Several epidemiologic studies support the emerging paradigm that current alcohol consumers have decreased risk of most types of non-Hodgkin lymphoma. The observed lower risk among people who drank alcohol does not seem to vary with beverage type. The mechanisms accounting for alcohol-induced decrease in the incidence of lymphomas remain largely unknown. We demonstrate that low-dose chronic exposure to ethanol inhibits mammalian target of rapamycin (mTOR) C1 complex formation, resulting in decreased phosphorylation events involved in mTOR pathway signaling in a lymphoid-tissue specific manner. These changes in mTOR signaling lead to a decrease in eIF4E associated with the translation initiation complex and a repression of global cap-dependent synthesis in both lymphoma cell lines and normal donor lymphocytes. We show that chronic exposure of ethanol at physiologically relevant concentrations in a xenograft model results in a striking inhibition of lymphoma growth. Our data support a paradigm in which chronic ethanol exposure inhibits mTOR signaling in lymphocytes with a significant repression of cap-dependent translation, reducing the tumorigenic capacity of non-Hodgkin lymphoma in a human xenograft model. The ethanol-mediated repression of mTOR signaling coupled with decreased in vivo lymphoma growth underscore the critical role of mTOR signaling and translation in lymphoma.
Introduction
Alcohol abuse is associated with diseases of the liver, pancreas, and breast, and many other malignant disorders.1,2 Interestingly, in contrast to solid tumors, several epidemiologic studies, including a large pooled analysis of case-control studies3 and a recent cohort study,4 indicate that persons who frequently consume small amounts of alcohol (1 or 2 drinks per day) have decreased risk of most types of non-Hodgkin lymphoma (NHL). The underlying biologic mechanism(s) for this negative association is (are) unknown at present.
Ethanol decreases protein synthesis, and this effect is associated with changes in the phosphorylation status of several key components of the translational machinery.5,6 Chronic alcohol intake is associated with disruption of the mammalian target of rapamycin (mTOR) signaling pathway; however, few mechanistic details are known about how ethanol interrupts this critical signaling pathway involved in translation.7 mTOR forms 2 functionally distinct multiprotein serine/threonine kinase complexes, mTORC1 and mTORC2. The mTORC1 complex controls protein synthesis by phosphorylating key downstream effector molecules, the translational inhibitor protein 4E-BP1 and, p70 S6 kinase in response to sensory inputs, such as nutritional, growth factor, and cellular energy status. Activation of the p70 S6 kinase results in phosphorylation of the ribosomal protein S6 (RPS6) and allows its interaction with other ribosomal subunits and ribosomal biogenesis.8 Phosphorylation of ribosomal protein S6 by p70 S6 kinase stimulates the translation of mRNAs with a 5′ oligopyrimidine tract, which typically encode components of the protein synthesis machinery.8
The signaling pathways that converge on mTOR are commonly activated in multiple human malignancies. The dysregulation of mTOR signaling leads to a selective increase in translation of messages that encode antiapoptotic proteins, activators of cell cycle progression, and growth factors, such as cyclin D1 and c-myc.9,10 This underscores the importance of targeted inhibition of mTOR as a therapy in cancer treatment. The mTOR inhibitor rapamycin and analogs have been shown to induce apoptosis in multiple tumor types.11
Recently, aberrations in the activation of the mTOR-signaling pathway have come to light in NHL.12,13 An in vivo model using the mTOR inhibitor CCI-779 demonstrated apoptosis and growth suppression of primary acute lymphocytic lymphoma cells engrafted in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice,14 providing a strong rationale for the development of mTOR-targeted therapy in lymphoid malignancies. Therefore, improved understanding of the underlying molecular mechanism(s) associated with the ability of ethanol to suppress mTOR activity may form the basis for the development of innovative approaches to prevent lymphoma growth.
Herein, we illustrate that ethanol, at levels comparable with those that are readily achievable in chronic low-dose alcohol consumers, inhibits mTOR phosphorylation and subsequent signaling events, reduces the availability of eIF4E bound to the 5′ 7-methyl-guanosine triphosphate (GTP) cap, and increases the association of the inactive eIF4E and 4E-BP1 complex in lymphoid but not breast cancer cells. We found that ethanol specifically affects cap-dependent translation and global polysomal distribution profiles of lymphoid cells. We demonstrate, in an in vivo xenograft mouse model, that chronic ethanol consumption can inhibit lymphoma tumor formation.
Methods
Cell culture and treatment
Burkitt lymphoma (Raji) cells and diffuse large B-cell lymphoma (SUDHL-4) cells were cultured in RPMI 1640 containing 10% fetal bovine serum and 1% penicillin/streptomycin. Normal peripheral blood lymphocyte (PBL) cells were isolated from healthy donors and cultured with IL-2 as described previously.15 Normal B cells were isolated from healthy donors by immunodepletion of normal T cells from PBLs. Cells were then treated with IL-4 (80 U/mL) to expand a B-cell population as previously described.16 Mammary epithelial adenocarcinoma (MCF-7, BT-474, and BT-20) cells were cultured in Dulbecco modified essential medium containing 10% fetal bovine serum and 1% penicillin/streptomycin.17 Cells were treated with ethanol concentrations (0-20 mM) for 10 days or 10 nM rapamycin for 24 hours. Cells were cultured in a closed flask system and centrifuged every other day with ethanol replenished in fresh media to prevent effects from evaporation as previously described.18
Western blotting
Western blots were carried out as previously described.19 Blots were probed with antibodies recognizing: phospho-mTOR (Ser 2448; no. 2971), mTOR, phospho-TSC2 (Ser 939), pAMPK (Thr 169), phospho-p70 S6 kinase (Thr 389), p70 S6 kinase, phospho-RPS6 (Ser 235/236), RPS6 (Cell Signaling Technology, Danvers, MA), phospho-AKT (Thr 308), TSC2 (Millipore, Billerica, MA), eIF4E, phospho-4E-BP1 (Thr 70), 4E-BP1 (Santa Cruz Biotechnology, Santa Cruz, CA), or β-actin (Abcam, Cambridge, United Kingdom). Signals were detected with enhanced chemiluminescence according to the manufacturer's instructions (Pierce Chemical, Rockford, IL). A minimum of 3 independent Western blots were performed.
7-methyl-GTP Sepharose 4B pull-down
After 10 days of ethanol treatment, cells were lysed with radio immunoprecipitation assay (RIPA) buffer, and whole-cell lysates were used for pull-down with 7-methyl-GTP cap analog Sepharose 4B beads (GE Healthcare, Little Chalfont, United Kingdom). Pull-down reactions were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and analyzed by immunoblotting for eIF4E.
Immunoprecipitation
After treatment, cells were lysed with RIPA buffer, and whole-cell lysates were subjected to immunoprecipitation. Immunoprecipitation was carried out overnight at 4°C using 4E-BP1 (Santa Cruz Biotechnology) antibody, mTOR (Cell Signaling Technology), and IgG as a control. After extensive washes in TNN buffer (50 mM Tris, pH 7.5, 250 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 0.5% NP-40), the IP material was resolved by SDS-PAGE, transferred onto polyvinylidene difluoride (PVDF) membranes, and immunoblotted for eIF4E and raptor, respectively.
Transient transfection assays of translation
A total of 4 × 106 Raji and MCF-7 cells were treated with ethanol (0-20 mM) for 9 days and then transfected with a bicistronic luciferase construct (cap-dependent firefly luciferase and internal ribosome entry site [IRES] Renilla luciferase). Six hours after transfection, cells were treated with appropriate amounts of ethanol for a further 24 hours. Cells were then harvested, and luciferase activity was measured using the Dual Luciferase Reporter Assay system (Promega, Madison, WI).
Polysome preparation
Raji and MCF-7 cells, either untreated or treated with 20 mM ethanol, were collected 10 days later by centrifugation and resuspended in a buffer containing 150 mM KOAc, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (pH 7.5), 2.5 mM Mg (OAc)2, 2 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 200 U/mL RNAsin as described.20 After addition of 100 μg/mL digitonin, lysates were pelleted (10 000g, 1 minute, 4°C), and the cytoplasmic extracts were then loaded onto 10% to 50% sucrose gradients and centrifuged (Beckman SW41, 35 000g, 3 hours, 4°C; Beckman Coulter, Fullerton, CA).21 Subsequently, the material was fractionated into 1-mL aliquots using a gradient fractionator (Brandel, Gaithersburg, MD) and monitored by optical density measurement (A254).
In vivo translation
Analysis of de novo translation was carried out using methodology previously described.22 Briefly, 106 Raji and SUDHL-4 cells were incubated with 1 mCi L-[35S]methionine and L-[35S]cysteine (Easy Tag EXPRESS; PerkinElmer Life and Analytical Sciences, Waltham, MA) per 6-well plate for 20 minutes, whereupon cells were lysed using RIPA buffer. Cell lysates were then resolved by SDS-PAGE, transferred onto PVDF membranes, and visualized by PhosphorImager (GE Healthcare).
In vivo tumor growth in the xenograft models
The Institutional Animal Care and Use Committee of the University of Maryland approved all procedures involving animals. SCID beige mice were housed in a pathogen-free environment under controlled conditions of light and humidity and received food and water ad libitum (control vs 5% EtOH vol/vol vs 10% EtOH vol/vol) for up to 45 days with a 2-week acclimation period before implantation of SUDHL-4 cells. Previous reports have shown that mice can consume water containing up to 20% ethanol without showing an aversion to the ethanol.6,7 All control mice were pair-fed with the ethanol consuming groups to ensure nutritional intake was similar among groups. SUDHL-4 cells (106) were resuspended in 100 μL phosphate-buffered saline and then mixed with an equal volume of Matrigel. The mixture was subcutaneously injected into the left dorsal flank of 5- to 7-week-old SCID mice. Once tumors were palpable, tumor growth was measured every other day with calipers, and tumor volumes were calculated by the formula ½ × r12 × r2 (r1 < r2), as described previously.23 No toxicity was observed at these doses of ethanol, as treated mice did not experience behavioral changes or significant weight loss compared with controls. The gross appearances of the livers were normal after necropsy. Thirty-one days after inoculation of SUDHL-4 cells, tumors were harvested for further analysis.
Immunohistochemistry
Three-millimeter paraffin sections were stained with a Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's protocol. The paraffin section slides were deparaffinized24 with xylene and rehydrated through graded alcohol washes followed by antigen retrieval by microwave for 25 minutes in sodium citrate buffer (10 mM, pH 6.0). Slides were then incubated in 3% hydrogen peroxide to quench endogenous horseradish peroxidase for 10 minutes. The slides were blocked by incubation in normal goat serum (dilution 1:10) in phosphate-buffered saline (pH 7.4) and subsequently incubated overnight at 4°C with rabbit anti–human pRPS6 (Ser 235/236) antibody (1:150) and rabbit anti–human pmTOR (Ser 2448) antibody (1:150; Cell Signaling Technology no. 2976). Slides were then treated with biotinylated anti–rabbit IgG and incubated with preformed avidin-peroxidase complex. The sections were counterstained with hematoxylin, dehydrated, and mounted. Negative controls were included. Photographs of representative tumor growth in mice were taken with a Nikon (Tokyo, Japan) Coolpix 5400 camera, and images were processed using Adobe (San Jose, CA) Photoshop CS, version 8. Photomicrographs of representative immunohistochemistry were observed with a Nikon eclipse50i microscope using a 40×/0.65 NA air objective; imaging medium, air. Photomicrographs were taken with a Nikon Digital Sight DS-2M digital camera, and NIS-Elements acquisition software was utilized. Images were processed with Adobe Photoshop CS, version 8.
Statistical analysis
Results were analyzed using Microsoft Excel (Microsoft, Redmond, WA). P values were calculated using a paired Student t test. Statistical significance was determined when a P value less than .05 was attained.
Results
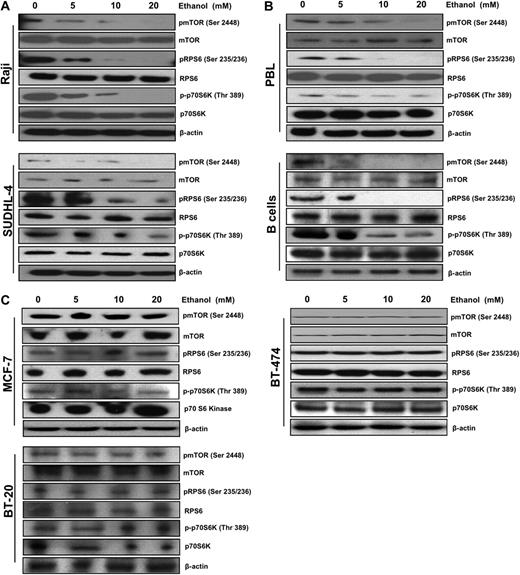
Ethanol treatment decreased phosphorylation of mTOR, p70 S6 kinase, and RPS6 in lymphoid cells
A multitude of signaling pathways converge to regulate translation initiation. mTOR is a critical pathway regulating translation by controlling the cellular availability of eIF4E. Given that ethanol exposure has been reported to modulate the mTOR signaling pathway7 and alcohol consumption is associated with a decreased incidence of NHL,3 we set out to examine the effect of ethanol on mTOR and its downstream targets in lymphoid cells. As demonstrated by Western blot analysis, ethanol exposure caused a strong dose-dependent decrease in the phosphorylation state of mTOR on Ser 2448 and of key downstream effectors of mTOR: p70 S6 kinase, and RPS6 in both Raji and SUDHL-4 cells (Figure 1A). We used both IL-4–stimulated normal B cells and IL-2–stimulated PBLs from normal human donors to examine whether activated normal lymphocytes responded similarly to ethanol exposure as the transformed lymphoma cell lines. Down-regulated mTOR signaling in activated PBLs and normal B cells after exposure to ethanol was observed as well, demonstrating that ethanol exposure had an inhibitory effect on nontransformed lymphocytes (Figure 1B). Notably, ethanol treatment had no significant down-regulation on the phosphorylation of mTOR, p70 S6 kinase, and RPS6 in MCF-7, BT-474, or BT-20 mammary epithelial adenocarcinoma cells (Figure 1C).
Ethanol decreases mTOR pathway signaling in lymphocytes. Representative Western blot analysis of (A) Raji and SUDHL-4, (B) B cells and PBLs, and (C) MCF-7, BT-474, and BT-20 cells after 10 days of ethanol treatment (0-20 mM). A total of 50 μg total protein lysates was loaded, and the abundance of pmTOR, mTOR, pRPS6, RPS6, p-p70S6K, p70S6K, and β-actin was assessed.
Ethanol decreases mTOR pathway signaling in lymphocytes. Representative Western blot analysis of (A) Raji and SUDHL-4, (B) B cells and PBLs, and (C) MCF-7, BT-474, and BT-20 cells after 10 days of ethanol treatment (0-20 mM). A total of 50 μg total protein lysates was loaded, and the abundance of pmTOR, mTOR, pRPS6, RPS6, p-p70S6K, p70S6K, and β-actin was assessed.
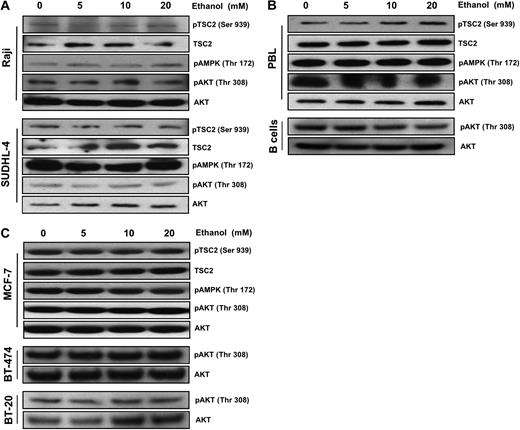
Many signaling pathways upstream of mTOR, such as those that sense cellular levels of ATP, growth factors, and amino acid abundance, regulate TSC1/2 complex formation and activity via phosphorylation. Interestingly, we observed no significant changes in AKT phosphorylation, the phosphorylation of TSC2 at Ser 939, or activation of the AMPK pathway in both Raji and SUDHL-4 cells (Figure 2A). Similar results were observed in normal lymphoid cells (Figure 2B) and mammary epithelial adenocarcinoma cells (Figure 2C).
Ethanol down-regulates mTOR in a TSC2-independent manner. Representative Western blot analysis of (A) Raji and SUDHL-4, (B) B cells and PBLs, and (C) MCF-7, BT-474, and BT-20 cells treated with ethanol (0-20 mM) for 10 days. A total of 50 μg total protein lysates was loaded, and the abundance of pTSC2, TSC2, pAMPK, pAKT, and AKT was assessed.
Ethanol down-regulates mTOR in a TSC2-independent manner. Representative Western blot analysis of (A) Raji and SUDHL-4, (B) B cells and PBLs, and (C) MCF-7, BT-474, and BT-20 cells treated with ethanol (0-20 mM) for 10 days. A total of 50 μg total protein lysates was loaded, and the abundance of pTSC2, TSC2, pAMPK, pAKT, and AKT was assessed.
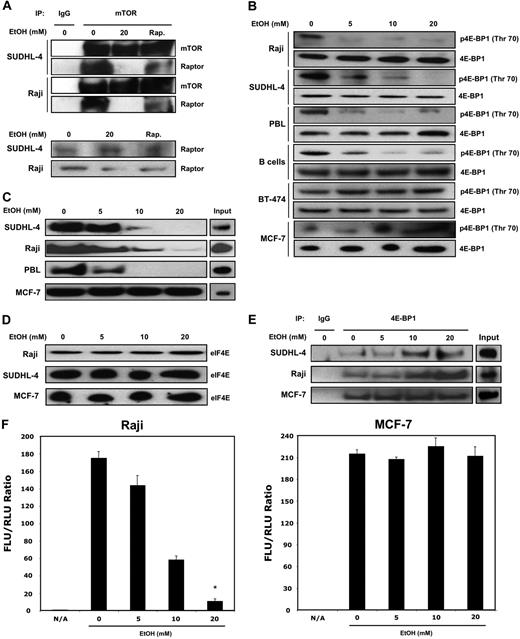
Chronic ethanol exposure reduced formation of mTORC1 complex, eIF4E availability, increased 4E-BP1/eIF4E association, and inhibited cap-dependent translation
mTOR exists as 2 structurally distinct complexes, mTORC1 and mTORC2. mTORC1, the main regulator of protein synthesis, comprises mTOR, raptor, and mLST8. Raptor is an essential scaffolding protein in the mTORC1 complex, and its absence from the complex inhibits propagation of mTORC1 signaling. We analyzed the molecular effects of low-dose ethanol exposure on mTORC1 complex formation through mTOR/raptor coimmunoprecipitation assays. Chronic ethanol exposure does inhibit mTORC1 complex formation, as shown by the decreased amount of raptor in the immunoprecipitates from SUDHL-4 and Raji cells (Figure 3A). Rapamycin is known to inhibit mTOR signaling through binding of FKBP12 and prevention of raptor association to mTORC1. Using a rapamycin-resistant mTOR construct, we demonstrated that ethanol inhibits mTOR activation in a rapamycin-independent manner (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). mTOR regulates cap-dependent translation through the phosphorylation and inactivation of the 4E-BPs, a family of translation inhibitors that act through their binding of eIF4E. Phosphorylation of 4E-BP1 results in a conformational change that releases eIF4E and an increase of eIF4E protein present at the 5′ 7-methyl-GTP cap. We observed hypo-phosphorylation of 4E-BP1 (Thr 70) in conjunction with decreased mTOR signaling in lymphoma cells with no changes observed in MCF-7 and BT-474 breast cancer cells (Figure 3B). The binding of eIF4E to the 5′ 7-methyl-GTP cap structure present on mRNA is essential for initiating cap-dependent translation. We hypothesized that ethanol-induced reduction of mTOR phosphorylation and downstream signaling molecules may reduce the availability of eIF4E for translation initiation. Indeed, in response to chronic ethanol exposure, the binding of eIF4E to mRNAs with a 5′ 7-methyl-GTP cap structure was significantly reduced in normal PBLs and lymphoma cells only (Figure 3C) with no substantial change in total eIF4E protein levels (Figure 3D).
Ethanol alters 4E-BP1 and eIF4E association and decreases cap-dependent translation in lymphocytes. (A) Immunoprecipitation (IP) of protein lysates with either anti-mTOR antibody or control IgG was assayed to analyze changes in association of mTOR and Raptor. (B) Raji, SUDHL-4, B cells, PBLs, MCF-7, and BT-474 cells were treated with ethanol (0-20 mM) for 10 days, and lysates were collected. Levels of p4E-BP1 and total 4E-BP1 were analyzed by Western blot. (C) A total of 250 μg total lysates was incubated with a 5′ 7mG cap analog conjugated to agarose beads and used to pull down cap-bound eIF4E. (D) Total eIF4E levels were measured by Western blot analysis. (E) Immunoprecipitation (IP) of protein lysates with either anti–4E-BP1 antibody or control IgG was assayed to analyze changes in association of 4E-BP1 and eIF4E. (F) Cap-dependent translation of ethanol-treated cells was measured by transfection with a bicistronic luciferase construct (cap-dependent firefly luciferase and IRES Renilla luciferase). Six hours after transfection, cells were treated with appropriate amounts of ethanol for a further 24 hours. Cells were then harvested, and luciferase activity was measured. Data points represent the mean of 3 independent experiments, with error bars corresponding to SD (P ≤ .002).
Ethanol alters 4E-BP1 and eIF4E association and decreases cap-dependent translation in lymphocytes. (A) Immunoprecipitation (IP) of protein lysates with either anti-mTOR antibody or control IgG was assayed to analyze changes in association of mTOR and Raptor. (B) Raji, SUDHL-4, B cells, PBLs, MCF-7, and BT-474 cells were treated with ethanol (0-20 mM) for 10 days, and lysates were collected. Levels of p4E-BP1 and total 4E-BP1 were analyzed by Western blot. (C) A total of 250 μg total lysates was incubated with a 5′ 7mG cap analog conjugated to agarose beads and used to pull down cap-bound eIF4E. (D) Total eIF4E levels were measured by Western blot analysis. (E) Immunoprecipitation (IP) of protein lysates with either anti–4E-BP1 antibody or control IgG was assayed to analyze changes in association of 4E-BP1 and eIF4E. (F) Cap-dependent translation of ethanol-treated cells was measured by transfection with a bicistronic luciferase construct (cap-dependent firefly luciferase and IRES Renilla luciferase). Six hours after transfection, cells were treated with appropriate amounts of ethanol for a further 24 hours. Cells were then harvested, and luciferase activity was measured. Data points represent the mean of 3 independent experiments, with error bars corresponding to SD (P ≤ .002).
To further investigate the mechanism underlying the reduced level of eIF4E protein bound to the cap structure, we coimmunoprecipitated cell lysates with antibody recognizing 4E-BP1. As shown in Figure 3E, there was a dose-dependent increase in the association of 4E-BP1 with eIF4E in ethanol-treated lymphoid cells.
We subsequently examined whether a reduction in eIF4E availability for translation initiation would result in a decrease of in vivo cap-dependent translation. We used a bicistronic luciferase reporter system with a cap-dependent firefly and IRES-mediated Renilla luciferase construct. Chronic alcohol exposure resulted in a dramatic dose-dependent reduction of in vivo cap-dependent translation of Raji cells with a gradual decrease in the firefly to Renilla luciferase ratio observed, whereas MCF-7 cells exhibited no substantial changes in translation of the reporter (Figure 3F).
Long-term ethanol treatment results in global translational repression of lymphoid cells
Having established that ethanol results in decreased mTOR signaling and a striking reduction in cap-dependent translation, we hypothesized that ethanol might negatively impact global translation in lymphoid cells. To explore this possibility, polysome distribution profiles were analyzed from both Raji and MCF-7 breast cancer cells treated with 20 mM ethanol for 10 days. The profile obtained for the Raji cells was consistent with translational repression indicated by the striking shift of ribosomes from the polysomal fractions to monosomes in ethanol-treated versus control cells (Figure 4A). In contrast, MCF-7 cells displayed no significant changes in the amount of actively translating polysomes (Figure 4A).
Chronic ethanol treatment leads to a suppression of global translation. (A) Raji and MCF-7 cells were treated with ethanol (0 or 20 mM) for 10 days. On the 10th day, cytoplasmic lysates were prepared and fractionated over sucrose gradients. From left to right, the distribution of free mRNA (not bound to ribosomes or ribosome subunits), ribosome subunits 40S and 60S, as well as monosomes (single ribosomes) and polysomes of increasing molecular weight is indicated. (B) Raji and SUDHL-4 cells were treated with ethanol (0 or 20 mM) for 10 days and then incubated for 20 minutes with 35S-labeled amino acids. Lysate aliquots of 50 μg of total protein were fractionated by SDS-PAGE, transferred onto PVDF membranes, and visualized and quantified using a PhosphorImager. Two independent experiments are shown.
Chronic ethanol treatment leads to a suppression of global translation. (A) Raji and MCF-7 cells were treated with ethanol (0 or 20 mM) for 10 days. On the 10th day, cytoplasmic lysates were prepared and fractionated over sucrose gradients. From left to right, the distribution of free mRNA (not bound to ribosomes or ribosome subunits), ribosome subunits 40S and 60S, as well as monosomes (single ribosomes) and polysomes of increasing molecular weight is indicated. (B) Raji and SUDHL-4 cells were treated with ethanol (0 or 20 mM) for 10 days and then incubated for 20 minutes with 35S-labeled amino acids. Lysate aliquots of 50 μg of total protein were fractionated by SDS-PAGE, transferred onto PVDF membranes, and visualized and quantified using a PhosphorImager. Two independent experiments are shown.
Another measure of global translation, the incorporation of 35S-labeled amino acids into nascent proteins, was used as an additional confirmatory measure to illustrate the global repression of translation present in lymphoma cells after ethanol treatment. In both Raji and SUDHL-4 cells, a significant reduction in global translation was evidenced by diminished incorporation of labeled amino acids into newly translated protein (Figure 4B).
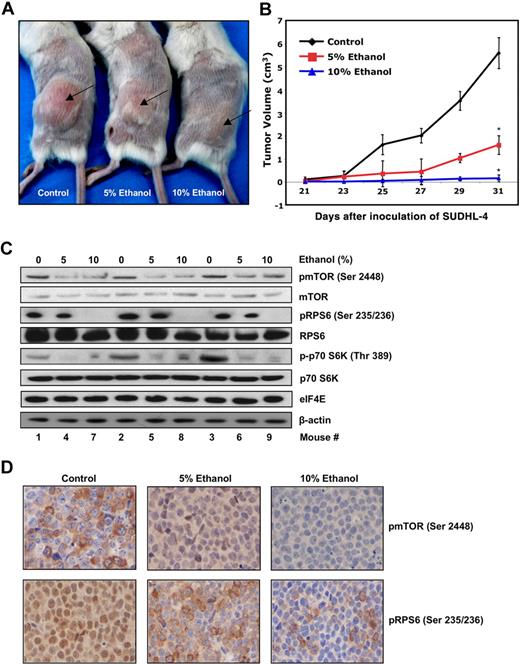
Chronic ethanol exposure results in diminished tumor formation in a human lymphoma xenograft model
A preclinical xenograft mouse model was established to examine whether ethanol could inhibit lymphomagenesis in SCID mice. A significant decrease in tumor formation was observed in mice with a diet supplemented with ethanol for a duration of 6.5 weeks (see “In vivo growth in the xenograft models” and Figure 5A,B). To confirm that the reduction in tumor formation was related to the interference with mTOR signaling by ethanol, we performed immunoblotting and immunohistochemistry on tumors harvested from xenografts 31 days after inoculation. Figure 5C illustrates a down-regulation of mTOR signaling in representative mice from each group, as shown by decreased levels of pmTOR (Ser 2448), pRPS6 (Ser 235/236), and p-p70S6K (Thr 389) from tumor lysates. Immunohistochemistry on slides prepared from tumor cross sections revealed corroborative evidence showing a marked decrease in pmTOR (Ser 2448) and pRPS6 (Ser 235/236) staining compared with controls (Figure 5D). Taken together, these data demonstrate that a significant decrease in lymphoma growth in a xenograft mouse model was the result of an inhibition of mTOR pathway signaling caused by low levels of ethanol.
Chronic ethanol treatment demonstrates significant antilymphoma activity in a human xenograft mouse model. Twenty-one mice were divided into 3 groups (control vs 5% EtOH vol/vol vs 10% EtOH vol/vol) with 7 mice each. Mice were injected subcutaneously with SUDHL-4 cells and continued on ethanol treatment for remainder of experiment. (A) Representative mice (mice 2, 5, and 8 from Western blots) were photographed before death to visually demonstrate tumor growth. (B) The average tumor volume of each group (n = 7) with SD is shown as a function of time (*P ≤ 2.17 × 10−5). (C) Xenograft tumors were excised from mice for further analysis. Tumors from control (1-3) 5% EtOH (4-6) and 10% EtOH (7-9) were lysated to obtain protein extracts. A total of 50 μg protein lysates was analyzed by Western blot and immunoblotted for pmTOR (Ser 2448), mTOR, pRPS6, RPS6, p-p70S6K, p70S6K, eIF4E, and β-actin. (D) Immunohistochemistry of paraffin-embedded xenograft tumors, excised from mice treated with ethanol (control vs 5% EtOH vol/vol vs 10% EtOH vol/vol), anti-pmTOR, and anti-pRPS6 antibody.
Chronic ethanol treatment demonstrates significant antilymphoma activity in a human xenograft mouse model. Twenty-one mice were divided into 3 groups (control vs 5% EtOH vol/vol vs 10% EtOH vol/vol) with 7 mice each. Mice were injected subcutaneously with SUDHL-4 cells and continued on ethanol treatment for remainder of experiment. (A) Representative mice (mice 2, 5, and 8 from Western blots) were photographed before death to visually demonstrate tumor growth. (B) The average tumor volume of each group (n = 7) with SD is shown as a function of time (*P ≤ 2.17 × 10−5). (C) Xenograft tumors were excised from mice for further analysis. Tumors from control (1-3) 5% EtOH (4-6) and 10% EtOH (7-9) were lysated to obtain protein extracts. A total of 50 μg protein lysates was analyzed by Western blot and immunoblotted for pmTOR (Ser 2448), mTOR, pRPS6, RPS6, p-p70S6K, p70S6K, eIF4E, and β-actin. (D) Immunohistochemistry of paraffin-embedded xenograft tumors, excised from mice treated with ethanol (control vs 5% EtOH vol/vol vs 10% EtOH vol/vol), anti-pmTOR, and anti-pRPS6 antibody.
Discussion
Chronic consumption of alcohol has been shown to be associated with diseases of the pancreas and liver, and multiple malignancies including squamous cell carcinoma, liver cancer, and breast cancer.1,25 However, recently, there have been epidemiologic data indicating that a low level of alcohol consumption (1 or 2 drinks per day) decreases the frequency of most types of NHL.3,4 The mechanisms accounting for such a paradoxical and alcohol-induced decrease in the incidence of lymphomas remain largely unknown. Alcohol has immunomodulatory effects, which may differ according to the degree of intake. Although heavy alcohol consumption impairs immune function, light or moderate alcohol use might improve cellular and humoral immune responses.26 Other mechanisms underlying a potential protective effect of moderate alcohol consumption may include antioxidants, such as resveratrol in wine or flavonoids in beer, and improvement of insulin sensitivity by alcohol.27,28 Current data suggest that there is no difference between the effects of consumption of red wine and white wine. In a pivotal pooled analysis study, the association between alcohol consumption and risk of NHL could not be attributed to consumption of a specific type of alcoholic beverage.3
Chronic alcohol intake is associated with disruption of the mTOR-signaling pathway in nonlymphoid tissue, such as the mouse cerebral cortex.7 However, there are limited published data describing the impact of ethanol exposure on this critical signaling pathway in human neoplasia. Importantly, many of the commonly characterized abnormalities present in cancer include loss of phosphatase with tensin homology, and constitutive activation of AKT, both of which can influence mTOR activity. Rapamycin treatment leads to the accumulation of cells at the G1 checkpoint, which is consistent with the fact that the drug inhibits global cap-dependent translation. Targeted therapy of the mTOR pathway has been shown to be effective in several preclinical studies and has emerging potential in the clinical setting.12,14 Indeed, rapamycin analogs have already been used in clinical trials of NHL with encouraging early results.29
In light of these findings, we set out to examine the mechanistic underpinnings of ethanol-induced down-regulation of signaling in the mTOR pathway. Chronically exposing lymphoma cell lines at levels readily attainable in human plasma (0-0.092 g/dL, or ∼3 drinks per day maximally)6 resulted in decreased phosphorylation of key downstream proteins involved in mTOR pathway signaling, including mTOR, p70 S6 kinase, RPS6, and 4E-BP1 (Figures 1,3B). Importantly, these levels of ethanol do not significantly impinge on cell viability (Figure S2).
The first major discovery into the upstream regulation of mTOR signaling came with the discovery that TSC1/TSC2 protein complex negatively controls mTOR activity.30 Mutations in either TSC1 or TSC2 proteins lead to hamartomatous benign tumors, such as Cowden disease.31 At present, the phosphorylation and inactivation of TSC1/2 through the AKT pathway is the most defined link between a deregulated pathway and mTOR in cancer.32,33 Our experimental observations support the notion that ethanol down-regulates mTOR signaling in a TSC1/2-independent manner, as exemplified by the lack of significant changes in phosphorylation levels of AKT, TSC2, and AMPK (Figures 2, S1B). Previous reports have shown that ethanol can inhibit mTOR phosphorylation and signaling without inducing changes in upstream regulatory pathways, such as AKT and AMPK in nonhuman tissue.34,35 We demonstrated that pAMPK levels do not significantly change between basal conditions compared with all doses of ethanol (5-20 mM). However, we did observe that on amino acid starvation these DLBCL cells activated the AMPK pathway, as shown by enhanced pAMPK levels. Furthermore, we were able to modulate this pathway using compound C, a well-described AMPK pharmacologic inhibitor (Figure S1B). The mechanism(s) underlying a specific down-regulation of the canonical AKT phosphorylation site, Ser 2448 on mTOR, in an AKT-independent manner is still poorly understood. A recent report demonstrates that, in human glioma cells, mTOR Ser 2448 is regulated in an EGFR-PKC-dependent pathway and is independent of AKT signaling.36 These aggregate data support alternative regulatory pathway(s) that can modulate the phosphorylation of Ser 2448. Given that mTOR is known to shuttle between the nucleus and cytoplasm, it is conceivable that ethanol could influence its subcellular localization, thereby impacting on the ability of membrane-bound AKT to phosphorylate mTOR.37 These questions remain to be formally studied and are an active area of investigation in our laboratory.
Signaling through the mTOR pathway is known to regulate the availability of eIF4E to form the ternary initiation cap complex eIF4F, the rate-limiting step in cap-dependent translation initiation. We demonstrated the decreased availability of eIF4E to associate with mRNAs containing a 5′ 7-methyl-GTP cap structure (Figure 3C) and increased association of eIF4E with 4E-BP1 in lymphoid cells exposed to ethanol (Figure 3E). Investigation of ethanol-induced perturbations of cap-dependent translation using a bicistronic luciferase reporter assay system confirmed in vivo that a lack in bioavailability of eIF4E leads to a decrease in the translation of cap-dependent messages (Figure 3F) in lymphoid but not breast cancer cells. In addition, we found that alcohol treatment resulted in a global decrease in protein translation in lymphoma cell lines (Figure 4A,B). Multiple epidemiologic studies have demonstrated a strong positive association with consumption of alcohol and incidence of breast cancer.1,2,38 In contrast, there have been little published data on the phenotypic effects of ethanol exposure in breast cancer. Singletary et al have shown that alcohol can induce a higher proliferative index in breast cancer cell lines.39,40 A recent report demonstrated that rapamycin treatment increased the cap-dependent translation of cervical carcinoma and embryonic kidney cell lines.41 We provide evidence that ethanol works to inhibit mTOR signaling in a lymphoid tissue-specific manner supported by the lack of mTOR-related signaling defects observed in several breast cancer cells under identical experimental conditions. This tissue specificity may be influenced by the differential expression of signaling pathways between lymphoma and breast cancer. Previous studies have shown that ethanol can lead to a more tumorigenic phenotype in breast cancer by both estrogen receptor and ErbB signaling pathways, both of which are not expressed in lymphoma.39,42 Conversely, B-cell lymphomas have been shown to express a multitude of signaling pathways different from those in breast cancer, such as the tyrosine kinase Syk.43 For example, in B-cell lymphoma, Syk provides a strong proliferative signal through the mTOR pathway; in contrast, in breast cancer, Syk has a tumor suppressor role negatively affecting mitotic progression and proliferation.44,45 Together, our findings support a model in which ethanol inhibits the formation of the translation initiation complex and prevents translation of cap-dependent messages in a tissue-specific manner.
The prevailing model for mTOR signaling implicates RPS6 activation in translational control of mRNAs characterized by the presence of an oligopyrimidine tract at the 5′ terminus (5′ TOP). These mRNAs encode multiple proteins that are associated with the assembly or functionality of the translational machinery.46 The 5′ oligopyrimidine tract in the mRNAs of ribosomal proteins contains an essential translational cis-regulatory element required for their translation.47 These mRNAs are selectively translated when cells in a resting state are thrust into a proliferative cycle or when amino acid–deprived cells are supplemented with the missing nutrients.48,49 The relationship between the translational regulation of mRNAs containing 5′ TOP sequences and RPS6 has led to a model describing RPS6 and 5′TOP mRNA interactions. However, there are several recent reports demonstrating that 5′ TOP mRNAs follow normal translational regulation in systems where RPS6 remains constitutively unphosphorylated, either where alanines are substituted at its 5 phosphorylation sites or in p70 S6 kinase null cells.8,49 Therefore, the role of RPS6 in translational regulation of 5′ TOP mRNAs is still an unresolved issue at present. One possible explanation for the apparent discrepancy could be that murine cell lines were used in experiments where RPS6 remained constitutively unphosphorylated and still maintained normal translational regulation of 5′ TOP mRNAs. The contribution of down-regulating RPS6 phosphorylation (Figure 1A) to global translational repression requires further investigation.
To investigate the in vivo influence of ethanol on signaling patterns, we used a preclinical model with a human lymphoma xenograft model. Mice receiving water supplemented with ethanol (10% vol/vol) can achieve blood alcohol concentrations that range from 0.05 to 0.15 g/dL, a level sufficient to impair motor skills in humans.6 De Feo et al have shown that consumption of 3 or 4 glasses of wine over the course of a meal, an amount commonly consumed by social drinkers, is sufficient to significantly inhibit hepatic protein translation.50 It has been previously shown that chronic ethanol consumption in mice alters the phosphorylation levels of mTOR, p70 S6 kinase, and 4E-BP1 in mouse cerebral cortex.7 Chronic ethanol exposure in rats has also been shown to sequester eIF4E from the cap-complex, resulting from an increase in eIF4E-4E-BP1 association in hepatocytes.51 In our human lymphoma xenograft model, we demonstrated, for the first time, that long-term, low-dose ethanol exposure suppresses lymphoma tumor formation, at least in part, through disruption of mTOR pathway signaling.
The data presented here support a model in which chronic ethanol exposure inhibits mTOR signaling in lymphocytes with a significant repression of cap-dependent translation and reduces the tumorigenic capacity of NHL in a human xenograft model. This suggests a mechanistic basis for the published epidemiologic studies showing that a low level of alcohol consumption is inversely correlated with incidence of NHL. The ethanol-mediated repression of mTOR signaling coupled with a decrease in lymphoma growth presented underscore the critical role of mTOR signaling and translation in lymphoma.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank J. Silvio Gutkind for providing the lentivirus vectors encoding vector, wild-type mTOR (wt-mTOR), and rapamycin-resistant mTOR mutant (rr-mTOR), containing an SI substitution in the FKBP12-rapamycin binding domain, as well as Dr Jeffrey Hasday for technical support.
This work was supported in part by a Merit Review Award from the Department of Veterans Affairs (R.B.G.).
Authorship
Contribution: P.R.H. and K.M.-M. performed research, analyzed data, and wrote the paper; X.F.Z. analyzed data; S.C. performed research; B.D. performed research and analyzed data; and R.B.G. conceived of and designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald B. Gartenhaus, Marlene and Stewart Greenebaum Cancer Center, 655 W Baltimore St, BRB 9-014, Baltimore, MD 21201; e-mail: rgartenhaus@som.umaryland.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal