a defect in survival or proliferation or both in response to pre-BCR/BCR signaling in Zfx-deficient B cells. This was further corroborated by the delayed GC formation and T-dependent antibody response in the absence of Zfx. In vitro, this was reflected in the increased apoptosis and reduced cycling of Zfx-deficient B cells in response to BCR stimulation. The increase in cell death occurred in response to other stimuli such as LPS and might reflect a general role of Zfx in the survival of select cell populations such as HSCs and B cells. However, reduced proliferation was specific for BCR ligation but not other stimuli in B cells; furthermore, normal proliferation was previously observed in all Zfx-deficient cells, including stem cells.21 Thus, Zfx may independently regulate B-cell survival and proliferation to promote the optimal long-term response of B cells to BCR signals. Indeed, Zfx loss disrupted the global expression dynamics at a late (12 hour) time point and delayed the accumulation of key proliferation/survival proteins cyclin D2 and Bcl-xL. A very similar phenotype has been observed in B cells deficient for transcription factor Mef2c, except that the expression of cyclin D2 and Bcl-xL was impaired at the transcriptional level in that case.8 These studies emphasize the complex transcriptional regulation of B-cell activation, in which some factors appear specifically required for BCR-induced signals.
Genomewide expression analysis of Zfx-deficient B cells showed the aberrant activation of ISR after BCR stimulation. The 2 common causes of ISR are nutrient deprivation and ER stress, which activate eIF2α kinases GCN2 and PERK, respectively.15 The precise cause of the ISR in Zfx-deficient B cells remains to be determined and is likely to include both pathways. However, the observed normal processing of XBP-1 argues against the primary role of ER stress and emphasizes selective activation of the proapoptotic ISR but not of the antiapoptotic UPR. Because ISR reduces the synthesis rate of most cellular proteins, the observed ISR activation would explain the posttranscriptionally reduced accumulation of cyclin D2 and Bcl-xL. Indeed, the kinetics of the ISR activation, with prominent ATF4 synthesis 2 hours after stimulation, is consistent with the delay in cyclin D2 and Bcl-xL appearance several hours later. Furthermore, ISR was shown to directly down-regulate Bcl2 family proteins such as MCL-1, thereby activating the mitochondrial apoptosis pathway.43 The combination of ISR-mediated CHOP induction and delayed Bcl-xL accumulation is likely to impair the survival of Zfx-deficient B cells after BCR stimulation.
Zfx protein was readily observable in the nuclei of splenic B cells and was required for the expression of its direct target genes such as Dis3l in both resting and activated B cells. Thus, Zfx protein appears constitutively active in the B-cell lineage, but is particularly important during pre-BCR/BCR-mediated B-cell activation. This is similar to its preferential role in undifferentiated HSCs and embryonic stem cells compared with their differentiated progeny.21 One possible explanation in both cases is increased Zfx expression levels, as observed in stem cells, Fraction C/C′ pre-B cells and BCR-activated mature B cells. In addition, Zfx protein activity is likely to be modulated by cell type–specific cofactors and chromatin modification machinery, a model currently under investigation. Finally, cell type–specific Zfx activity may be determined by the nature of its direct target genes. Although the functionally relevant targets of Zfx remain to be defined, recent genomewide analysis showed several thousand genes bound by Zfx.44 This and our previous analysis21 suggest that Zfx primarily activates transcription, consistent with the presence of a strong activation domain. In contrast, ISR target genes up-regulated in Zfx-deficient B cells neither represent major targets of Zfx binding nor contain a consensus Zfx (Chen et al44 binding site and B.R., unpublished data, March 2009). Thus, the induction of these genes appears indirect and reflects the ISR activation caused by the loss of putative primary target genes.
It has been proposed that some aspects of lymphocyte biology, such as B- and T-cell memory maintenance, resemble and may have a shared genetic basis with HSC self-renewal.45,46 Indeed, some transcription factors appear to control both HSC function and lymphoid development or homeostasis or both. For instance, Polycomb group protein Bmi1 is essential for adult HSC self-renewal and for lymphocyte development and proliferation, but largely dispensable for erythromyeloid development.47 A similarly selective role in HSCs and lymphocytes was reported for Ikaros and related proteins.48 However, the cell-intrinsic roles of these factors in lymphocytes remain to be confirmed by conditional gene targeting. Our studies show an essential, cell-intrinsic requirement for Zfx in HSCs and in the B-cell lineage, providing further evidence for the shared genetic basis of HSC and lymphocyte maintenance. In particular, both HSCs and B cells showed higher levels of Zfx expression (the latter following pre-BCR/BCR signaling), common targets of Zfx transcriptional activity (such as Dis3l), and impaired survival in the absence of Zfx. Further studies should elucidate Zfx-dependent targets and pathways controlling survival and activity of stem cells and lymphocytes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank H. Gu and H. Babbe for providing mice, M. Reth for permission to use the Mb1-Cre strain, K. Calame for advice and reagents, and all members of the Reizis laboratory for helpful discussions.
This work was supported by National Institutes of Health grant HL084353 (B.R.), training grant AI007525 (T.L.A) and training grant GM007367 (M.R.S), and fellowship AI066459 (T.L.A.).
National Institutes of Health
Authorship
Contribution: T.L.A. designed research, performed experiments, analyzed results, and wrote the paper; M.R.S.-R. performed experiments; and B.R. designed research, analyzed results, and wrote the paper
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Boris Reizis, Department of Microbiology, Columbia University Medical Center, 701 W 168th St, New York, NY 10032; e-mail: bvr2101@columbia.edu.