Abstract
Interleukin-7 (IL-7) plays a central role in the homeostasis of the T-cell compartment by regulating T-cell survival and proliferation. Whether IL-7 can influence T-cell receptor (TCR) signaling in T cells remains controversial. Here, using IL-7–deficient hosts and TCR-transgenic T cells that conditionally express IL-7R, we examined antigen-specific T-cell responses in vitro and in vivo to viral infection and lymphopenia to determine whether IL-7 signaling influences TCR-triggered cell division events. In vitro, we could find no evidence that IL-7 signaling could costimulate T-cell activation over a broad range of conditions, suggesting that IL-7 does not directly tune TCR signaling. In vivo, however, we found an acute requirement for IL-7 signaling for efficiently triggering T-cell responses to influenza A virus challenge. Furthermore, we found that IL-7 was required for the enhanced homeostatic TCR signaling that drives lymphopenia-induced proliferation by a mechanism involving efficient contacts of T cells with dendritic cells. Consistent with this, saturating antigen-presenting capacity in vivo overcame the triggering defect in response to cognate peptide. Thus, we demonstrate a novel role for IL-7 in regulating T cell–dendritic cell interactions that is essential for both T-cell homeostasis and activation in vivo.
Introduction
The cytokine interleukin-7 (IL-7) plays a vital role in regulating the homeostasis and function of the T-cell compartment. Mice lacking either the cytokine1 or its specific receptor,2 IL-7Rα (CD127) have a profound block at the CD4−CD8− double-negative stage of thymic development. Consequently, thymi are severely reduced in size and the mice are profoundly lymphopenic, having very few mature peripheral T cells. IL-7 also plays a central role in regulating the homeostasis of the peripheral T-cell compartment. It is essential for survival of naive CD4 and CD8 T cells3-5 and is also an important factor in the long-term survival of CD46 and CD87-9 memory cells. In addition, IL-7 has been implicated in the generation of memory cells from effectors.10,11
During immune responses, IL-7R is down-regulated after activation3 and is not thought to participate in the effector response, rather handing over its responsibilities to other γc cytokines, such as IL-2 and IL-15. It is unclear, however, whether IL-7 signals play any role in the initial priming and activation events, a point at which T cells are still expressing IL-7Rα and receiving IL-7 signals. Initial studies of polyclonal Il7ra−/− mice found their T cells to be hyporesponsive to T-cell receptor (TCR)-mediated signaling,12 suggesting that IL-7 can influence TCR signaling. However, these mice are profoundly lymphopenic and their T cells have an abnormal activated phenotype often associated with lymphopenic mice. Later analysis of naive Il7ra−/− OT1 TCR transgenic T cells reported normal T-cell activation.3 However, studies of tumor immunity find that lymphopenia can enhance antitumor responses,13-15 and in some studies increased bioavailability of IL-7 has been directly implicated in the mechanism.14 In the homeostasis of naive T cells, there is evidence that IL-7 can synergize with TCR signals to promote both survival4 and induction of TCR-dependent homeostatic proliferation.3,16 IL-7 signaling can affect cell cycle by inhibiting p27kip, a negative regulator of cell cycle,17 whereas evidence for convergence of TCR and IL-7 signaling comes from studies of human memory cells, which show that FoxO3a, a proapoptotic transcription factor, is a downstream target of both TCR and IL-7 signaling.18 A more recent study has suggested that IL-7 can directly influence sensitivity of TCR signaling by tuning CD8 coreceptor expression.19 In humans, IL-7 production by dendritic cells (DCs) was found to affect cytomegalovirus-specific CD8 T-cell responses in vitro.20
Previously, we have described a tetracycline-inducible IL-7R transgenic mouse model in which TCR transgenic T cells conditionally express IL-7R. T cells from these mice generate effector cells with comparable function to controls, but examining primary responses to live influenza virus in vivo revealed that far few effectors were generated compared with wild-type control cells early in the response at day 7.10 In the present study, we used the same system to investigate whether there was any evidence that IL-7R signaling could influence TCR-dependent T-cell activation and proliferation. In vitro, we could find no evidence that either induction or blockade of IL-7 signaling had any effect on TCR sensitivity or activation. In contrast, in vivo an absence of IL-7R expression or IL-7 cytokine resulted in a significant defect in triggering of T cell–proliferative responses both to influenza A virus and to lymphopenia. Rather than influencing TCR signaling directly, we found evidence that, for both antigen and lymphopenia-induced proliferation, IL-7 was indeed required for efficient interactions of T cells with DCs and that the failure to trigger responses was a failure to make sufficient contacts with antigen-presenting DCs.
Methods
Mice
F5 Il7r−/− TreIL-7R rtTAhuCD2 tetracycline-inducible IL-7R transgenic mice (TetIL-7R) have been described previously.10 Breeders and weaned pups were fed doxycycline in food (3 mg/g) to induce IL-7R expression. (F5 Rag1−/−xC57Bl/6JCD45.1)F1 mice were used as controls throughout. These strains and recombinase activating gene-1–deficient (Rag1−/−) mice, F5 Rag1−/−, β2m−/−Rag1−/−, Il7−/−Rag1−/−, Il15ra−/−Rag1−/− mice were bred in a conventional colony free of pathogens at the National Institute for Medical Research (NIMR, London, United Kingdom). All lines used were of H-2b haplotype. Animal experiments were done according to institutional guidelines, with ethical approval from the Home Office.
In vitro activation of F5 T cells
Lymphocytes were teased from lymph nodes and spleen of donor mice and single-cell suspensions prepared. Cells were labeled with 2 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA) in Dulbecco phosphate-buffered saline (PBS; Invitrogen) for 10 minutes at 37°C and washed twice and unfractionated cells cultured (106 T cells/mL) in complete RPMI 1640 media (Sigma-Aldrich, St Louis, MO) supplemented with 10% fetal calf serum, glutamine, 2-mercaptoethanol, and antibiotics (all Sigma-Aldrich). Where used, IL-7 and IL-15 were supplemented at 10 ng/mL. T cells were activated with either NP6821 or NP3422 peptide in PBS for short-term stimulation (< 4 hours) or complete media proliferation assays.
Flow cytometry
Flow cytometry was carried out using 2 to 5 × 106 lymph node or spleen cells. Cell concentrations were determined using a Scharfe Instruments Casy Counter (Scharfe Instruments, Reutlingen, Germany). Cells were incubated with saturating concentrations of antibodies in 100 μL PBS-bovine serum albumin (0.1%)–azide (1 mM) for 1 hour at 4°C followed by 3 washes in PBS-bovine serum albumin-azide. Monoclonal antibodies used in this study were as follows: allophycocyanin (APC)–TCR (H57-597; eBioscience, San Diego, CA), fluorescein isothiocyanate-CD11c (eBioscience), phycoerythrin (PE)–CD69 (eBioscience), PE-CD86 (eBioscience), PE-IL-7R (eBioscience), bio-I-Ab (eBioscience), APC- and bio-CD45.1 (eBioscience), APC-CD5 and APC-CD44 (Leinco Technologies, St Louis, MO), bio-CD44 (eBioscience), APC-, and peridinin chlorophyll protein (PerCP)– and PE-CD8 (eBioscience). Biotinylated monoclonal antibody staining was detected using PerCP steptavidin (BD Biosciences PharMingen, San Diego, CA) and PE-Texas Red steptavidin (Invitrogen). bio-I-Ab staining was detected using PE-steptavidin (BD Biosciences PharMingen) for T cells and PerCP steptavidin for mature DCs. PE-pZap70 (BD Biosciences, San Jose, CA), PE-Bcl-2 (BD Biosciences PharMingen), active PE-caspase 3 (BD Biosciences PharMingen), and APC-pSTAT5 (BD Biosciences) staining of paraformaldehyde fixed samples was carried out according to the manufacturer's instructions. Four- and 5-color cytometric staining was analyzed on a FACSCalibur and LSR Instruments (BD Biosciences), respectively, and data analysis was performed using FlowJo version 8.5 software (TreeStar, Ashland, OR).
Labeling and adoptive transfer of T cells
Lymphocytes were teased from lymph nodes and spleen of donor mice and single-cell suspensions prepared. Cells were labeled with 2 μM CFSE and transferred into recipient mice via tail vein injections. Mice further challenged with influenza A virus (A/NT/60-68) were injected intravenously with 1 to 100 hemagglutin (HA) units of virus or 2 intraperitoneal injections of NP68 peptide at the dose indicated at 0 and 18 hours after cell transfer. After 72 hours, spleens of recipient mice were taken from host mice and splenocytes analyzed by fluorescence-activated cell sorter (FACS) for expression of CD8, TCR, CD45.1, and CFSE. Triggering and burst size of proliferative responses were calculated using FlowJo analysis software and refer to the precursor population.
Results
IL-7 does not affect TCR sensitivity in vitro
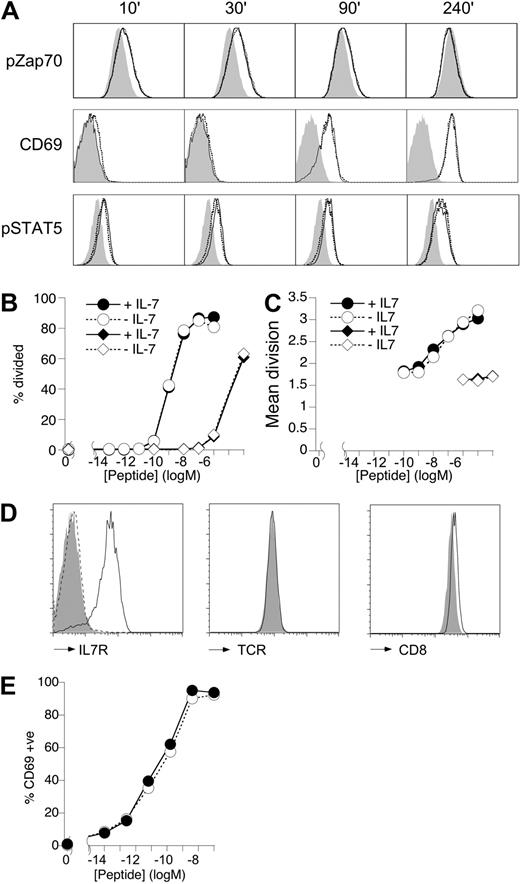
To determine whether IL-7 could affect T-cell activation, we first assessed the effects of IL-7 signaling on proximal TCR signaling, triggering, and proliferation of TCR transgenic T cells in vitro. T cells of F5 mice express a transgenic TCR specific for a peptide of nucleoprotein (NP) of influenza A (flu) virus.21 T cells from these mice were stimulated with 10 nM agonist peptide (NP68)21 in the presence or absence of a high dose of IL-7 (10 ng/mL). Neither phosphorylation of Zap70 kinase, which peaked at 30 minutes, nor subsequent induction of CD69 at 90 minutes after stimulation was affected by the presence of IL-7 (Figure 1A). IL-7 signaling was intact in these activated T cells because both IL-7R expression (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) and induction of phosphoSTAT5 (pSTAT5) were identical to non–NP68-stimulated cells during these early stages of activation (Figure 1A). IL-2 also induces STAT5 phosphorylation,23 and pSTAT5 could be detected in IL-7–free cultures by 4 hours (Figure S1B). However, this pSTAT5 level was also unaffected by the presence of IL-7 either at 4 hours (Figure 1A) or 24 hours (Figure S1C). Consistent with this, Bcl2 expression levels were not modulated by IL-7 in peptide-stimulated cultures.
IL-7 does not affect T-cell activation in vitro. (A) Total lymph node cells from F5 Rag1−/− mice were cultured (106/mL) with 10 nM NP68 in the presence or absence of IL-7 (10 ng/mL). Histograms are of pZap70 and CD69 levels on CD8-gated T cells in unstimulated (gray fill) or NP68-stimulated cultures with (solid line) or without (broken line) IL-7 for the times indicated. pSTAT5 staining is shown for IL-7–stimulated cells cultured with (solid line) or without (broken line) NP68 peptide compared with unstimulated cultures lacking IL-7 and NP68 (gray fill). (B,C) Total F5 lymph node cells were labeled with CFSE and cultured (106/mL) with a range of peptide doses. At day 3, CFSE profile of viable CD8+ T cells was analyzed by FACS. Graphs show the percentage cells triggered into division (B) and mean division of triggered cells (C) in response to different doses of agonist NP68 peptide (circles) or the weak agonist NP34 (diamonds) in the absence (empty symbols) or presence (filled symbols) of IL-7 (10 ng/mL). (D) Histograms show expression of IL-7R, TCR, and CD8 by IL-7R+ F5 T cells from control (solid line) and IL-7R− F5 T cells from F5 TetIL-7R mice off doxycycline for 7 days (gray fill). Histogram of IL-7R by CD4+CD8+ DP F5 thymocytes (broken line) is shown as negative control. (E) The graph shows CD69 expression at 18 hours by IL-7R+ F5 splenocytes of control mice (●) and CD8+ IL-7R− F5 splenocytes from F5 TetIL-7R mice off doxycycline for 7 days (○), stimulated with different doses of NP68 peptide. Data are representative of 3 or more experiments.
IL-7 does not affect T-cell activation in vitro. (A) Total lymph node cells from F5 Rag1−/− mice were cultured (106/mL) with 10 nM NP68 in the presence or absence of IL-7 (10 ng/mL). Histograms are of pZap70 and CD69 levels on CD8-gated T cells in unstimulated (gray fill) or NP68-stimulated cultures with (solid line) or without (broken line) IL-7 for the times indicated. pSTAT5 staining is shown for IL-7–stimulated cells cultured with (solid line) or without (broken line) NP68 peptide compared with unstimulated cultures lacking IL-7 and NP68 (gray fill). (B,C) Total F5 lymph node cells were labeled with CFSE and cultured (106/mL) with a range of peptide doses. At day 3, CFSE profile of viable CD8+ T cells was analyzed by FACS. Graphs show the percentage cells triggered into division (B) and mean division of triggered cells (C) in response to different doses of agonist NP68 peptide (circles) or the weak agonist NP34 (diamonds) in the absence (empty symbols) or presence (filled symbols) of IL-7 (10 ng/mL). (D) Histograms show expression of IL-7R, TCR, and CD8 by IL-7R+ F5 T cells from control (solid line) and IL-7R− F5 T cells from F5 TetIL-7R mice off doxycycline for 7 days (gray fill). Histogram of IL-7R by CD4+CD8+ DP F5 thymocytes (broken line) is shown as negative control. (E) The graph shows CD69 expression at 18 hours by IL-7R+ F5 splenocytes of control mice (●) and CD8+ IL-7R− F5 splenocytes from F5 TetIL-7R mice off doxycycline for 7 days (○), stimulated with different doses of NP68 peptide. Data are representative of 3 or more experiments.
To measure triggering and proliferation, T cells from these mice were labeled with CFSE and stimulated with a wide range of NP68 concentrations in the presence or absence of high-dose IL-7 (10 ng/mL). Analysis of T-cell proliferation at 72 hours revealed that the addition of IL-7 had no effect on either the proportion of cells triggered into division (Figure 1B) or the size of their proliferative burst at any antigen dose (Figure 1C). It was possible that IL-7 signaling might only modulate suboptimal TCR stimuli but not stimuli induced by high avidity ligands, such as NP68. Therefore, we also tested the effects of IL-7 stimulation on T-cell responses to NP34, an NP peptide with weak agonist/antagonist properties.22 As expected, triggering (Figure 1B) and proliferation (Figure 1C) of F5 T cells to this peptide were much reduced compared with NP68-induced responses. However, IL-7 signaling had no influence on proliferative responses to the weaker NP34 peptide ligand. Furthermore, titrating IL-7 (1-50 ng/mL) also failed to reveal any effects on F5 T-cell responses (data not shown).
We could find no evidence that induction of IL-7 signaling could affect T-cell activation, so we next asked whether a complete loss of IL-7 signaling had any effect. Recent studies have implicated a role for IL-7R signaling in tuning T-cell TCR responsiveness by modulating CD8 expression.19 To examine this possibility further, we took advantage of a mouse model in which F5 mice conditionally express IL-7R using the tetracycline regulatory system (F5 Il7r−/− TreIL-7R rtTAhuCD2, F5 TetIL-7R here on; “Mice”).10 Induction of IL-7R expression by feeding mice doxycycline throughout life overcomes the block in thymic development that normally occurs in Il7r−/− F5 mice and allows the generation of a normal peripheral compartment of F5 T cells.10 Peripheral T cells from F5 TetIL-7R mice taken off doxycycline food for 7 days (IL-7R− F5 T cells here on) cease to express IL-7R (Figures 1D, S2). Analysis of IL-7R− F5 T cells did reveal a subtle reduction in CD8 expression (Figure 1D), although not to the extent described elsewhere.19 We have previously assessed the proliferative capacity of IL-7R− F5 T cells in experiments similar to those described here (Figure 1B,C) and could find no defect in their ability to proliferate to antigen in vitro.10 However, to be certain that the slight reduction in CD8 expression by IL-7R− F5 T cells was not having an influence earlier in the response, we also examined CD69 up-regulation at 24 hours. Induction of CD69 expression was entirely normal over a wide range of antigen doses, regardless of IL-7R expression (Figure 1E). In conclusion, we could find no evidence that either blockade or stimulation of IL-7 signaling in F5 T cells had any direct effect on TCR responsiveness or activation in vitro.
Impaired triggering of IL-7R− F5 T cells in response to flu challenge
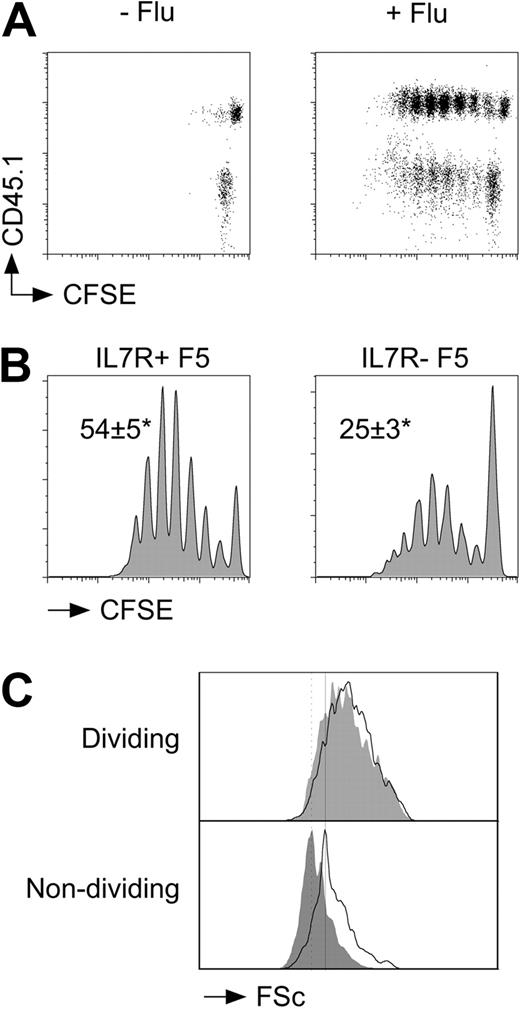
We next tested whether IL-7 signaling could affect T-cell responses to viral challenge in vivo. F5 T cells from CD45.1+ control F5 mice (IL-7R+ F5 T cells) and CD45.2+ IL-7R− F5 T cells were labeled with CFSE and cotransferred into Rag1−/− recipients. Host mice were then immediately challenged with A/NT/60-68 influenza A virus. To assess triggering and proliferation of F5 T cells, recipients were culled at day 3 and CFSE profile of donor populations assessed by FACS. Mice challenged with flu virus underwent a clear burst of cell divisions at day 3 not observed in unchallenged hosts (Figure 2A). When CFSE profiles of control and IL-7R− F5 T cells in the same host were compared, a highly significant (P < .001) and reproducible difference in the frequency of cells triggered to proliferate was apparent. The proportion of F5 T cells triggered into division in the absence of IL-7R expression was reduced more than 2-fold compared with control F5 T cells (Figure 2B). Although there was a clear reduction in the frequency of triggered cells, the profile of dividing IL-7R− F5 T cells appeared normal. The average burst size by control F5 T cells was 2.4 (± 0.4) divisions at day 3 compared with 2.4 (± 0.3) for IL-7R− F5 T cells from F5 TetIL-7ROFF mice. This selective defect in triggering was also reflected in the physical size of the cells responding. Dividing cells from both populations exhibited identical increases in cell size after their activation, whereas undivided IL-7R− F5 T cells were noticeably smaller than undivided control F5 T cells, most probably resulting from the requirement for IL-7 signaling for the maintenance of naive T-cell size.24 Significantly, we could find no evidence that death of undivided or dividing IL-7R− F5 T cells could account for the observed triggering defect (Figures S3, S4).
Defective triggering of F5 T cells in the absence of IL-7R expression. F5 T cells from CD45.1+ control F5 and CD45.1− F5 TetIL-7R mice off doxycycline for 7 days were CFSE-labeled, mixed at a 1:1 ratio, and transferred (3 × 106 total T cells/mouse) to groups of Rag1−/− hosts. Groups of recipient mice (n = 3) were further challenged with flu virus intravenously (24 U/mouse). At day 3 after transfer, mice were culled and donor populations among host splenocytes analyzed by FACS. (A) Dot plots are of CFSE versus CD45.1 expression by CD8+TCRhi cells from naive or flu-challenged recipients. (B) Histograms are of CFSE profiles for CD45.1+ control F5 T cells (IL-7R+ F5) and CD45.1− F5 T cells from F5 TetIL-7R donors (IL-7R− F5). Numbers indicate the average percentage of F5 T cells triggered into division plus or minus SD in each case. *P < .008; n = 6. (C) Histograms show cell size as determined by Forward Scatter (FSc) signal for divided and undivided control (solid line) and IL-7R− (gray fill) F5 T cells. Data are representative of 6 independent experiments.
Defective triggering of F5 T cells in the absence of IL-7R expression. F5 T cells from CD45.1+ control F5 and CD45.1− F5 TetIL-7R mice off doxycycline for 7 days were CFSE-labeled, mixed at a 1:1 ratio, and transferred (3 × 106 total T cells/mouse) to groups of Rag1−/− hosts. Groups of recipient mice (n = 3) were further challenged with flu virus intravenously (24 U/mouse). At day 3 after transfer, mice were culled and donor populations among host splenocytes analyzed by FACS. (A) Dot plots are of CFSE versus CD45.1 expression by CD8+TCRhi cells from naive or flu-challenged recipients. (B) Histograms are of CFSE profiles for CD45.1+ control F5 T cells (IL-7R+ F5) and CD45.1− F5 T cells from F5 TetIL-7R donors (IL-7R− F5). Numbers indicate the average percentage of F5 T cells triggered into division plus or minus SD in each case. *P < .008; n = 6. (C) Histograms show cell size as determined by Forward Scatter (FSc) signal for divided and undivided control (solid line) and IL-7R− (gray fill) F5 T cells. Data are representative of 6 independent experiments.
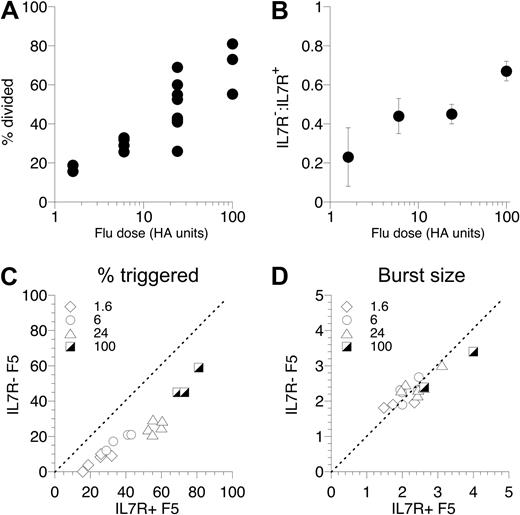
To test how robust the defect in triggering of IL-7R− F5 T cells was, we extended the experiment to challenge groups of mice with a range of different flu doses. Immunizing recipient mice with flu doses between 1 U and 100 U resulted in a range of T cell–triggering responses broadly proportional to the dose of flu administered (Figure 3A). Triggering of IL-7R− F5 T cells was significantly reduced over all the doses of flu tested, compared with control F5 T cells cotransferred in the same host (Figure 3B,C). Interestingly, of cells successfully triggered to divide, we found no difference in proliferation between IL-7R+ and IL-7R− F5 T cells (Figure 3D) at any of the flu doses, suggesting that the defect observed in the absence of IL-7R expression was restricted to initial triggering and not subsequent proliferation.
T-cell triggering is defective over a wide range of antigen doses. (A) Control F5 T cells were CFSE-labeled and transferred to Rag1−/− hosts (3 × 106/mouse), and groups of mice were challenged with a range of flu doses. At day 3, mice were culled and the responding T-cell population in the spleen analyzed by FACS. The scatter plot shows the percentage of F5 T cells triggered into division in individual mice challenged with different doses of flu virus. (B-D) F5 T cells from CD45.1+ control F5 and CD45.1− F5 TetIL-7R mice off doxycycline 7 days were CFSE-labeled, mixed at a 1:1 ratio, and transferred (3 × 106 total T cells/mouse) to groups of Rag1−/− hosts challenged with 1.6, 6, 24, or 100 U flu virus. At day 3, mice were culled and CFSE profile of CD8+ TCRhi CD45.1+ control (IL-7R+ F5) and CD45.1− F5 TetIL-7R F5 T cells (IL-7R− F5) analyzed by FACS. Plot shows ratio of IL-7R− F5/IL-7R+ F5 cells triggered into division as a function of flu dose (B). Scatter plot shows percentage of F5 T cells triggered into division for IL-7R+ F5 (x-axis) versus IL-7R− F5 (y-axis) cells in the same recipient challenged with the flu dose indicated (C). The scatter plot shows the mean division of triggered IL-7R+ F5 (x-axis) versus IL-7R− F5 T cells in individual hosts (D). Data are pooled from 3 independent experiments.
T-cell triggering is defective over a wide range of antigen doses. (A) Control F5 T cells were CFSE-labeled and transferred to Rag1−/− hosts (3 × 106/mouse), and groups of mice were challenged with a range of flu doses. At day 3, mice were culled and the responding T-cell population in the spleen analyzed by FACS. The scatter plot shows the percentage of F5 T cells triggered into division in individual mice challenged with different doses of flu virus. (B-D) F5 T cells from CD45.1+ control F5 and CD45.1− F5 TetIL-7R mice off doxycycline 7 days were CFSE-labeled, mixed at a 1:1 ratio, and transferred (3 × 106 total T cells/mouse) to groups of Rag1−/− hosts challenged with 1.6, 6, 24, or 100 U flu virus. At day 3, mice were culled and CFSE profile of CD8+ TCRhi CD45.1+ control (IL-7R+ F5) and CD45.1− F5 TetIL-7R F5 T cells (IL-7R− F5) analyzed by FACS. Plot shows ratio of IL-7R− F5/IL-7R+ F5 cells triggered into division as a function of flu dose (B). Scatter plot shows percentage of F5 T cells triggered into division for IL-7R+ F5 (x-axis) versus IL-7R− F5 (y-axis) cells in the same recipient challenged with the flu dose indicated (C). The scatter plot shows the mean division of triggered IL-7R+ F5 (x-axis) versus IL-7R− F5 T cells in individual hosts (D). Data are pooled from 3 independent experiments.
Acute requirement for IL-7 for efficient T-cell triggering
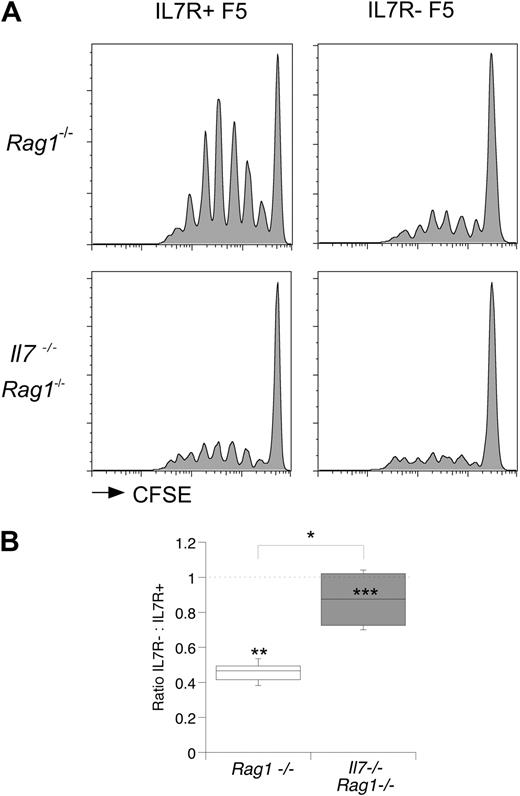
Cessation of IL-7R expression in F5 TetIL-7R mice was achieved by withdrawal of doxycycline from the diet of mice for 7 days. We therefore wished to determine whether the requirement for IL-7 signaling was acute, acting at the time of challenge, or whether chronic stimulation from IL-7 was required to condition T cells for optimal T-cell responsiveness. To determine the temporal requirement for IL-7 signaling for efficient T-cell triggering, we compared responses to flu challenge of mixtures of IL-7R+ and IL-7R− F5 T cells in either Rag1−/− or Il7−/−Rag1−/− hosts. As expected, in Rag1−/− hosts, IL-7R− F5 T cells exhibited a clear defect in triggering (Figure 4A) as already described. Strikingly, in Il7−/−Rag1−/− hosts, the frequency of control IL-7R+ F5 and IL-7R− F5 T cells triggered after flu challenge was virtually identical (Figure 4A). The ratio of IL-7R− F5 to control IL-7R+ F5 T cells successfully triggered into division was 0.87 in IL-7–deficient hosts compared with 0.45 in control hosts (Figure 4B), suggesting an acute IL-7 requirement for efficient triggering of T cells at the time of antigen encounter.
Acute requirement for IL-7 for optimal triggering of T cells. F5 T cells from CD45.1+ control F5 and CD45.1− F5 TetIL-7R mice off doxycycline 7 days were CFSE-labeled, mixed at a 1:1 ratio, and transferred (3 × 106 total T cells/mouse) to groups of Rag1−/− or Il7−/−Rag1−/− hosts. Recipient mice were further challenged with flu virus (24 U/mouse). At day 3, mice were culled and F5 T cells populations in spleen analyzed by FACS. (A) Histograms show CFSE profiles of CD8+ TCRhi F5 T cells from the donor mice and in the hosts indicated. (B) Box plot shows the ratio of control F5 to F5 TetIL-7R donor T cells triggered into division in the indicated hosts. Statistics: *P < .016, comparing ratios in Rag1−/− and Il7−/−Rag1−/−. Comparison of frequency of triggered control and F5 TetIL-7R T cells in Rag1−/− hosts (**P < .001) and in Il7−/−Rag1−/− hosts (***P = .66).
Acute requirement for IL-7 for optimal triggering of T cells. F5 T cells from CD45.1+ control F5 and CD45.1− F5 TetIL-7R mice off doxycycline 7 days were CFSE-labeled, mixed at a 1:1 ratio, and transferred (3 × 106 total T cells/mouse) to groups of Rag1−/− or Il7−/−Rag1−/− hosts. Recipient mice were further challenged with flu virus (24 U/mouse). At day 3, mice were culled and F5 T cells populations in spleen analyzed by FACS. (A) Histograms show CFSE profiles of CD8+ TCRhi F5 T cells from the donor mice and in the hosts indicated. (B) Box plot shows the ratio of control F5 to F5 TetIL-7R donor T cells triggered into division in the indicated hosts. Statistics: *P < .016, comparing ratios in Rag1−/− and Il7−/−Rag1−/−. Comparison of frequency of triggered control and F5 TetIL-7R T cells in Rag1−/− hosts (**P < .001) and in Il7−/−Rag1−/− hosts (***P = .66).
IL-7 signaling facilitates efficient T cell–DC interaction
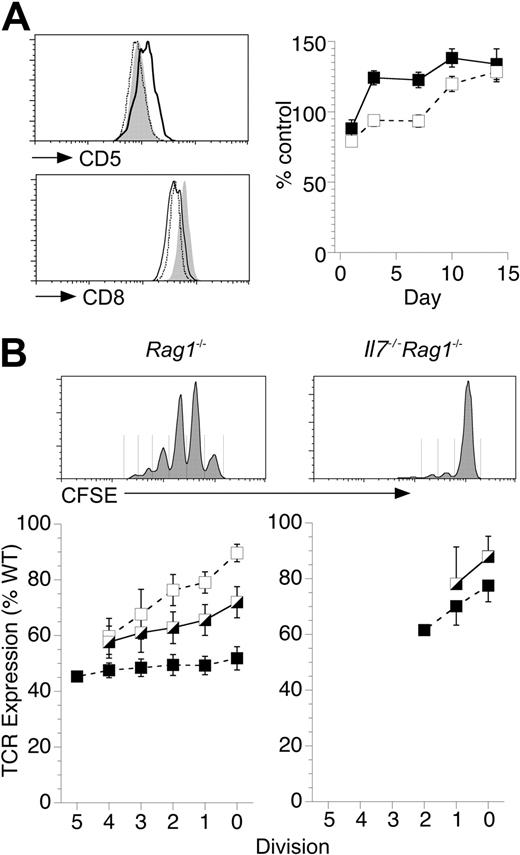
To further investigate the mechanism by which IL-7 could affect T-cell triggering, we examined F5 T-cell proliferative responses to lymphopenia. Lymphopenia-induced proliferation (LIP) is driven by TCR signals from self-peptide major histocompatibility complexes (spMHC) but also has a profound requirement for IL-7 signals.3,25 In the absence of IL-7, F5 T cells also fail to undergo LIP.16 Therefore, we first asked whether the homeostatic TCR signals that drive LIP were affected by the absence of IL-7 or whether IL-7 facilitated LIP by a mechanism independent of TCR signaling. To assess homeostatic TCR signaling, we examined CD5 expression levels. CD5 is a negative regulator of TCR signaling, and its expression levels are tuned by homeostatic signals through the TCR.4,26 F5 T cells transferred to Rag1−/− hosts up-regulated CD5 consistent with an increase in homeostatic TCR signals from spMHC driving LIP (Figure S5A). CD5 up-regulation was class I MHC dependent as levels were down-regulated on the same cells transferred to class I MHC-deficient β2m−/−Rag1−/− lymphopenic hosts (Figure S5A). Whereas F5 T cells transferred to Rag1−/− hosts underwent a rapid and sustained up-regulation of CD5 (Figure 5A), the same cells transferred to Il7−/−Rag1−/− hosts remained unchanged for the first week and only started to gradually increase expression thereafter (Figure 5A), suggesting that homeostatic TCR signaling to donor F5 T cells was not increased in lymphopenic hosts in the absence of IL-7, despite being completely devoid of host T cells. CD8 expression by F5 T cells was similar after transfer to Rag1−/− or Il7−/−Rag1−/− hosts but up-regulated in class I–deficient hosts (Figure 5A).
IL-7 enhances T-cell stimulation by DCs to induce LIP. (A) T cells from F5 Rag1−/− donors were transferred (2 × 106 /mouse) to Rag1−/−, β2m−/−Rag1−/−, or Il7−/−Rag1−/− hosts (n = 5/group). At various days, spleens were taken from recipient mice and stained for CD8, TCR, and CD5. Histograms of CD5 expression 7 days after transfer of F5 T cells to Rag1−/− hosts (solid line) and Il7−/−Rag1−/− (broken line) hosts compared with expression by F5 T cells from control F5 Rag1−/− mice (gray fill). Histograms of CD8 expression are of F5 T cells 7 days after transfer to Rag1−/− hosts (solid line), β2m−/−Rag1−/− hosts (gray fill), and Il7−/−Rag1−/− hosts (broken line). Graph shows CD5 expression after transfer to Rag1−/− (solid) or Il7−/−Rag1−/− hosts (empty) as percentage of expression by F5 T cells from control mice. (B) F5 T cells were CFSE-labeled and transferred (2 × 106 cells/mouse) to Rag1−/− and Il7−/−Rag1−/− hosts. At days 7, 14, and 21, lymphocytes were recovered and stained for expression of CD8 and TCR. Histograms are of representative CFSE profiles at day 14 and indicate gates used to examine cells that had undergone different number of division. Line graphs show TCR expression by F5 T cells that have undergone different numbers of divisions at day 7 (□), day 14 ( ), and day 21 (■) after transfer into the hosts indicated normalized to expression in control F5 Rag1−/− mice.
), and day 21 (■) after transfer into the hosts indicated normalized to expression in control F5 Rag1−/− mice.
IL-7 enhances T-cell stimulation by DCs to induce LIP. (A) T cells from F5 Rag1−/− donors were transferred (2 × 106 /mouse) to Rag1−/−, β2m−/−Rag1−/−, or Il7−/−Rag1−/− hosts (n = 5/group). At various days, spleens were taken from recipient mice and stained for CD8, TCR, and CD5. Histograms of CD5 expression 7 days after transfer of F5 T cells to Rag1−/− hosts (solid line) and Il7−/−Rag1−/− (broken line) hosts compared with expression by F5 T cells from control F5 Rag1−/− mice (gray fill). Histograms of CD8 expression are of F5 T cells 7 days after transfer to Rag1−/− hosts (solid line), β2m−/−Rag1−/− hosts (gray fill), and Il7−/−Rag1−/− hosts (broken line). Graph shows CD5 expression after transfer to Rag1−/− (solid) or Il7−/−Rag1−/− hosts (empty) as percentage of expression by F5 T cells from control mice. (B) F5 T cells were CFSE-labeled and transferred (2 × 106 cells/mouse) to Rag1−/− and Il7−/−Rag1−/− hosts. At days 7, 14, and 21, lymphocytes were recovered and stained for expression of CD8 and TCR. Histograms are of representative CFSE profiles at day 14 and indicate gates used to examine cells that had undergone different number of division. Line graphs show TCR expression by F5 T cells that have undergone different numbers of divisions at day 7 (□), day 14 ( ), and day 21 (■) after transfer into the hosts indicated normalized to expression in control F5 Rag1−/− mice.
), and day 21 (■) after transfer into the hosts indicated normalized to expression in control F5 Rag1−/− mice.
If IL-7 signaling could directly tune or amplify TCR signaling induced by spMHC, this would explain the failure of F5 T cells to receive enhanced homeostatic TCR signaling in Il7−/−Rag1−/− hosts. An alternative possibility was that, in the absence of IL-7, there was a failure of spMHC ligand to induce the enhanced TCR signaling required for LIP. To ask whether IL-7 was affecting TCR engagement with spMHC ligand, we measured TCR down-modulation on donor F5 T-cell populations. TCR is down-modulated after stimulation by peptide MHC,27,28 and we found the same was also true of spMHC-mediated stimulation of TCR during LIP. After transfer of F5 T cells to Rag1−/− hosts, TCR levels were down-modulated because of increased engagement by spMHC in the absence of host T cells because the same cells transferred to class I–deficient Rag1−/− hosts up regulated TCR expression in the absence of spMHC ligands (Figure S5A). Maximal down-modulation of TCR in Rag1−/− hosts took between 14 and 21 days but at early time points was greatest on those cells that had divided the most, possibly because that had received more stimulation from spMHC to divide (Figure 5B). However, all T cells down-modulated TCR to a similar level by day 21, regardless of division history, suggesting that the whole donor population was subject to enhanced engagement of TCR by spMHC in these lymphopenic hosts. In contrast, in IL-7–deficient hosts, there was only limited down-modulation of TCR (Figure 5B), suggesting that, in the absence of IL-7, TCR engagement by spMHC was hardly increased. Even undivided F5 T cells in Rag1−/− underwent greater down-modulation of TCR than undivided cells in Il7−/−Rag1−/− hosts.
We next asked whether the failure of TCR to engage spMHC in the absence of IL-7 was indeed secondary to a failure of F5 T cells to make sufficient contacts with spMHC-expressing DCs. We quantified T cell–DC contact on the basis of passive acquisition of class II MHC molecules by T cells that occurs as a consequence of T cell–DC interactions.29 Mouse T cells cannot synthesize class II MHC themselves29 but do acquire surface expression as a consequence of cell-cell interactions with class II MHC+ cells, mostly DCs in Rag1−/− lymph nodes, and specific staining was not observed on CD8 T cells from in I-Ab−/− mice (Figure S5B). Higher levels of class II MHC were found on F5 T cells in Rag1−/− hosts than in replete F5 Rag1−/− hosts; and interestingly, enhanced expression appeared dependent on host class I MHC expression (Figure S5C), also implying a role for spMHC recognition for enhancing T cell–DC interactions. After transfer to T cell–deficient Rag1−/− hosts, F5 T cells had higher levels of I-Ab molecules on their surface, suggesting increased contact with DCs. It is probable that this increased T cell–DC interaction in the absence of competing host T cells resulted in the increase in TCR-spMHC engagement that delivers enhanced homeostatic TCR signals and ultimately drives LIP. Significantly, little increase in I-Ab staining of F5 T cells was observed on cells transferred to IL-7–deficient hosts in the first week (Figure 6A), suggesting that IL-7 was also required for the enhanced T cell–DC contacts that result in the induction of LIP. The failure to enhance class II acquisition in the absence of IL-7 could not be attributed to changes in host DCs in Il7−/−Rag1−/− hosts, as DCs in these mice were present in normal numbers and of normal phenotype compared with those in Rag1−/− hosts (Figure S6). However, as for CD5 expression, class II expression did gradually increase thereafter, and it is notable that a small subset of F5 T cells did undergo divisions in IL-7–deficient hosts, most probably induced by IL-15 (Figure S3A). To confirm that acquisition of class II molecules was dependent on IL-7R signaling to F5 T cells, we also compared I-Ab staining on IL-7R+ and IL-7R− F5 cells after cotransfer to Rag1−/− hosts. Interestingly, analysis before transfer revealed that IL-7R− F5 T cells had slightly higher basal I-Ab staining than IL-7R+ F5 controls, probably resulting from reduced competition for DCs in F5 TetIL-7Rind mice that have 3- to 4-fold fewer F5 T cells than controls.10 Significantly, though, only IL-7R+ F5 T cells increased class II expression after transfer (Figure 6B) and, consistent with our experiments using IL-7–deficient hosts (Figure 6A), levels were significantly greater than on IL-7R− F5 T cells in the same host.
IL-7 enhances T cell–DC interactions. (A) Histogram shows class II MHC expression by F5 T cells recovered from spleen 7 days after transfer to Il7−/−Rag1−/− (broken line) or Rag1−/− (solid line) hosts (n = 5 per group) compared with F5 T cells from control F5 mice (gray fill). Graph shows I-Ab staining after transfer to Rag1−/− (■) or Il7−/−Rag1−/− hosts (□) as percentage of expression by F5 T cells from control mice. Data are representative of 3 or more experiments. (B) CD45.1+ IL-7R+ and CD45.1− IL-7R− F5 T cells were cotransferred to Rag1−/− hosts (n = 4) and cells analyzed for class II MHC expression 1 day later. Histograms show I-Ab staining by IL-7R+ and IL-7R− F5 T cells before (broken line) and after (solid line) transfer to Rag1−/− hosts. Bar chart is MFI of I-Ab staining by the indicated F5 T cell donor after transfer to Rag1−/− hosts (*P < .02).
IL-7 enhances T cell–DC interactions. (A) Histogram shows class II MHC expression by F5 T cells recovered from spleen 7 days after transfer to Il7−/−Rag1−/− (broken line) or Rag1−/− (solid line) hosts (n = 5 per group) compared with F5 T cells from control F5 mice (gray fill). Graph shows I-Ab staining after transfer to Rag1−/− (■) or Il7−/−Rag1−/− hosts (□) as percentage of expression by F5 T cells from control mice. Data are representative of 3 or more experiments. (B) CD45.1+ IL-7R+ and CD45.1− IL-7R− F5 T cells were cotransferred to Rag1−/− hosts (n = 4) and cells analyzed for class II MHC expression 1 day later. Histograms show I-Ab staining by IL-7R+ and IL-7R− F5 T cells before (broken line) and after (solid line) transfer to Rag1−/− hosts. Bar chart is MFI of I-Ab staining by the indicated F5 T cell donor after transfer to Rag1−/− hosts (*P < .02).
Antigen dose overcomes the triggering defect in IL-7R–deficient F5 T cells
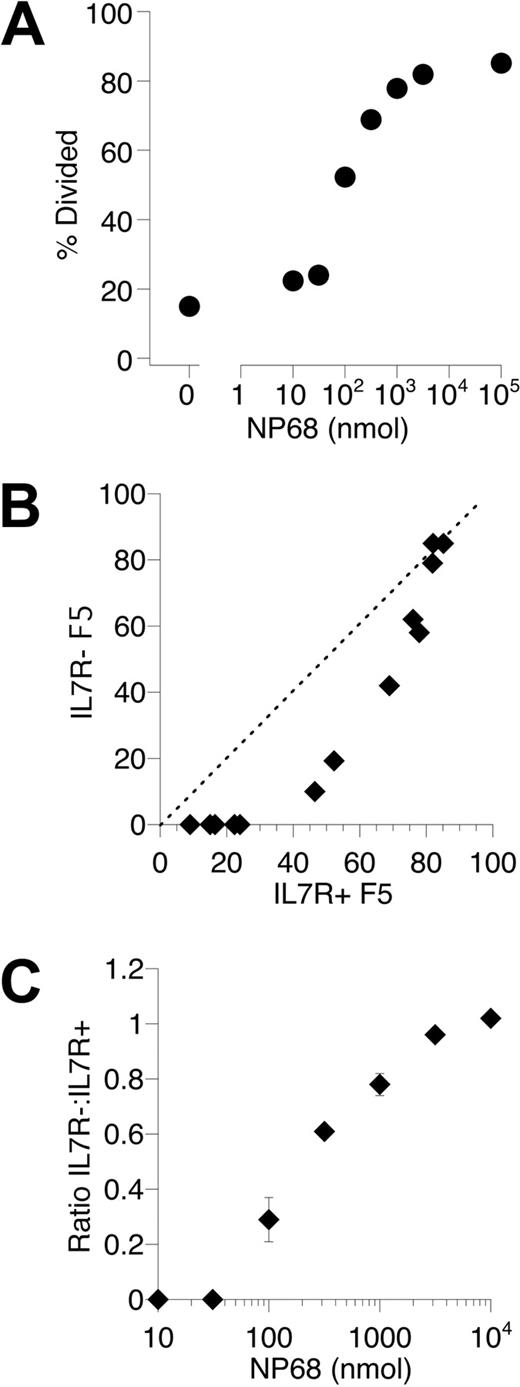
The evidence from our studies of LIP by F5 T cells suggested a novel role for IL-7 influencing efficient T cell–DC interactions. Normal activation of IL-7R− F5 T cells in vitro suggested that T cell–DC conjugate formation was essentially normal in the absence of IL-7R signaling. The reduced DC contact we detected in vivo may therefore reflect a reduction in the number of T cell–DC contacts rather than a qualitative reduction in individual interactions. Only a proportion of control F5 T cells were successfully triggered during flu challenge (Figure 3), suggesting that antigen is limiting in this challenge; therefore, successful activation would depend on T cells finding DCs presenting antigen. The triggering defect in IL-7R− F5 T cells to flu challenge may therefore reflect impairment in efficient location of DCs presenting flu antigen compared with IL-7R+ control F5 T cells. If true, then the triggering defect should be overcome by saturating the antigen-presenting capacity in vivo. To test this hypothesis, we examined antigen responses of IL-7R− F5 cells in a setting in which antigen-presenting capacity would not be limiting in vivo. This was achieved by challenging mice with a range of doses of soluble NP68 peptide, such that, at high doses, antigen-presenting capacity would become saturated in vivo. As before, CFSE-labeled IL-7R− F5 T cells and control IL-7R+ F5 cells were cotransferred to Rag1−/− hosts. Mice were challenged with a broad range of NP68 peptide doses; and at day 3, the frequency of cells triggered into divisions was determined. As was the case for responses to flu, the size of the responding population was proportional to antigen dose (Figure 7A). When triggering responses of control IL-7R+ and IL-7R− F5 T cells were compared over a range of NP68 doses, a similar defect in triggering was evident among the IL-7R− F5 T cells as observed in response to flu challenge. Significantly, however, as antigen dose increased, the defect was overcome such that triggering of control IL-7R+ F5 and IL-7R− F5 T cells became indistinguishable (Figure 7B,C). Crucially, this also confirmed that IL-7R− F5 T cells in these mice were normally responsive and demonstrated that the observed triggering defect was not a consequence of nonresponsiveness in a subset of the IL-7R− F5 T cells.
Saturating antigen-presenting capacity overcomes the triggering defect in IL-7R− F5 T cells. (A) Control F5 T cells were CFSE-labeled and transferred to Rag1−/− hosts (3 × 106 cells/mouse), and groups of mice were challenged with a range of NP68 peptide doses at 0 and 18 hours after transfer. At day 3, mice were culled and the responding T-cell population analyzed by FACS. The scatter plot shows the percentage of control F5 T cells triggered into division in individual mice challenged with different doses of peptide. (B) F5 T cells from CD45.1+ control F5 (IL-7R+ F5) and CD45.1− F5 TetIL-7R mice off doxycycline 7 days (IL-7R− F5) were CFSE-labeled, mixed at a 1:1 ratio, and transferred (3 × 106 total T cells/mouse) to groups of Rag1−/− hosts challenged with a range of NP68 peptide doses. At day 3, mice were culled and CFSE profile of IL-7R+ F5 and IL-7R− F5T cells analyzed by FACS. Scatter plot shows the percentage of F5 T cells triggered into division for IL-7R+ F5 (x-axis) versus IL-7R− F5 (y-axis) T cells in the same recipient (B) and ratio of IL-7R− F5: IL-7R+ F5 as a function of peptide dose (C). Data are representative 2 independent experiments.
Saturating antigen-presenting capacity overcomes the triggering defect in IL-7R− F5 T cells. (A) Control F5 T cells were CFSE-labeled and transferred to Rag1−/− hosts (3 × 106 cells/mouse), and groups of mice were challenged with a range of NP68 peptide doses at 0 and 18 hours after transfer. At day 3, mice were culled and the responding T-cell population analyzed by FACS. The scatter plot shows the percentage of control F5 T cells triggered into division in individual mice challenged with different doses of peptide. (B) F5 T cells from CD45.1+ control F5 (IL-7R+ F5) and CD45.1− F5 TetIL-7R mice off doxycycline 7 days (IL-7R− F5) were CFSE-labeled, mixed at a 1:1 ratio, and transferred (3 × 106 total T cells/mouse) to groups of Rag1−/− hosts challenged with a range of NP68 peptide doses. At day 3, mice were culled and CFSE profile of IL-7R+ F5 and IL-7R− F5T cells analyzed by FACS. Scatter plot shows the percentage of F5 T cells triggered into division for IL-7R+ F5 (x-axis) versus IL-7R− F5 (y-axis) T cells in the same recipient (B) and ratio of IL-7R− F5: IL-7R+ F5 as a function of peptide dose (C). Data are representative 2 independent experiments.
Discussion
Whether cytokines such as IL-7 can influence the sensitivity of T cells to TCR stimulation remains controversial. There are conflicting reports from a variety of different systems, and little consensus on mechanism. In the present study, we examined both antigen and LIP of F5 T cells to determine whether IL-7 could affect the TCR signaling that is required to induce these responses. We found no evidence that IL-7 signaling could directly tune TCR responsiveness in vitro but found evidence in vivo of a novel mechanism by which IL-7 could augment CD8 T-cell stimulation by affecting the ability of CD8 T cells to interact with antigen-presenting DCs.
IL-7 has several pleiotropic effects on T cells that could, in principle, contribute to the enhancement of TCR stimulation we observed. Although IL-7 is an essential survival factor for naive T cells,24 we found no evidence that this played any role in the regulating T-cell triggering. Although IL-7R− F5 T cells died gradually in the long-term, we observed no detectable loss of IL-7R− F5 T cells over the 3-day duration of the flu challenge. After successful triggering in vivo, proliferation and blast transformation were normal in the IL-7R− F5 T cells, as is effector generation from these cells.10 Apoptosis of proliferating IL-7R− F5 T cells could not account for the triggering defect because both Bcl2 expression and caspase-3 activation were identical between IL-7R+ and IL-7R− F5 cells during flu responses. As well as promoting survival, IL-7 signaling pathways have been described to influence cell division through the inhibition of the cell cycle inhibitor p27kip, and some rescue of proliferation in IL-7–deficient hosts is observed in p27kip-deficient T cells.17 However, we found that IL-7R− F5 T cells express normal levels of p27kip (data not shown). Other factors, such as FoxO3a,18 have also been identified as targets downstream of both TCR and IL-7 signaling pathways. We do not therefore exclude the possibility that pathways activated downstream of IL-7R signaling may, under certain circumstances, converge with TCR-induced pathways and be a mechanism to enhance T-cell proliferation. Indeed, we have suggested such a model in the past.4,16 However, in the present study, we could find no obvious role for IL-7 as a costimulator of T-cell activation with either high or low avidity ligands, over a broad range of antigen and cytokine doses. Other studies have suggested that IL-7 can tune T-cell reactivity by modulating coreceptor expression.19 Although we did observe a small but reproducible reduction in CD8 expression by IL-7R− F5 T cells (∼15%), neither this nor the complete absence of IL-7R expression had any detectable effect on CD69 up-regulation or T-cell proliferation in vitro,10 neither was CD8 expression by IL-7R+ F5 T cells influenced by IL-7 deficiency in vivo. Therefore, we could find no evidence to suggest that activating IL-7 signaling can directly influence T-cell responses to TCR ligands.
Arguably the strongest evidence for cross talk between TCR and IL-7 signaling comes from studies of LIP. LIP is triggered by TCR signals induced after engagement of receptor with spMHC in lymphopenia. IL-7 is also essential for induction of LIP,3,16,25 but its precise role is not understood. Although p27kip is clearly a target of IL-7 signaling,17 its ablation in T cells could only slightly rescue proliferation in the absence of IL-7 and so does not completely account for the role IL-7 in facilitating LIP. We found that induction of LIP was associated with increased homeostatic TCR signaling resulting from spMHC recognition,30 which occurred as a result of enhanced interactions of T cells with DCs in secondary lymphoid organs. It is not surprising that such increased contact should occur in the absence of host T-cell competition, and we previously found LIP to be inversely proportional to the number of F5 T cells transferred and hence level of T-cell competition.31 What was surprising was that, in the absence of IL-7, the increase in homeostatic TCR signaling was not observed, and neither was there any evidence of enhanced contact with DCs despite the lack of competing host T cells. It therefore appears that a key role for IL-7 exists at the level of initial delivery of TCR signaling by influencing the ability of T cells to interact and receive signals from pMHC on DCs, rather than tuning or modulating ongoing TCR signaling.
The mechanism by which IL-7 is influencing T-cell–DC interactions to enhance priming remains to be fully elucidated. Chemokines CCL19 and CCL21 regulate both migration and motility of naive T cells32 and have been recently implicated in regulating delivery of IL-7 signaling in lymph nodes by specific attraction of T cells to IL-7–secreting fibroblastic reticular cells,33 whereas inactivation of Gαi-coupled receptors, such as CCR7, with pertussis toxin, efficiently inhibits LIP but not T-cell survival.34 In addition, Foxo1 and IL-7 signaling can regulate CD62L and Klf2 expression, implying a role for IL-7 signaling in lymph node homing and trafficking.35 However, we found that neither IL-7 nor IL-7R deficiency had any effect on CCL21-induced transmigration of F5 T cells in vitro (M.S. and B.S., unpublished data, 2008). Another study found evidence that IL-7 specifically regulates CD4 T-cell homeostasis by directly modulating class II expression on plasmacytoid DCs,36 raising the intriguing possibility that homeostasis of CD4 and CD8 T cells by DCs and IL-7 could be achieved by distinct mechanisms relying on different DC subpopulations.
Another function of IL-7 is in the control of T-cell size and metabolism independently of survival, through the activation of PI3 kinase-dependent pathways24 and regulation of glucose metabolism.37 Consistent with this, IL-7R− F5 T cells were smaller than control IL-7R+ F5 T cells and probably also had reduced metabolism. Indeed, we found that CFSE labeling of IL-7R− F5 T cells, a process dependent on active uptake of dye, was consistently lower compared with control F5 T cells. The reduced size did not affect the ability of cells to activate or blast transform, but reduced cellular metabolism may have had an impact on the ability of the naive T cells to traffic around lymphoid tissues and thereby the rate with which they could interact with DCs. The ability of T cells to actively migrate around lymph nodes scanning DCs for antigen is vital for efficient initiation of immune responses. In lymphopenia, enhanced IL-7 signaling to F5 T cells results in significant growth,31 and the increase in metabolism may allow a more rapid scanning rate of DCs that would account for the increased level of DC contact we detected and, consequently, the delivery of more intense signaling from spMHC. This would also explain how spMHC ligands can trigger T cells into division in lymphopenia but not replete conditions. A failure to efficiently scan DCs would also explain why IL-7R− F5 T cells were less successful at finding DCs presenting peptide under conditions of limiting antigen, such as after flu challenge. However, for those cells that did successfully find a flu antigen-presenting DC, priming and activation would proceed normally, as we observed. Consistent with this view, when we saturated the antigen-presenting compartment of the mice with soluble peptide, thereby removing any advantage conferred to more motile cells that could scan more DCs, triggering rates among IL-7R− and IL-7R+ F5 T cells became identical. In conclusion, our data reveal a novel role for IL-7 in regulating the ability of T cells to interact with DCs and thereby influence both T-cell priming and homeostasis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rosemary Murphy and the Biological Services staff for assistance with mouse breeding and typing.
This work is supported by the MRC United Kingdom.
Authorship
Contribution: M.S. performed experiments and interpreted data; C.P. performed experiments; and B.S. performed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benedict Seddon, Division of Immune Cell Biology, MRC NIMR, The Ridgeway, London NW7 1AA, United Kingdom; e-mail: bseddon@nimr.mrc.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal