Abstract
Class A scavenger receptor (SRA), also known as CD204, has been shown to participate in the pathogenesis of atherosclerosis and the pattern recognition of pathogen infection. However, its role in adaptive immune responses has not been well defined. In this study, we report that the lack of SRA/CD204 promotes Toll-like receptor (TLR)4 agonist–augmented tumor-protective immunity, which is associated with enhanced activation of CD8+ effector T cell and improved inhibition of tumor growth. Dendritic cells (DCs) deficient in SRA/CD204 display more effective immunostimulatory activities upon TLR4 engagement than those from wild-type counterparts. Silencing of SRA/CD204 by RNA interference improves the ability of DCs to prime antigen-specific CD8+ T cells, suggesting that antigen-presenting cells, for example, DCs, play a major role in SRA/CD204-mediated immune modulation. Our findings reveal a previously unrecognized role for SRA/CD204, a non-TLR pattern recognition receptor, as a physiologic negative regulator of TLR4-mediated immune consequences, which has important clinical implications for development of TLR-targeted immunotherapeutic intervention.
Introduction
Class A scavenger receptor (SRA), also known as CD204 or macrophage scavenger receptor, is a prototypic member of a family of structurally diverse transmembrane receptors collectively termed scavenger receptors.1 SRA/CD204 is preferentially expressed in immune cells of myeloid lineage, including dendritic cells (DCs) and macrophages (Mφs). SRA/CD204 has been shown to act as a pattern recognition receptor (PRR) capable of binding a broad range of ligands, including chemically modified or altered molecules, bacterial surface components, apoptotic cells, and endogenous danger molecules such as stress protein.2,3 The role of SRA/CD204 in atherosclerosis has been extensively studied, because it was the first receptor identified for modified lipoproteins that are pertinent to the development of vascular disease.4 Several studies have shown that SRA/CD204 deficiency resulted in impaired protection against pathogen infection,5,6 which has been partially attributed to the increased susceptibility of SRA/CD204-deficient animals to the overproduction of proinflammatory cytokines during endotoxic shock.7 Emerging evidence also implicates SRA/CD204 as a suppressor in an inflammatory response.8,9
During studies of SRA/CD204 as a binding structure on antigen-presenting cells (APCs) for immunostimulatory heat shock/stress protein and its contribution to the antitumor efficacy of heat shock protein (HSP)–formulated vaccines, we made an unexpected observation that the lack of SRA/CD204 significantly enhances HSP vaccine-generated immunity against poorly immunogenic tumors.10,11 In addition to HSPs, SRA/CD204 appears also capable of dampening the adjuvant effects derived from exogenous danger molecules of nonmammalian origins (eg, lipopolysaccharide [LPS]).11
The mammalian Toll-like receptor (TLR) family is the best characterized class of PRRs.12 Recognition of the microbial pattern molecules (ie, pathogen-associated molecular patterns) by TLRs triggers an intracellular signaling cascade involving adaptor molecules, protein kinases, and transcription factors.13 The significance of TLR signaling in enhancing antigen presentation and activating adaptive immunity has been well established. TLR-mediated activation of DCs includes up-regulation of major histocompatibility complex (MHC) class II and costimulatory molecules and secretion of proinflammatory cytokines or chemokines.14 Many established and experimental vaccines incorporate agonists for TLRs, not only to protect against infectious diseases, but also in therapeutic immunization against cancer.15
Although several lines of evidence suggest a role of SRA/CD204 in the host innate immune response, the functional significance of SRA/CD204 in adaptive immunity triggered by TLR signaling has not been established. In this study, we have directly assessed the contribution of SRA/CD204 to antigen-specific CD8+ T-cell responses augmented by TLR4 agonist used as an adjuvant. We demonstrate that SRA/CD204 deficiency promotes expansion and activation of endogenous as well as adoptively transferred antigen-specific CD8+ T lymphocytes. Moreover, SRA/CD204-deficient DCs upon TLR4 activation are more potent in priming naive T cells than their wild-type (WT) counterparts. Lastly, RNA interference-mediated silencing of SRA/CD204 in DCs also leads to improved T-cell activation. The results provide the first evidence that SRA/CD204 is capable of modifying adaptive immune responses that arose from the TLR4 activation.
Methods
Mice and cell lines
WT C57BL/6 mice were obtained from National Cancer Institute (Bethesda, MD). SRA/CD204 knockout (KO) mice (SRA−/−) have been backcrossed to C57BL/6 mice for at least 10 generations. SRA−/− mice, recombination-activating gene (RAG)-1 (C57BL/6J-Rag1tm1mom) mice, and Pmel mice that carry T-cell receptor (TCR) transgene specific for the mouse homologue (pmel-17) of human glycoprotein (gp)10016 were purchased from The Jackson Laboratory (Bar Harbor, ME). All experiments and procedures involving mice were approved by the Institutional Animal Care and Use Committee of Roswell Park Cancer Institute. B16-ovalbumin (OVA; H-2b) and EG7 cells were maintained in Dulbecco modified Eagle medium and RPMI 1640, respectively, supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Antibodies and reagents
Mouse monoclonal antibodies (mAbs) to CD8 (53-6.7), CD11c (HL3), CD44 (IM7), CD69 (H1.2F3), and isotype control rat immunoglobulin (Ig)G2b (RTK4530) were purchased from BioLegend (San Diego, CA). I-A/I-E (MHC class II, M5/114.15.2), CD40 (1C10), and CD86 (PO3.1) were purchased from eBioscience (San Diego, CA); CD62L (MEL-14-H2.100), mouse plasmacytoid dendritic cell antigen-1 (JF05-IC2.2.1), and CD11c microbeads (N418) were purchased from Miltenyi Biotec (Auburn, CA). CD80 (16-10A1) was purchased from BD Pharmingen (San Jose, CA). SRA polyclonal antibody for immunoblotting and mAb (2F8) for fluorescence-activated cell sorter (FACS) analysis were purchased from R&D Systems (Minneapolis, MN) and AbD Serotec (Raleigh, NC), respectively. iTag MHC tetramer (OVA, SIINFEKL) was purchased from Beckman Coulter (Fullerton, CA). Recombinant human interleukin (IL)-2 was purchased from Novartis Pharmaceuticals (Emeryville, CA). The gp100 protein was prepared, as previously described.11 Monophosphoryl lipid A (MPL) and LPS were purchased from Sigma-Aldrich (St Louis, MO). CellTrace 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE) cell proliferation kit was purchased from Molecular Probes (Eugene, OR). Collagenase D was purchased from Roche Applied Science (Indianapolis, IN).
Immunization and tumor studies
For immunization with OVA protein, mice were vaccinated subcutaneously (s.c.) with OVA (50 μg) and MPL adjuvant (10 μg) twice at weekly intervals. For immunization with OVA-pulsed DCs, bone marrow (BM)-DCs were incubated with 10 μg/mL OVA protein for 4 h, followed by LPS (100 ng/mL) stimulation overnight. Mice received 2 × 106 OVA-loaded DCs s.c. twice at weekly intervals. At indicated time points, spleen and lymph nodes were harvested for analysis of OVA-specific T-cell responses. For tumor challenge, 1 week after the second immunization, 3 × 105 B16-OVA tumor cells were injected s.c. into mice.
BM transplantation
Recipient mice were irradiated with a total of 10 Gy from a cesium irradiator and received 107 BM cells from donor mice. Total BM isolated from the femurs and tibias of donor mice was washed, triturated using a 20-gauge needle, and passed through a 70-μm nylon mesh cell strainer (BD Biosciences, San Jose, CA) to produce a single-cell suspension in phosphate-buffered saline (PBS). Eight to 12 weeks after transplantation, mice were immunized and followed by tumor challenge, as described above.
T-cell function assays
For enzyme-linked immunosorbent spot (ELISPOT) assay, splenocytes were isolated from immunized mice 1 week after immunization to determine antigen-specific interferon (IFN)-γ–secreting T cells, as previously described.17 An in vivo cytotoxic T lymphocyte (CTL) assay was performed to determine the cytolytic activity of OVA-specific CD8+ T cells. Syngeneic naive splenocytes were labeled with either 5 μM CFSE (CFSEhigh cells as in vivo target cells) and then pulsed for 1 hour at 37°C with 1 μg SIINFEKL or 0.5 μM CFSE (CFSElow cells) that were pulsed with a control peptide. A mixture of 2 cell populations at 1:1 ratio (total 5 × 106 cells) was injected intravenously into the immunized animals (n = 5). Spleen or lymph nodes were analyzed 16 hours later by FACS. The peptide-specific lysis was calculated according to the following formula: lysis % = [1 − (Rnaive/Rimmunized)] × 100, in which R is the ratio of CFSElow/CFSEhigh. For in vitro CTL assay, splenocytes were stimulated with 1 μg/mL OVA257-264 in the presence of IL-2 (20 U/mL) for 5 days and serially diluted in V-bottomed 96-well plates containing 51Cr-labeled target cells (4 × 103/well) in triplicate with various effector to target ratios. Percentage of specific lysis was calculated as 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
DC preparation
BM-DCs were generated by culture of mouse BM cells in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF), as described previously.11 To prepare splenic DCs, spleens were minced and digested in RPMI media containing collagenase D (1 mg/mL) and DNase I (100 ng/mL) for 90 minutes at 37°C. After filtration through a 70-μm sieve, CD11c+ DCs were isolated by positive selection with CD11c antibody-coated microbeads on magnetic-activated cell-sorting columns (Miltenyi Biotec). The purity of CD11C+ was consistently greater than 95%, as indicated by staining with fluorescein isothiocyanate (FITC)-conjugated anti-CD11c mAb (clone HL3). For phenotype analysis, cells were resuspended in PBS with 2% fetal calf serum and 0.1% sodium azide, and subjected to staining with relevant antibodies or appropriate isotype controls.
Adoptive T-cell transfer
Lymph node cells from OT-I mice were prepared in a single-cell suspension and labeled with 5 μM CFSE. A total of 2 × 106 OT-I cells was transferred into recipient mice by tail vein injection. Next day, mice were immunized s.c. with OVA-MPL. Spleen and lymph nodes were harvested at indicated time points. OT-I cell proliferation was measured using flow cytometry based on the dilution of CFSE florescence intensity.
In vitro priming of OT-I cells
BM-DCs were pulsed with OVA protein (10 μg/mL) or OVA257-264 peptide (0.1 μg/mL) for 3 hours, followed by stimulation with LPS for another 2 hours. After washing 2 times, serially diluted DCs were incubated with 5 × 104 OT-I cells in 200 μL of RPMI 1640 medium in a round-bottom 96-well microtiter plate. Cells were cultured for 60 hours and pulsed with 3H-thymidine (0.5 μCi/well) during the last 16 hours of culture period. T-cell proliferation was assessed by 3H-thymidine incorporation assays. Levels of cytokine IL-2 in the DC/OT-I coculture system were determined using an enzyme-linked immunosorbent assay (ELISA) kit (eBioscience). For some experiments, OT-I cells were labeled with 2.5 μM CFSE before coculturing with OVA-pulsed DCs. T-cell proliferation was measured by flow cytometric analysis.
OVA tetramer staining
Peripheral blood was harvested via retro-orbital bleeding, and red blood cells were lysed. A total of 5 × 105 peripheral blood lymphocytes (PBLs) was stained with phycoerythrin-conjugated H-2Kb/OVA tetramer and FITC-labeled anti-CD8 mAb at 4°C for 30 minutes. Cells were washed twice with PBS before fixation in 1% formaldehyde. Flow cytometric analysis was performed using FACSCalibur and CellQuest software (BD Biosciences). The frequency of CTL precursors was calculated as the number of tetramer-positive cells divided by the number of CD8+ cells.
Reverse transcription-polymerase chain reaction analysis
Total RNA was extracted from BM-DCs using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instruction. For reverse transcription, 1 μg of total RNA and 50 ng of oligo-dT primer were used for 20-μL reaction volumes with Superscript II reverse transcriptase (Invitrogen). The sequences of the polymerase chain reaction (PCR) primer pairs used to amplify the respective cDNAs were as follows: mTNF-α, forward 5′-AGCACAGAAAGCATGATCCG-3′, reverse 5′-GTTGTCTTTGAGATCCATGCCG TTGG-3′; mIL-6, forward 5′-TTGGGACTGATGCTGGTGA-3′, reverse 5′-TCCTTAGCCACTCCTTCTG-3′; mIL-12p40, forward 5′-TGTTGTAGAGGTGGAC TGGAC-3′, reverse 5′-GGGAACTGCTACTGCTCTTGA-3′; mIL-12p35, forward 5′-CTCCTAAACCACCTCAGTTTGGCCAGGGTC, reverse 5′-TAGATGCTACCAA GGCACAGGGTCATCATC; mIP10, forward 5′-AGTGCTGCCGTCATTTTCTG-3′, reverse 5′-AGGCTCTCTGCTGTCCATC-3′; β-actin, forward 5′-GTCCCTCACC CTCCCAAAAG-3′, reverse 5′-GCTGCCTCAACACCTCAACC-3′, respectively. PCRs were carried out using a thermal cycler, and the resultant products were analyzed in a 1.5% agarose gel.
Short hairpin RNA–mediated gene silencing
Mouse SR-A lentiviral (LV) short hairpin RNA (shRNA) plasmids were purchased from Open Biosystem (Huntsville, AL). The pLKO.1-scrambled plasmid was used as negative controls. Lentiviruses were packaged using Phoenix cells cotransfected with pLKO.1 constructs and pMD.G and pCMVΔR8.91. Day 6 BM-DCs were infected with LV-scramble shRNA or LV-SRA shRNA in the presence of 4 μg/mL polybrene and 20 ng/mL GM-CSF. Cells were collected 48 hours later, washed extensively, and used for immunoblotting and FACS analysis.
Statistical analysis
Differences between groups within experiments were tested for significance with analysis of variance (ANOVA) test or Student t test using GraphPad Prism software (GraphPad, San Diego, CA). P values less than .05 were considered statistically significant.
Results
Hematopoietic cells are involved in the enhancement of TLR4 agonist-stimulated antitumor immunity in SRA/CD204-deficient mice
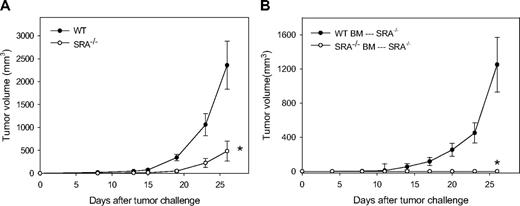
Based on our earlier observations that the absence of SRA/CD204 promoted HSP and complete Freund adjuvant–based vaccine potency, we first evaluated antitumor immunity generated by vaccination targeting TLR4 signaling pathways. MPL is a chemically modified derivative of the lipid A moiety of LPS, and has been used extensively in clinical trials as a vaccine adjuvant.18 Both WT and SRA/CD204 KO (SRA−/−) mice were immunized with OVA protein as a model antigen mixed with MPL, followed by B16-OVA tumor challenge. Consistent with our previous report, WT mice vaccinated with OVA-MPL developed aggressively growing tumors, whereas tumor growth in all the SRA−/− mice was significantly inhibited (Figure 1A).
Hematopoietic cells are involved in SRA/CD204 deficiency-enhanced antitumor immunity after OVA-MPL vaccination. (A) Enhanced antitumor efficacy in immunized SRA−/− mice. Age-matched WT and SRA−/− C57BL/6 mice (n = 5) were immunized twice at a weekly interval with 50 μg of OVA protein mixed with 10 μg of MPL. One week after booster immunization, mice were challenged with 3 × 105 B16-OVA tumor cells (*P < .001, SRA−/− vs WT mice, ANOVA test). Experiments were performed twice, and a representative experiment is shown. (B) Hematopoietic cells required for the enhanced vaccine activities in SRA−/− mice. Recipient SRA−/− mice (n = 5) were whole body irradiated and received 107 total BM cells from donor WT or SRA−/− mice. Eight weeks later, recipient mice were immunized with OVA-MPL and challenged with 2 × 105 B16-OVA cells (P < .001, SRA−/− BM vs WT BM, ANOVA test). Results are representative of 2 separate experiments.
Hematopoietic cells are involved in SRA/CD204 deficiency-enhanced antitumor immunity after OVA-MPL vaccination. (A) Enhanced antitumor efficacy in immunized SRA−/− mice. Age-matched WT and SRA−/− C57BL/6 mice (n = 5) were immunized twice at a weekly interval with 50 μg of OVA protein mixed with 10 μg of MPL. One week after booster immunization, mice were challenged with 3 × 105 B16-OVA tumor cells (*P < .001, SRA−/− vs WT mice, ANOVA test). Experiments were performed twice, and a representative experiment is shown. (B) Hematopoietic cells required for the enhanced vaccine activities in SRA−/− mice. Recipient SRA−/− mice (n = 5) were whole body irradiated and received 107 total BM cells from donor WT or SRA−/− mice. Eight weeks later, recipient mice were immunized with OVA-MPL and challenged with 2 × 105 B16-OVA cells (P < .001, SRA−/− BM vs WT BM, ANOVA test). Results are representative of 2 separate experiments.
BM transplantation experiment was performed to assess whether the enhanced vaccine efficacy in SRA−/− mice was attributed to hematopoietic cells. Whereas all SRA−/− mice receiving WT BM cells developed tumors, no tumor was seen in SRA−/− mice 25 days after receiving SRA−/− BM cells, indicating that reconstitution with WT BM in SRA-deficient mice abrogated the enhanced antitumor response (Figure 1B).
SRA/CD204 deficiency promotes MPL-stimulated proliferation of OVA-specific CD8+ T cells
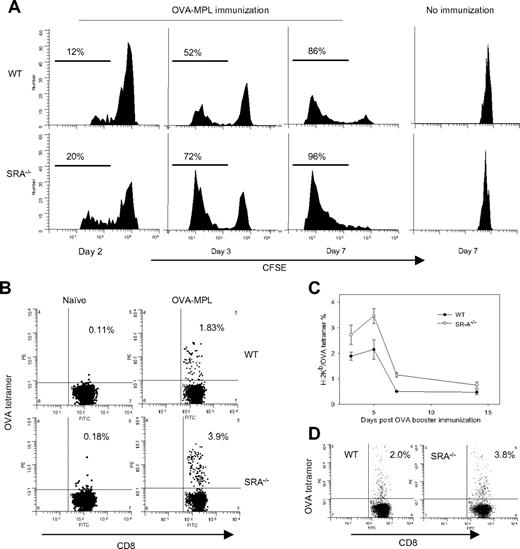
To determine whether the improved antitumor response in SRA−/− mice correlated with an enhanced antigen-specific immune response, an adoptive T-cell transfer assay was carried out using OT-I cells with a transgenic TCR that recognizes OVA257-264 in the context of H2Kb. CFSE-labeled OT-I cells were transferred to WT or SRA−/− mice, followed by OVA (50 μg/mouse)-MPL immunization. CD8+CFSE+ OT-I cells in spleens started to proliferate on day 2, and reached the peak at approximately day 7 in WT and SRA−/− mice. It is evident that OT-I cells displayed a stronger proliferation in SRA−/− mice compared with WT mice (Figure 2A). In contrast to the immunized mice, there was no OT-I cell proliferation observed in nonimmunized WT and SRA−/− mice (Figure 2A right column), indicating that SRA deficiency has little effect on T-cell response in the absence of vaccination. Despite the enhanced OT-I cell expansion in SRA−/− mice, these T cells in WT and SRA−/− mice similarly expressed an activated phenotype with high expression of CD44, CD69, and reduced expression of CD62L (data not shown).
Enhanced proliferation of OVA-specific CD8+ T cells in SRA/CD204 KO mice. (A) Enhanced expansion of adoptively transferred OT-I cells in SRA−/− mice. Mice received CFSE-labeled OT-I cells on day −1, and were immunized with OVA-MPL on day 0. Spleens were harvested at different time points and subjected to FACS analysis by gating on CD8+ cells. OT-I cells were also transferred to mice without immunization and used as controls. Data are representative of 2 separate experiments in which at least 3 mice in each group were analyzed. (B) Increased frequency of endogenous OVA-specific CD8+ T cells in SRA−/− mice. Representative FACS dot plots of tetramer staining. WT and SRA−/− mice (n = 3) were immunized with OVA-MPL. PBLs were collected on day 5 and stained with anti-CD8 antibodies and OVA tetramer. (C) Kinetic analysis of the frequency of OVA-specific CD8+ T cells. After immunization, CD8+OVA tetramer+ T cells in PBLs were assessed on days 3, 5, 7, and 14. Percentage of tetramer-positive cells gated on CD8+ T cells. Data shown as mean plus or minus SD are representative of 2 experiments (P < .001, SRA−/− vs WT mice, ANOVA test). (D) Stronger recall response against OVA in SRA−/− mice. Two months after vaccination, mice were immunized with OVA, and analyzed for CD8+OVA tetramer+ T cells in PBLs.
Enhanced proliferation of OVA-specific CD8+ T cells in SRA/CD204 KO mice. (A) Enhanced expansion of adoptively transferred OT-I cells in SRA−/− mice. Mice received CFSE-labeled OT-I cells on day −1, and were immunized with OVA-MPL on day 0. Spleens were harvested at different time points and subjected to FACS analysis by gating on CD8+ cells. OT-I cells were also transferred to mice without immunization and used as controls. Data are representative of 2 separate experiments in which at least 3 mice in each group were analyzed. (B) Increased frequency of endogenous OVA-specific CD8+ T cells in SRA−/− mice. Representative FACS dot plots of tetramer staining. WT and SRA−/− mice (n = 3) were immunized with OVA-MPL. PBLs were collected on day 5 and stained with anti-CD8 antibodies and OVA tetramer. (C) Kinetic analysis of the frequency of OVA-specific CD8+ T cells. After immunization, CD8+OVA tetramer+ T cells in PBLs were assessed on days 3, 5, 7, and 14. Percentage of tetramer-positive cells gated on CD8+ T cells. Data shown as mean plus or minus SD are representative of 2 experiments (P < .001, SRA−/− vs WT mice, ANOVA test). (D) Stronger recall response against OVA in SRA−/− mice. Two months after vaccination, mice were immunized with OVA, and analyzed for CD8+OVA tetramer+ T cells in PBLs.
We next determined the impact of SRA/CD204 on the endogenous OVA-specific CD8+ T-cell response using tetramer staining. At day 5 after immunization, PBLs were collected and labeled with anti-CD8 and Kb-SIINFEKL tetramers. OVA-MPL vaccination induced a significantly higher percentage of CD8+ tetramer-positive population in SRA−/− mice compared with WT mice (Figure 2B). Similar results were obtained when kinetic changes in the frequency of OVA-specific CTLs were analyzed at different time points postvaccination (Figure 2C). The recall response of OVA-specific CD8+ T cells was examined in OVA-MPL–immunized WT and SRA−/− animals 2 months later. The number of tetramer-positive CD8+ T cells was higher in SRA−/− mice than in WT mice after OVA vaccination (Figure 2D).
SRA/CD204 deficiency enhances functions of OVA-specific CD8+ effector T cells
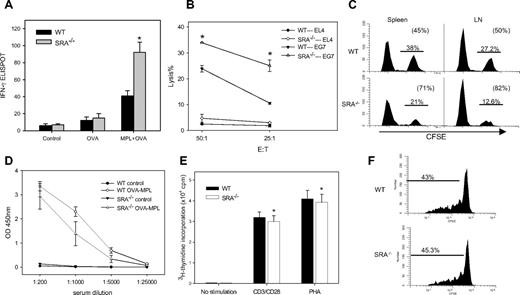
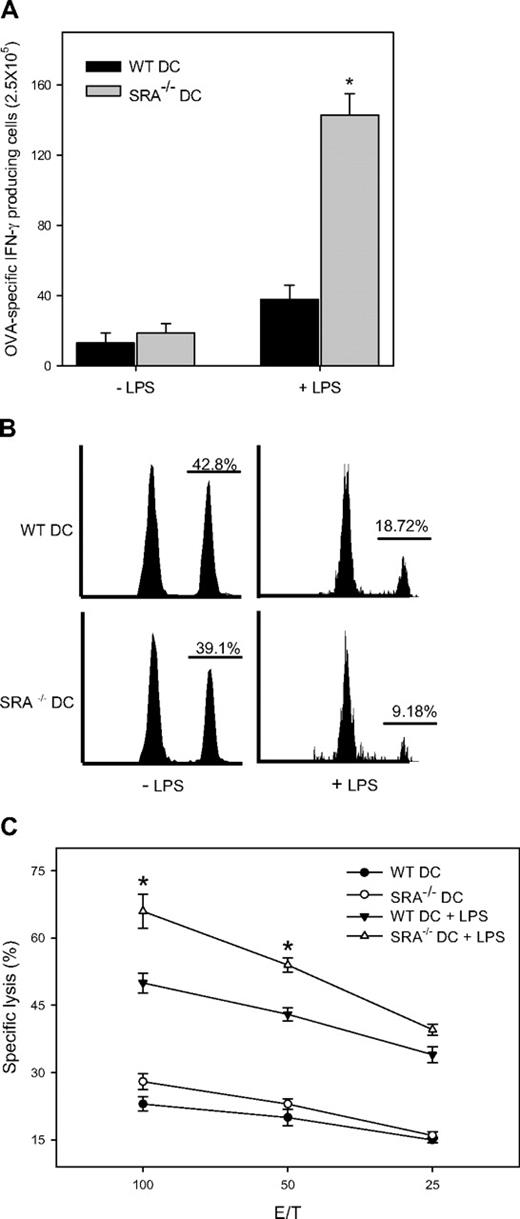
We assessed the effect of SRA/CD204 on the functional status of antigen-specific CD8+ T cells in immunized mice using an ELISPOT assay. The number of IFN-γ spots was counted to evaluate the frequency of OVA257-264(SIINFEKL)-specific CD8+ T cells that were able to produce IFN-γ. Cells from naive mice or mice immunized with OVA protein in the absence of adjuvant produced no or very low amounts of IFN-γ. However, CD8+ T cells from OVA-MPL–immunized SRA−/− mice showed a more robust production of IFN-γ than those from immunized WT mice (Figure 3A), suggesting that TLR4 activation or adjuvant activity is required for the different T-cell response. Effector T-cell function, that is, cytolytic activity, was then examined by chromium release assay. Compared with WT counterparts, T cells from immunized SRA−/− mice exhibited significantly higher cytolytic activities against OVA-expressing EG7 cells, but not antigen-negative targets (Figure 3B), indicating an antigen-specific recognition.
Enhanced effector functions of OVA-specific CD8+ T cells in SRA/CD204 KO mice. (A) Increased IFN-γ production by OVA-specific CTLs from SRA−/− mice. Animals (n = 5) were immunized with OVA alone, OVA-MPL, or left untreated. One week after booster immunization, splenocytes were restimulated with OVA257-264 and subjected to an ELISPOT assay (*P < .01, SRA−/− vs WT mice; error bars indicate SE). (B) Increased cytolytic activity of effector T cells from SRA−/− mice. Splenocytes were restimulated with OVA257-264 in the presence of IL-2 for 5 days, and chromium release assays were carried out using OVA-expressing EG7 cells as targets. OVA-negative EL4 cells were used as controls. Data shown as mean plus or minus SD are representative of 2 experiments. (C) Increased OVA-specific cytolytic activity in immunized SRA−/− mice. Immunized WT and SRA−/− mice (n = 3) were injected intravenously with 5 × 106 OVA257-264-pulsed, CFSEhigh splenocytes mixed with nonpulsed, CFSElow splenocytes at 1:1 ratio. Splenocytes and lymph node (LN) cells were analyzed 12 hours later by FACS. Representative histogram profiles from 2 experiments are shown. (D) Antibody response to OVA antigen not affected by SRA/CD204 deficiency. WT and SRA−/− mice (n = 5) were immunized with OVA-MPL or left untreated. Sera were collected 7 days after booster immunization and analyzed for IgG levels against OVA by ELISAs (*P > .5, WT vs SRA−/−). (E) Intrinsic T-cell function not altered by SRA/CD204 deficiency. Naive T cells (> 98% purity) were stimulated with 10 μg/mL anti-CD3, anti-CD28 mAb in precoated wells, or PHA (1 μg/mL) at 37°C for 72 hours. Cell proliferation was determined by measuring thymidine incorporation (counts per minute) using a scintillation counter (*P > .5, WT vs SRA−/−). (F) Similar in vivo proliferation of T cells from WT and SRA−/− mice. CFSE-labeled CD3+ T cells from WT or SRA−/− mice were adoptively transferred to RAG-1 mice (n = 3). The recipient mice were then immunized with OVA-MPL. CFSE intensity was measured 5 days later using FACS by gating on CD3- and CFSE-positive cells (P > .5, WT vs SRA−/−).
Enhanced effector functions of OVA-specific CD8+ T cells in SRA/CD204 KO mice. (A) Increased IFN-γ production by OVA-specific CTLs from SRA−/− mice. Animals (n = 5) were immunized with OVA alone, OVA-MPL, or left untreated. One week after booster immunization, splenocytes were restimulated with OVA257-264 and subjected to an ELISPOT assay (*P < .01, SRA−/− vs WT mice; error bars indicate SE). (B) Increased cytolytic activity of effector T cells from SRA−/− mice. Splenocytes were restimulated with OVA257-264 in the presence of IL-2 for 5 days, and chromium release assays were carried out using OVA-expressing EG7 cells as targets. OVA-negative EL4 cells were used as controls. Data shown as mean plus or minus SD are representative of 2 experiments. (C) Increased OVA-specific cytolytic activity in immunized SRA−/− mice. Immunized WT and SRA−/− mice (n = 3) were injected intravenously with 5 × 106 OVA257-264-pulsed, CFSEhigh splenocytes mixed with nonpulsed, CFSElow splenocytes at 1:1 ratio. Splenocytes and lymph node (LN) cells were analyzed 12 hours later by FACS. Representative histogram profiles from 2 experiments are shown. (D) Antibody response to OVA antigen not affected by SRA/CD204 deficiency. WT and SRA−/− mice (n = 5) were immunized with OVA-MPL or left untreated. Sera were collected 7 days after booster immunization and analyzed for IgG levels against OVA by ELISAs (*P > .5, WT vs SRA−/−). (E) Intrinsic T-cell function not altered by SRA/CD204 deficiency. Naive T cells (> 98% purity) were stimulated with 10 μg/mL anti-CD3, anti-CD28 mAb in precoated wells, or PHA (1 μg/mL) at 37°C for 72 hours. Cell proliferation was determined by measuring thymidine incorporation (counts per minute) using a scintillation counter (*P > .5, WT vs SRA−/−). (F) Similar in vivo proliferation of T cells from WT and SRA−/− mice. CFSE-labeled CD3+ T cells from WT or SRA−/− mice were adoptively transferred to RAG-1 mice (n = 3). The recipient mice were then immunized with OVA-MPL. CFSE intensity was measured 5 days later using FACS by gating on CD3- and CFSE-positive cells (P > .5, WT vs SRA−/−).
We next conducted in vivo CTL assays to measure more directly the cytotoxic capacity of OVA-specific CD8+ T cells. Immunized mice were injected intravenously with a mixed population of CFSE-labeled splenocytes: 50% CFSEhigh cells pulsed with SIINFEKL and 50% CFSElow cells without peptide pulsing as an internal control. FACS analysis indicated that OVA-MPL vaccination resulted in a higher efficiency of peptide-specific killing in SRA−/− mice than in WT mice (Figure 3C). We also examined OVA-specific humoral response in the immunized mice. No significant change was seen in the levels of total IgG specific for OVA in WT and SRA−/− mice (Figure 3D).
Absence of SRA/CD204 promotes activation of DCs
We assessed the effect of SRA/CD204 deficiency on cellular composition of the immune compartments and found that SRA−/− mice showed similar lymphocyte composition in spleen and lymph nodes (eg, numbers and ratios of CD4+ and CD8+ T cells) as WT mice (data not shown). No difference was observed in proliferation response of T cells from WT and SRA−/− mice after CD3/CD28 engagement or phytohemagglutinin (PHA) stimulation (Figure 3E). WT and SRA−/− mice-derived T cells also displayed similar expansion in OVA-MPL–vaccinated RAG-1 mice (Figure 3F), indicating that the absence of SRA/CD204 does not affect intrinsic T-cell functions.
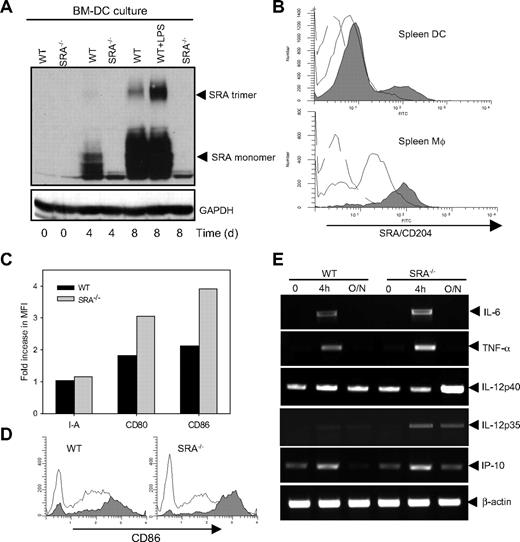
Given the high expression of SRA/CD204 in phagocytes, we hypothesized that APCs are involved in the enhanced CD8+ T-cell response in TLR4 agonist-stimulated SRA−/− mice. SRA/CD204 expression was initially detected at day 4 of culture and increased incrementally through day 8, while DCs matured/differentiated in the presence of GM-CSF (Figure 4A). LPS stimulation up-regulated SRA/CD204 expression in BM-DCs, as indicated by elevated levels of the trimeric form of the receptor (Figure 4A). After LPS challenge, spleen-derived DCs showed a significant increase in surface expression of SRA/CD204 (Figure 4B). Similar results were seen in spleen Mφ (Figure 4B) and peritoneal Mφ (data not shown) from LPS-challenged WT mice.
SRA/CD204 dampens TLR4 agonist-stimulated inflammatory response in DCs. (A) Increased SRA/CD204 expression during DC differentiation and activation. BM cell culture in the presence of GM-CSF was analyzed for SRA/CD204 expression by immunoblotting. Day 8 WT BM-DCs were treated with LPS overnight. (B) Increased SRA/CD204 expression in APCs in response to LPS stimulation in vivo. WT mice were challenged with 10 μg of LPS by tail vein injection. Splenic DCs and Mφ were isolated using magnetic beads 48 hours later, and stained for SRA/CD204 expression (broken line, isotype control; solid line, no LPS treatment; gray filled, LPS treatment). (C) Increased up-regulation of costimulatory molecules in SRA−/− splenic DCs. After LPS challenge, splenic DCs were isolated and stained with antibodies for MHC class II and costimulatory molecules. Fold increase in mean fluorescence intensity (MFI) is shown. Data shown are representative of 3 experiments. (D) Representative histogram profiles showing higher levels of CD86 in SRA−/− splenic DCs after LPS stimulation in vivo (open histogram, no LPS treatment; gray histogram, LPS treatment). (E) Enhanced LPS-induced gene transcription of inflammatory mediators in SRA−/− DCs. BM-DCs were stimulated with LPS (100 ng/mL) for 4 hours or overnight. Total RNA was prepared and subjected to RT-PCR analysis using specific primers. β-Actin was used as an internal control.
SRA/CD204 dampens TLR4 agonist-stimulated inflammatory response in DCs. (A) Increased SRA/CD204 expression during DC differentiation and activation. BM cell culture in the presence of GM-CSF was analyzed for SRA/CD204 expression by immunoblotting. Day 8 WT BM-DCs were treated with LPS overnight. (B) Increased SRA/CD204 expression in APCs in response to LPS stimulation in vivo. WT mice were challenged with 10 μg of LPS by tail vein injection. Splenic DCs and Mφ were isolated using magnetic beads 48 hours later, and stained for SRA/CD204 expression (broken line, isotype control; solid line, no LPS treatment; gray filled, LPS treatment). (C) Increased up-regulation of costimulatory molecules in SRA−/− splenic DCs. After LPS challenge, splenic DCs were isolated and stained with antibodies for MHC class II and costimulatory molecules. Fold increase in mean fluorescence intensity (MFI) is shown. Data shown are representative of 3 experiments. (D) Representative histogram profiles showing higher levels of CD86 in SRA−/− splenic DCs after LPS stimulation in vivo (open histogram, no LPS treatment; gray histogram, LPS treatment). (E) Enhanced LPS-induced gene transcription of inflammatory mediators in SRA−/− DCs. BM-DCs were stimulated with LPS (100 ng/mL) for 4 hours or overnight. Total RNA was prepared and subjected to RT-PCR analysis using specific primers. β-Actin was used as an internal control.
We next assessed the effect of SRA/CD204 on phenotype of splenic DCs after LPS challenge in vivo. Splenic DCs from untreated WT and SRA−/− mice showed similar levels of I-A (MHC class II), CD80, and CD86 (data not shown). However, splenic DCs from SRA−/− mice were more responsive to LPS stimulation, as indicated by a more significant up-regulation of costimulatory molecules (ie, CD86; Figure 4C,D).
Reverse transcription-PCR (RT-PCR) analysis was carried out to determine mRNA levels of inflammatory mediators in DCs after LPS stimulation (Figure 4E). Compared with WT DCs, SRA−/− DCs showed higher levels of gene transcription for cytokines IL-6, tumor necrosis factor-α, IL-12p35, and chemokine IFN-γ–inducible protein-10 after 4-hour LPS stimulation, which is consistent with our earlier results from ELISAs.11 After DCs were cocultured with LPS overnight, IL-12p40 transcription returned to basal levels in WT DCs, whereas the mRNA levels of the gene remained much higher in SRA−/− DCs, implicating a longer duration of IL-12p40 transcription response in TLR4-engaged SRA−/− cells.
SRA/CD204 inhibits T cell–stimulating activity of DCs in vitro after TLR4 activation
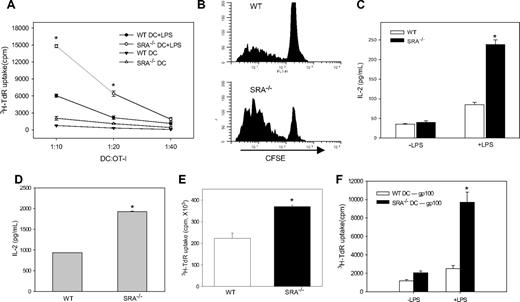
To determine whether SRA/CD204 negatively regulates immunostimulatory functions of APC upon activation of TLR4 signaling, we compared the ability of BM-DCs generated ex vivo to prime OVA-specific naive T cells. OVA protein-pulsed DCs were treated with or without ultrapure LPS, followed by coculture with OT-I cells (Figure 5A). In the absence of LPS stimulation, OT-I cells showed only marginally increased proliferation when cultured with WT and SRA−/− DCs. However, upon LPS treatment, SRA−/− DCs were much more effective than WT DCs in driving OT-I cell proliferation, as indicated by significantly increased incorporation of 3H-thymidine into T cells (Figure 5A). The result was confirmed by FACS analysis of CFSE-labeled OT-I cells as responder cells, in which cell expansion was measured by CFSE dilution (Figure 5B). In addition, levels of cytokine IL-2 secreted by T cells were measured by ELISAs after incubation with LPS-stimulated DCs. Consistent with the data from T-cell proliferation assays, more IL-2 was detected in the supernatants when OT-I cells were cocultured with SRA−/− DCs than with WT DCs (Figure 5C). Similarly, OVA257-264 peptide-pulsed SRA−/− BM-DC-stimulated OT-I cells also produced more IL-2 than did WT DCs (Figure 5D), suggesting that antigen-processing machinery may not play a significant role in the SRA deficiency-enhanced immunogenicity of DCs. In addition, comparable levels of MHC I levels were seen in both DCs after LPS stimulation (data not shown).
SRA/CD204 suppresses immunostimulatory activity of TLR4-engaged DCs in vitro. (A) Increased proliferation of OT-I cells primed by LPS-activated SRA−/− DCs. BM-DCs derived from WT or SRA−/− mice were pulsed with OVA protein (10 μg/mL) for 3 hours and subsequently activated with or without LPS for 2 hours. Cells were cocultured with OT-I cells for 72 hours. OT-I cell proliferation was measured by 3H-thymidine incorporation assays (*P < .005, LPS-treated SRA−/− DC vs LPS-treated WT DC). (B) OVA-pulsed, LPS-stimulated BM-DCs were cocultured with 5 μM CFSE-labeled OT-I cells. OT-I cell proliferation was assessed on day 3 by FACS based on the dilution of CFSE intensity. Representative histograms from 2 independent experiments are shown (P < .001, SRA−/− vs WT). (C) Increased IL-2 production by SRA−/− DC-stimulated OT-I cells. After incubation, the levels of IL-2 in the supernatant were determined by ELISA. (D) DCs were pulsed with OVA257-264 (1 μg/mL) and activated with LPS. After coculturing with OT-I cells, IL-2 levels were determined using an ELISA. Data shown as mean plus or minus SD (*P < .005, SRA−/− vs WT). (E) Enhanced immunostimulatory capability of DCs from immunized SRA/CD204 KO mice. Splenic DCs were isolated by magnetic selection from immunized WT or SRA−/− mice, and cocultured with OT-I cells for 3 days. 3H-TdR incorporation assays were used to determine OT-I cell proliferation was assayed (*P < .01, SRA−/− vs WT). (F) Increased Pmel cell proliferation by LPS-stimulated SRA−/− DCs. DCs were pulsed with gp100 protein antigens (20 μg/mL), and treated with or without LPS. DCs were then cocultured with gp100-specific CD8+ T cells purified from Pmel transgenic mice, followed by 3H-TdR incorporation assays (*P < .005, LPS-treated SRA−/− DC vs LPS-treated WT DC). Representative results from 2 independent experiments are shown.
SRA/CD204 suppresses immunostimulatory activity of TLR4-engaged DCs in vitro. (A) Increased proliferation of OT-I cells primed by LPS-activated SRA−/− DCs. BM-DCs derived from WT or SRA−/− mice were pulsed with OVA protein (10 μg/mL) for 3 hours and subsequently activated with or without LPS for 2 hours. Cells were cocultured with OT-I cells for 72 hours. OT-I cell proliferation was measured by 3H-thymidine incorporation assays (*P < .005, LPS-treated SRA−/− DC vs LPS-treated WT DC). (B) OVA-pulsed, LPS-stimulated BM-DCs were cocultured with 5 μM CFSE-labeled OT-I cells. OT-I cell proliferation was assessed on day 3 by FACS based on the dilution of CFSE intensity. Representative histograms from 2 independent experiments are shown (P < .001, SRA−/− vs WT). (C) Increased IL-2 production by SRA−/− DC-stimulated OT-I cells. After incubation, the levels of IL-2 in the supernatant were determined by ELISA. (D) DCs were pulsed with OVA257-264 (1 μg/mL) and activated with LPS. After coculturing with OT-I cells, IL-2 levels were determined using an ELISA. Data shown as mean plus or minus SD (*P < .005, SRA−/− vs WT). (E) Enhanced immunostimulatory capability of DCs from immunized SRA/CD204 KO mice. Splenic DCs were isolated by magnetic selection from immunized WT or SRA−/− mice, and cocultured with OT-I cells for 3 days. 3H-TdR incorporation assays were used to determine OT-I cell proliferation was assayed (*P < .01, SRA−/− vs WT). (F) Increased Pmel cell proliferation by LPS-stimulated SRA−/− DCs. DCs were pulsed with gp100 protein antigens (20 μg/mL), and treated with or without LPS. DCs were then cocultured with gp100-specific CD8+ T cells purified from Pmel transgenic mice, followed by 3H-TdR incorporation assays (*P < .005, LPS-treated SRA−/− DC vs LPS-treated WT DC). Representative results from 2 independent experiments are shown.
We also examined T cell–priming capability of splenic DCs derived from WT or SRA−/− mice after OVA-MPL immunization. Similar to BM-DCs, splenic DCs isolated from vaccinated SRA−/− mice were able to induce a more robust OT-I cell proliferation (Figure 5E) and higher levels of IL-2 production (data not shown) than those from vaccinated WT animals.
In addition to the model antigen OVA, a clinically relevant, tumor-associated antigen (ie, gp100) was used to determine the effect of SRA absence on the capability of DCs to activate gp100-specific T cells. Consistent with our observations in the OVA system, LPS-stimulated SRA−/− DCs were more efficient than WT cells in promoting the proliferation of the TCR transgenic Pmel cells (Figure 5F).
SRA/CD204 suppresses immunostimulatory capability of DCs in vivo
We next assessed the impact of SRA/CD204 on the immunogenicity of DCs in vivo by vaccination of WT mice with OVA protein-pulsed WT or SRA−/− BM-DCs. Immunization with non-LPS–treated DCs induced only a weak response in mice. Upon OVA257-264 peptide stimulation, splenocytes derived from mice immunized with LPS-activated SRA−/− DC produced more IFN-γ compared with those from WT DC-immunized mice (Figure 6A). To assess OVA-specific CTL function in vivo, CFSE differentially labeled splenocytes containing OVA-pulsed CFSEhigh cells, and untreated CFSElow cells were injected intravenously into all experimental groups. Mice immunized with LPS-activated SRA−/− DCs developed a more potent cytotoxic response against OVA-positive targets than did WT DC-immunized mice (Figure 6B). The enhanced tumor-killing activities were also observed in a 51Cr release assay in vitro using OVA257-264 peptide-pulsed syngeneic B16 tumor cells as targets (Figure 6C).
SRA/CD204 attenuates immunostimulatory activity of TLR4-engaged DCs in vivo. (A) Increased IFN-γ production by OVA-specific CTLs from SRA−/− DC-immunized mice. WT mice (n = 3) were immunized with OVA-pulsed DCs activated with or without LPS. Splenocytes were harvested and restimulated with OVA257-264 and subjected to an ELISPOT assay (*P < .001, LPS-treated SRA−/− DC vs LPS-treated WT DC). (B) Improved OVA-specific cytolytic activity in SRA−/− DC-immunized mice. After vaccinations with WT or SRA−/− DCs, mice were inoculated intravenously with 5 × 106 OVA257-264-pulsed, CFSEhigh splenocytes and nonpulsed, CFSElow population. Lymph node cells were analyzed by FACS after 12 hours. Data represent an example of the FACS data from 3 mice per experimental group. (C) Increased tumor-lytic activity of T-effector cell from SRA−/− DC-immunized WT mice. Splenocytes from WT or SRA−/− DC-immunized mice were restimulated with OVA257-264, and subjected to chromium release assays using OVA257-264-pulsed B16 cells as targets. Data shown as mean plus or minus SD are representative of 2 experiments.
SRA/CD204 attenuates immunostimulatory activity of TLR4-engaged DCs in vivo. (A) Increased IFN-γ production by OVA-specific CTLs from SRA−/− DC-immunized mice. WT mice (n = 3) were immunized with OVA-pulsed DCs activated with or without LPS. Splenocytes were harvested and restimulated with OVA257-264 and subjected to an ELISPOT assay (*P < .001, LPS-treated SRA−/− DC vs LPS-treated WT DC). (B) Improved OVA-specific cytolytic activity in SRA−/− DC-immunized mice. After vaccinations with WT or SRA−/− DCs, mice were inoculated intravenously with 5 × 106 OVA257-264-pulsed, CFSEhigh splenocytes and nonpulsed, CFSElow population. Lymph node cells were analyzed by FACS after 12 hours. Data represent an example of the FACS data from 3 mice per experimental group. (C) Increased tumor-lytic activity of T-effector cell from SRA−/− DC-immunized WT mice. Splenocytes from WT or SRA−/− DC-immunized mice were restimulated with OVA257-264, and subjected to chromium release assays using OVA257-264-pulsed B16 cells as targets. Data shown as mean plus or minus SD are representative of 2 experiments.
SRA/CD204 silencing enhances the immunogenicity of LPS-activated DCs
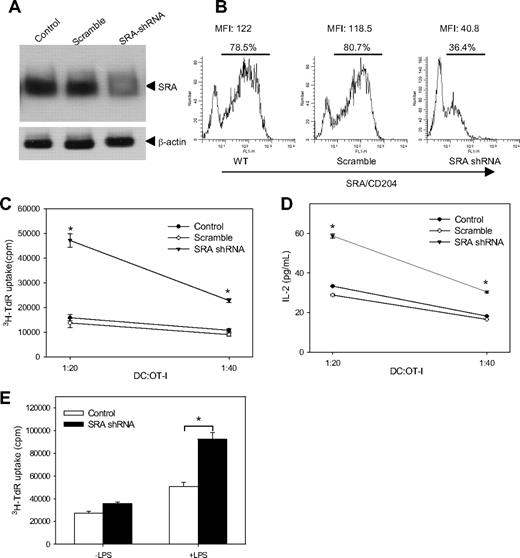
To further validate the contribution of SR-A to the immunostimulatory capability of DCs, self-inactivating LV encoding shRNA were used for SRA/CD204 silencing because of their safety and superior transduction efficiency in both dividing and nondividing cells. We have screened and identified a shRNA capable of specifically down-regulating SRA/CD204 in DCs. As indicated by immunoblotting assays, the level of SRA/CD204 protein in DCs infected with LV-SRA-shRNA (hairpin sequence CCGGGCAGTTCAGAATCCGTGAAATCTCGAGATTTCACGGATTCTGAACTGCTTTTTG) was decreased by approximately 90%, compared with that in untreated or scramble shRNA-transfected cells (Figure 7A). The reduction of SRA/CD204 expression was confirmed by FACS analysis using SRA/CD204 antibodies (Figure 7B).
SRA/CD204 silencing enhances immunostimulatory capability of TLR4-activated DCs. (A) Lentivirus-mediated RNA interference efficiently down-regulates SRA/CD204 expression in DCs. BM-DCs (105 cells/well) were transfected with LV-scramble shRNA, LV-SRA shRNA at a multiplicity of infection of 50, or left untreated. Cells were harvested 2 days later and analyzed by immunoblotting using anti-SRA/CD204 antibodies. (B) Silencing of SRA/CD204 in DCs by lentivirus encoding SRA shRNA, as indicated by FACs analysis. (C) SRA/CD204 silencing enhances ability of DCs to prime naive OT-I cells. BM-DC cells were transfected with LV-SRA shRNA, LV-scramble shRNA, or left untreated. DCs were harvested 2 days after infection and pulsed with OVA protein. After LPS stimulation and extensive washes, DCs were cocultured with OT-I cells at different ratios. OT-I cell proliferation was measured by 3H-thymidine incorporation assays. (D) Increased IL-2 production by OT-I cells stimulated with SRA/CD204-silenced DCs. After incubation, the levels of IL-2 in the supernatant were determined using an ELISA (*P < .005, SRA shRNA vs scramble control). (E) SRA-silenced DCs were stimulated with or without LPS, followed by coculture with OT-I cells at a ratio of 1:10. T-cell proliferation was assayed by 3H-thymidine uptake (*P < .005, SRA shRNA vs control). Representative results from 2 independent experiments are shown. Error bars indicate SE.
SRA/CD204 silencing enhances immunostimulatory capability of TLR4-activated DCs. (A) Lentivirus-mediated RNA interference efficiently down-regulates SRA/CD204 expression in DCs. BM-DCs (105 cells/well) were transfected with LV-scramble shRNA, LV-SRA shRNA at a multiplicity of infection of 50, or left untreated. Cells were harvested 2 days later and analyzed by immunoblotting using anti-SRA/CD204 antibodies. (B) Silencing of SRA/CD204 in DCs by lentivirus encoding SRA shRNA, as indicated by FACs analysis. (C) SRA/CD204 silencing enhances ability of DCs to prime naive OT-I cells. BM-DC cells were transfected with LV-SRA shRNA, LV-scramble shRNA, or left untreated. DCs were harvested 2 days after infection and pulsed with OVA protein. After LPS stimulation and extensive washes, DCs were cocultured with OT-I cells at different ratios. OT-I cell proliferation was measured by 3H-thymidine incorporation assays. (D) Increased IL-2 production by OT-I cells stimulated with SRA/CD204-silenced DCs. After incubation, the levels of IL-2 in the supernatant were determined using an ELISA (*P < .005, SRA shRNA vs scramble control). (E) SRA-silenced DCs were stimulated with or without LPS, followed by coculture with OT-I cells at a ratio of 1:10. T-cell proliferation was assayed by 3H-thymidine uptake (*P < .005, SRA shRNA vs control). Representative results from 2 independent experiments are shown. Error bars indicate SE.
We next examined the ability of SRA/CD204-silenced DCs to prime antigen-specific CD8+ T cells after TLR4 activation. Naive OT-I cells were cocultured with scramble shRNA, SRA/CD204 shRNA-transduced DCs, or untreated DCs. It was seen that SRA/CD204-silenced DCs were more efficient in driving OT-I cell proliferation (Figure 7C) and stimulating OT-I cells to produce IL-2 (Figure 7D) than scramble shRNA-treated or untreated DCs, indicating that SRA/CD204 deficiency-mediated immune regulation in vivo was not due to the genetic influence on mouse development derived from SRA/CD204 KO. To further confirm that TLR4 activation is important for the improved immune stimulation by SRA−/− DCs, shRNA-transduced DCs were treated with or without LPS before coculture with OT-I cells. Compared with non-LPS–stimulated cells, SRA-silenced DCs upon TLR4 activation resulted in a much more robust T-cell proliferation (Figure 7E).
Discussion
It has been well documented that triggering TLR signaling can lead to the generation of a robust immune response against tumor-associated antigens. In the present study, we have demonstrated that SRA/CD204 can dampen the immunostimulatory activities of adjuvant targeting TLR4 signaling cascades; therefore, down-regulating TLR4 agonist augmented adaptive immune response to soluble protein antigens. This is the first report that illustrates the impact of a non-TLR PRR on TLR4 engagement-induced immunologic consequences and antigen-specific tumor immunity.
After OVA-MPL vaccination, SRA/CD204 KO mice developed an enhanced antitumor response compared with their WT counterparts, which was associated with a robust activation of both adoptively transferred and endogenous OVA-specific CD8+ T cells. SRA/CD204 appears to mainly regulate MPL-induced cellular immunity rather than a humoral response, because no significant changes were observed in OVA-specific IgG levels in both WT and SRA KO mice. That the lack of SRA/CD204 enhances T-cell responses against both a model antigen OVA and a tumor-associated antigen gp100 lends strong support for our hypothesis that SRA/CD204 plays a negative regulatory role in TLR4 agonist-activated adaptive immunity. Our earlier findings showed that SRA/CD204 is also capable of attenuating stress protein/HSP-augmented vaccine efficacy targeting a tumor-associated antigen (ie, gp100).11 Thus, our results confirm that SRA/CD204 can sense both endogenous and exogenous stressors (eg, HSP, LPS), and suppress antigen-specific immune responses activated by both mammalian and nonmammalian adjuvants. Given the immunostimulatory adjuvant activities of TLR ligands, it is of interest to determine the impact of SRA/CD204 on an adaptive immune response promoted by other TLR agonists.
In the present study, we have compared the immunostimulatory capability of DCs from WT and SRA−/− mice. In the absence of LPS stimulation, both WT and SRA−/− DCs exhibited weak activities in driving T-cell proliferation. After TLR4 activation, SRA−/− DCs appear to be much more effective than WT counterparts in priming OVA-specific CD8+ T cells, suggesting that DCs are most likely to mediate the enhanced T-cell activation in SRA−/− mice. Given that there is no intrinsic defect in T-cell functions in WT and SRA−/− mice, our results indicate that an altered response of APCs upon TLR4 activation contributes, at least partially, to the differences in T-cell responses in vivo. Furthermore, SRA/CD204 silencing by lentivirus-mediated shRNA recapitulates the immune enhancing effects observed in DCs derived from SRA/CD204 KO mice, arguing for an antagonistic role of SRA/CD204 in TLR4-initiated T-cell immunity. SRs have been implicated in antigen uptake and presentation.3,19 However, the lack of SRA/CD204 does not appear to impair the endocytic/phagocytic capability of APCs (data not shown), suggesting that other redundant endocytic or SRs sufficiently compensate for the loss of SRA/CD204.7,20-22 The fate and processing routes of soluble antigens captured by SRA/CD204 are unknown. In addition, to what extent antigen cross-presentation in the absence of SRA/CD204 contributes to the enhanced T-cell activation remains to be determined.
The lack of SRA/CD204 appears to render DCs more responsive to stimulation by TLR4 agonist, as indicated by higher expression of costimulatory molecules in splenic DCs from LPS-challenged mice as well as increased transcriptional levels of several proinflammatory cytokines and chemokines in LPS-stimulated BM-DCs. These results are in line with previous reports implicating that SRA/CD204 serves as a negative regulator during inflammatory responses7,8,23,24 and as a limiting factor in DC maturation.9 Interestingly, deletion of type A SRs has been shown to render mice more susceptible to systemic lupus erythematosus.25 We speculate that the enhanced functional activation of DC after the engagement of TLR signaling pathways in the absence of SRA/CD204 contributes qualitatively and quantitatively to antigen presentation and subsequent CTL activation, leading to an enhanced antitumor response in SRA−/− mice. It also should be pointed out that the involvement of nonhematopoietic compartment (eg, SRA/CD204-expressing endothelial cells) is not clear and remains to be addressed, even though the hematopoietic cells are clearly required for the enhanced vaccine activities in SRA KO mice.
Although it has been recognized that certain endocytic receptors can perform dual functions, ie, internalizing ligand and triggering signaling transduction cascades,26,27 the molecular mechanisms by which the SRA/CD204 modulates TLR-activating signaling pathways remain largely unknown. We also made an observation that SRA/CD204 inhibits TLR4 agonist-induced activation of transcription factor nuclear factor-κB, which is known to control a large array of genes involved in inflammation and immunity (X.Y., H.Y., J.R.S., and X.-Y.W., unpublished data, January 2009). It is tempting to postulate that there exists a functional cross-regulation between these 2 PRRs. TLR4 activation can have effects on antigen uptake and presentation by APCs, as indicated by transient up-regulation of endocytic receptors, including SRA/CD204,28 which in turn modulates the consequences of TLR activation by interfering with the TLR signaling cascade either directly or indirectly, resulting in down-regulation of inflammatory signals. Modification of TLR4 signaling by SRA/CD204 is also supported by a recent observation from Kobzik's group that engagement of SRA/CD204 led to inhibition of IL-12 release by Mφ upon LPS stimulation.23
The control exerted by TLRs in linking innate and adaptive immunity is instrumental in the efficacy of therapeutic vaccines containing TLR ligands. Our results provide the first evidence that SRA/CD204 can impact on the outcome of adaptive immune responses triggered by TLR4 signaling activation. The cross-talk and inter-regulation of these 2 types of PPRs may play a broad role in coordinating the immune response during pathogen infection, progression of cardiovascular disease, as well as active immunotherapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Cancer Institute grants CA121848 (to X.-Y.W.), CA129111 (X.-Y.W.), and CA099362 (J.R.S.); American Cancer Society grant RSG-08-187-01-LIB (to X.-Y.W.); Roswell Park Alliance Foundation (to X.-Y.W.); and National Cancer Institute Cancer Center support grant to the Roswell Park Cancer Institute CA016056.
National Institutes of Health
Authorship
Contribution: H.Y., X.Y., P.G., Y.W., S.-H.B., and X.C. conducted experiments; X.-Y.W., H.L.K., and J.R.S. analyzed data; X.-Y.W. planned and supervised experiments; and X.-Y.W. and H.Y. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Xiang-Yang Wang, Department of Cellular Stress Biology, Elm & Carlton Sts, Roswell Park Cancer Institute, Buffalo, NY 14263; e-mail: xiang-yang.wang@roswellpark.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal