What are the rules that govern a naive T cell's prospects for survival or division after export from the thymus into the periphery? To help address these questions, we combine data from existing studies with robust mathematical models to estimate the absolute contributions of thymopoiesis, peripheral division, and loss or differentiation to the human naive CD4+ T-cell pool between the ages of 0 and 20 years. Despite their decline in frequency in the blood, total body numbers of naive CD4+ T cells increase throughout childhood and early adulthood. Our analysis shows that postthymic proliferation contributes more than double the number of cells entering the pool each day from the thymus. This ratio is preserved with age; as the thymus involutes, the average time between naive T-cell divisions in the periphery lengthens. We also show that the expected residence time of naive T cells increases with time. The naive CD4+ T-cell population thus becomes progressively less dynamic with age. Together with other studies, our results suggest a complex picture of naive T-cell homeostasis in which population size, time since export from the thymus, or time since the last division can influence a cell's prospects for survival or further divisions.
Introduction
An adult human has a population of approximately 1011 naive T cells circulating in the peripheral lymphoid organs and blood. From early in development, this population is generated and sustained by thymic export and division on the periphery, and is estimated to comprise at least 108 different T-cell receptor specificities,1 providing a broad spectrum of protection in a diverse pathogen environment.
The rate of export of naive T cells from the thymus declines substantially with age in healthy persons,2 but estimates of the total number of cells exported from the thymus over a person's lifetime are still approximately 10-fold greater than the total number of naive T cells in an adult at any one time; an estimate of daily thymic output based on the study of Steinmann et al2 integrated over 80 years is 5 × 1012 cells. Furthermore, at least a subset of naive cells continues to divide slowly after release from the thymus into the periphery. These 2 observations imply that turnover and replacement occurs in the naive T-cell pool. What are the rules that govern a circulating naive cell's prospects for survival and proliferation? Do these rules change as we age and, if so, how? Identifying these rules requires a combination of experimental approaches and mathematical models, and will provide an essential background for understanding the dynamics of the T-cell pool when it is dysregulated—for example, during the reconstitution of the T-cell pools after medical interventions that induce lymphopenia, or after antiretroviral therapy in HIV infection.3
As a step toward answering these questions, here we quantify the contributions of proliferation, loss, and thymic input to the development of the healthy naive T-cell compartment. We focus on naive CD4+ T-cell dynamics in persons up to age 20. The youngest age groups might be expected to have the most dynamic T-cell populations because rates of thymic export are highest and physiologic growth, in particular growth of blood volume and lymphoid tissue, is continuously altering the environment in which the T cells circulate and encounter homeostatic signals.
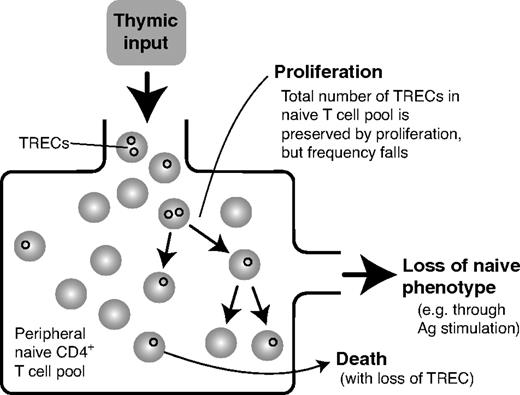
Currently, the most direct methods for measuring lymphocyte dynamics in vivo use deuterated glucose or heavy water to label dividing cells (see, for example, Macallan et al4 ). Interpretation of some of the data from these studies is controversial, however, and for ethical reasons the approach is difficult to use in children. Much of our understanding of the population dynamics of the human naive T-cell pool from early in development to young adulthood has been gained through more indirect methods and through extrapolation from mouse studies. Most commonly, the contribution of thymic export has been assessed using the frequency of T cells in blood expressing markers of recent exit from the thymus and the T-cell receptor excision circle (TREC) content of naive cells. TRECs are circular nuclear DNA fragments that are stable remnants of the recombination events that generate T-cell receptor diversity in the thymus and are shared randomly between daughter cells on division (Figure 1). The mean TREC content per cell of the naive T-cell pool is a poor measure of thymic function, however, because TRECs are diluted by division in the periphery. This is particularly problematic when trying to compare thymic function across different levels of lymphopenia because rates of peripheral division may vary substantially. In contrast, total TREC numbers are not affected by peripheral division. Measurement of total TRECs in the naive pool, rather than TREC frequency, thus provides (somewhat noisy) information regarding the balance between thymic export and the removal of naive cells through loss, cell death, or differentiation.5
Naive T-cell and TREC dynamics. TRECs (small black circles) are present at low frequencies in naive T cells leaving the thymus and are preserved in total numbers by cell division in the periphery. Assuming the rate of intracellular degradation of TRECs is negligible, loss of TRECs from the naive pool is caused only by loss of naive cells, either by death or differentiation into effector phenotype.
Naive T-cell and TREC dynamics. TRECs (small black circles) are present at low frequencies in naive T cells leaving the thymus and are preserved in total numbers by cell division in the periphery. Assuming the rate of intracellular degradation of TRECs is negligible, loss of TRECs from the naive pool is caused only by loss of naive cells, either by death or differentiation into effector phenotype.
In this paper, we combine naive T-cell and TREC data from several existing human studies with a simple and quite general mathematical model of naive T-cell population dynamics. This approach allows us to estimate the absolute contributions of thymic export, proliferation, and loss to total body naive CD4+ T-cell numbers, and how these contributions change between birth and young adulthood. In contrast to previous modeling studies, this model makes no assumptions regarding how cell proliferation and loss of naive cells in the periphery depend on cell numbers or densities through competition for homeostatic resources, and so provide robust estimates of the age-dependent rates of these fundamental processes in humans.
Methods
Estimating total body naive CD4+ T-cell numbers
To measure the contributions of division and loss to the naive pool, we first require estimates of total naive cell numbers as a function of age. Peripheral naive T-cell levels are typically quoted in units of cells per milliliter or microliter of blood, and these values decline with age.6,7 However, as noted previously,8 to estimate total body naive T-cell numbers, we need the additional information of blood volume, which increases during early life. Further, naive T cells circulate continuously between blood and lymphoid organs, and approximately 2% are found in the blood at any moment.9 There is some evidence that this proportion may be age-dependent; in the Appendix, we show that this has only a minor effect on our conclusions. In what follows, then, we estimate total body naive T-cell numbers by multiplying the cells per unit volume of blood by 50× total blood volume.
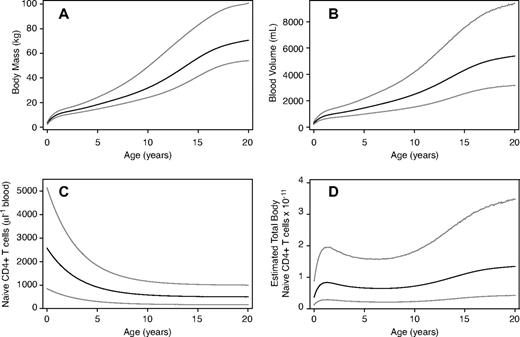
To our knowledge, however, there are no published studies directly specifying the dependence of mean blood volume on age. Instead, we use published estimates of the dependence of blood volume on body weight (Figure 2B), and of mean body mass on age (Figure 2A). These are brought together with an estimate of the age dependence of naive cell density per microliter of blood in healthy children (Figure 2C) to give a nonlinear function for mean total body naive cell numbers as a function of age (Figure 2D). We used a Monte Carlo approach to combine the uncertainties in these regression estimates and the population-level variations in physiology to estimate the distribution of total naive T-cell pool sizes across persons of a given age. Details of this procedure can be found in the Appendix.
Estimating total body naive CD4+ T cells as a function of age. (A) Body weight as a function of age, from Kuczmarski et al.10 (B) Blood volume as a function of age, estimated from panel A and the regression relation between weight and blood volume in Linderkamp et al11 ( Appendix). (C) Mean naive CD4+ T-cell density in blood with age, using the relation in Huenecke et al7 ( Appendix). (D) Estimated total body naive CD4+ T-cell numbers, using the data in panels B and C. Panels A, B, and D show the mean, 2.5 and 97.5 percentiles at the population level; panel C shows the mean and its associated 95% confidence interval.
Estimating total body naive CD4+ T cells as a function of age. (A) Body weight as a function of age, from Kuczmarski et al.10 (B) Blood volume as a function of age, estimated from panel A and the regression relation between weight and blood volume in Linderkamp et al11 ( Appendix). (C) Mean naive CD4+ T-cell density in blood with age, using the relation in Huenecke et al7 ( Appendix). (D) Estimated total body naive CD4+ T-cell numbers, using the data in panels B and C. Panels A, B, and D show the mean, 2.5 and 97.5 percentiles at the population level; panel C shows the mean and its associated 95% confidence interval.
Estimating total body naive CD4+ T-cell TRECs
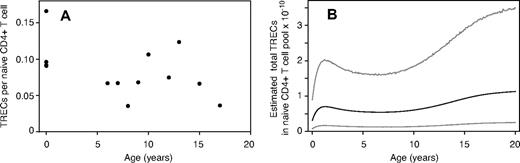
Douek et al12 measured the frequencies of T-cell receptor rearrangement excision circles in naive CD4+ T cells (as TRECs per μg of DNA) in healthy persons 0 to 80 years of age. Using their data only for persons 0 to 20 years of age, we find that there is no significant change in the TREC content of naive CD4+ T cells within this age range (Figure 3A; P = .11). Similar stability of TREC frequency was observed by Douek et al13 for naive CD4+ T cells in children 0 to 8 years of age, by Zhang et al14 in PBMC up to the age of 15 years, and by McFarland et al15 for naive CD8+ T cells up to age 20 years. Assuming that 1 μg of DNA represents 150 000 cells, we estimate the mean TREC number (τ) per naive CD4+ T cell in this age range to be 0.084 (±0.010). The values of τ among the pooled persons were well described by a log-normal distribution with parameters μ = −2.4 and σ = 0.46. Again, using a Monte Carlo approach, we combined this distribution with our empirical distribution of total naive T-cell numbers to generate an empirical distribution for total body TRECs across the population as a function of age (Figure 3B).
TREC dynamics in the young. (A) Measurements of TRECs per naive CD4+ T cell, from Douek et al.12 (B) Estimated distribution of total TRECs in the naive CD4+ T-cell pool across the human population with age, showing the mean, 2.5 and 97.5 percentiles.
TREC dynamics in the young. (A) Measurements of TRECs per naive CD4+ T cell, from Douek et al.12 (B) Estimated distribution of total TRECs in the naive CD4+ T-cell pool across the human population with age, showing the mean, 2.5 and 97.5 percentiles.
Naive CD4+ T-cell and TREC dynamics
We consider a very general model of naive T-cell population dynamics with a term for thymic input, a term for population growth (division in the periphery that preserves naive phenotype), and one for cell loss. As above, we let N(t) be the total number of naive CD4+ T cells in the body at age t measured in days. We describe changes in this population with age t with a generalized version of the standard model of naive cell dynamics used by several authors.5,8,16,17
Rate of change of total naive cell numbers = thymic input + proliferation − loss, or
where θ(t) is the rate of export of cells from the thymus (cells/day), ρ(t) is the per-cell rate of addition to the naive pool through successful cell division (day−1), and δ(t) is the per-cell rate of disappearance of cells from the naive pool (day−1). The latter is a composite of cell death, change of phenotype, and loss. From here on, we refer to these processes collectively as “loss.”
In differential equation models of this sort, the per-cell “rate” of a process is a measure of the probability any given cell will undergo that process in a short time interval. The quantity 1/ρ(t) can be interpreted as approximately the expected time to the next successful division of a naive cell in a person of age t, and 1/δ(t) is approximately the expected residence time of a CD4+ T cell in the naive pool in a person of age t.
We assume that the mean rates of division and loss in the periphery may change with age, but in this model we do not impose functional forms for this age dependence, nor do we assume any particular dependence of these rates on the pool size N(t). Note also that here we do not distinguish between T cells of different specificities or recent thymic emigrant and “central” naive status18 ; we are interested in the mean rates of division and loss across the whole naive CD4+ T-cell compartment.
We let the total TRECs in the naive CD4+ T-cell pool at age t be T(t). T is influenced by thymic export and loss, but not division. We use the model used by Hazenberg et al19 to describe TREC dynamics in the naive population:
Rate of change of total TRECs in peripheral naive CD4+ T-cell pool = (rate of thymic input × mean TREC content per thymic emigrant) − rate of TREC loss, or
where c is the mean TREC content of cells emerging from the thymus and θ(t) and δ(t) are defined above. We assume that intracellular degradation of TRECs over time is negligible and that the average TREC concentration of CD4+ T cells emerging from the thymus, c, is constant up to age 20 years.20 Junge et al21 reported an average 250 TRECs per 1000 recent thymic emigrant (CD31+) CD4+ T cells at birth, giving an estimated lower bound of c = 0.25. Okamoto et al22 estimated the TREC content of single-positive CD4+ thymocytes in neonates to be approximately 0.6; this provides a conservative upper bound on c because cells undergo divisions between acquisition of single-positive status and release from the thymus. In what follows, we use the estimate c = 0.25.
Modeling thymic export
We assume that thymic function is proportional to the volume of the thymic epithelial space (TES). Steinmann et al2 showed that the involution of the TES with age could be described well by a modified exponential decay function. There remains an unknown constant of proportionality that relates the volume of the TES to the rate of naive CD4+ T-cell export from the thymus in cells/day. We estimate this by scaling the Steinmann decay function to obtain a daily total thymic export rate of 108 cells per day at age 30, in line with the studies of Clark et al23 and Dutilh and de Boer.17 This is the combined output of both CD4+ and CD8+ T cells. Jamieson et al20 showed that the ratio of CD4 to CD8 single-positive mature thymocytes in fetal and adult thymi were similar, with a CD4+ proportion of 0.68. This provides an estimated rate of CD4+ T cell exported from the thymus of 6.8 × 107 cells per day at age 30. We use this to calibrate the thymic export function and derive the after expression for the mean rate of thymic export in cells per day as a function of age t (in days):
The constant of proportionality scaling θ(t) directly influences our estimates of the absolute values of mean division and loss rates in the periphery.
Using this estimated rate of thymic export in Equations 1 and 2, we can estimate the rates of cell division and loss, without making any further assumptions, by numerically estimating the rate of change of total cells dN/dt. That is, using our estimated dependence of total naive cells on age, we calculate the rate of growth of the total body naive pool.
Results
The interdivision time of naive CD4+ T cells increases with a person's age
If TRECs per naive cell are constant, as suggested by the data from Douek et al,12 the rate of cell division, ρ(t), becomes proportional to thymic export and inversely proportional to the size of the naive pool (see Appendix);
Hence, to maintain the observed constant TREC frequency τ, the rate of successful cell division in the periphery must decrease as thymic export wanes with age. We estimate ρ(t) numerically from Equation 4 using the estimated thymic export function θ(t) defined in Equation 3. We find that the mean interdivision time for naive cells, 1/ρ, is approximately 125 days for children between the ages of 1 and 5 years, or that approximately 0.8% of the naive CD4+ T-cell population is dividing on any given day. By the age of 20 years, the mean interdivision time increases to approximately 500 days (Figure 4A), corresponding to 0.2% of the naive population dividing per day. This is an independent estimate, comparable with the rate of naive T-cell production of 0.2% per day in young adults obtained from deuterated glucose labeling.24 However, in vivo labeling studies are unable to distinguish between mature cells dividing in the periphery and thymocytes that divided and were exported during the labeling period. The estimated cell production rate from these assays is a composite of both processes and so represents an upper bound on the rate of successful division in the periphery, ρ.
Estimated mean rates. The estimated mean rates (black lines) of successful naive CD4+ T-cell division, ρ(t) (A) and loss, δ(t) (B) as functions of age, t. The 2.5 and 97.5 percentiles are shown in gray. A proliferation rate of, for example, 0.02 means that approximately 2% of cells divide per day.
Estimated mean rates. The estimated mean rates (black lines) of successful naive CD4+ T-cell division, ρ(t) (A) and loss, δ(t) (B) as functions of age, t. The 2.5 and 97.5 percentiles are shown in gray. A proliferation rate of, for example, 0.02 means that approximately 2% of cells divide per day.
The expected residence or survival time of naive CD4+ T cells increases with age
From Equation 2 and the relation T(t) = τN(t), we can calculate the expected rate of loss of naive T cells:
This is a decreasing function of age (Figure 4B), which can be understood as follows. The denominator N(t) increases with t. Furthermore, whereas both terms in parentheses—thymic input (θ(t)) and the net rate of growth of the naive T-cell pool (dN/dt)—are decreasing functions of age, the thymic input term dominates because cθ(t)/τ is at least of the order 108 and our numerical estimates of the rate of change of total cell numbers are of the order or less than 107 cells per day after the age of 6 months. Thus, the term in parentheses is a decreasing function of age; hence, so is the loss rate δ(t).
From Figure 4B, we see that the expected residence time (1/δ) of a naive CD4+ T cell in the periphery is of the order of 70 days between the ages of 1 to 5 years and increases to approximately 400 days at the age of 20 years, corresponding to loss rates of approximately 1.4% and 0.3% of the population per day, respectively.
Assuming homeostasis over short timescales, the total rate of loss of naive cells is approximately equal to the net rate of production (thymic input and proliferation) at any one time. This is usually referred to as the rate of turnover. Our estimate of the loss rate in young adults, δ ∼ 0.0025 day−1, agrees well with the turnover rate estimated in the deuterated glucose labeling study of Macallan et al24 but is 5-fold higher than the estimate obtained by Vrisekoop et al using heavy water.25 The discrepancy between these experimental studies remains to be resolved. However, our estimates of the absolute values of division and loss rates depend directly on the estimated thymic export function, Equation 3, and in particular scale linearly with the constant of proportionality used to calibrate this function to a given mean total rate of thymic export at the age of 30 years. Additional uncertainty in this constant will broaden the confidence intervals on our estimates of ρ and δ. Our conclusion that both rates decline with age is independent of this scaling constant, however.
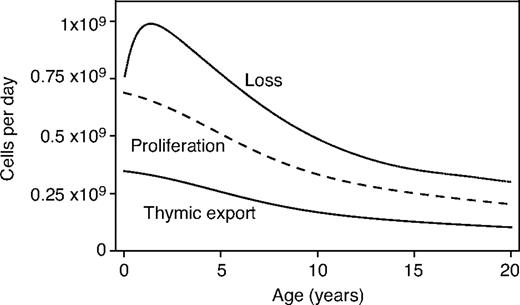
Between ages 0 and 20 years, the absolute daily contribution to the naive CD4+ T-cell population from peripheral division is greater than the contribution from thymic export
From Equation 4 and using τ ∼ 0.08 and the estimate c more than or equal to 0.25, the total number of cells produced by successful cell division in the periphery each day is
The ratio c/τ is a measure of the TREC dilution that occurs as a result of proliferation in the periphery. From Equation 6, the daily contribution of peripheral division to the pool will necessarily be greater than that of thymic export if c is greater than 2τ.
We see that the contribution of cells to the naive pool through proliferation at any given age is at least 2-fold larger than the contribution from the thymus (Figure 5) and may be as much as 6-fold larger if we use the conservative upper bound on the TREC content of recent thymic emigrants, c = 0.6. Because both c and τ appear to be constant up to age 20, Equation 6 tells us that the ratio of the total number of cells dividing per day to the total cells exported by the thymus per day remains constant with age in children.
Estimated mean absolute daily contributions of thymic export (solid line), peripheral division (dashed), and peripheral loss (dotted) to the dynamics of the naive CD4+ T-cell pool with age, using the lower bound on the TREC content of RTEs; c = 0.25.
Estimated mean absolute daily contributions of thymic export (solid line), peripheral division (dashed), and peripheral loss (dotted) to the dynamics of the naive CD4+ T-cell pool with age, using the lower bound on the TREC content of RTEs; c = 0.25.
Discussion
Our analysis shows that as the naive T-cell pool matures it becomes progressively less dynamic. First, we show that the mean interdivision time of these cells increases almost continuously with age; in young children, naive T-cell proliferation is relatively vigorous, with an interdivision time of the order a few months, but it slows continuously into adulthood. This is consistent with other studies; Hazenberg et al8 showed division rates falling between the ages of 0 and 5 years, whereas Macallan et al24 and Vrisekoop et al25 both demonstrated that average rates of naive CD4+ T-cell division are very low in adults. Second, we show here that the total rate of exit from the naive CD4+ T-cell pool (the combination of apoptosis, loss, and differentiation) declines with time. That is, the mean residence time of a naive T cell increases with a person's age. We also show, as has been noted previously,8 that total body numbers of naive CD4+ T cells increase almost continuously from (before) birth well into early adulthood, despite the decline in thymic output with age and the associated slowing of cell turnover.
Furthermore, it is usually assumed that thymic export makes the dominant contribution to naive T-cell production in children, but Hazenberg et al8 showed that peripheral division also provides a substantial fraction of the daily production of new cells. Our analysis confirms and quantifies this: up to the age of 20 years, at most 30% of the new naive cells that appear each day are exported from the thymus. A similar value has been estimated in young adult mice.26 Interestingly, the invariance of TREC frequencies in human peripheral naive T cells suggests that this proportion remains fixed during early life. There is apparently no increase in rates of division in the periphery to compensate for the drop in thymic production; on the contrary, division rates fall with age.
Modeling naive T-cell homeostasis
Naive CD4+ T cells are maintained homeostatically in the periphery by competition for cytokines and possibly self-peptide/major histocompatibility complex (MHC) signals.27,28 Cell survival times and average rates of division are then expected to depend on the total number or density of cells competing for these resources; in mice, manipulation of the availability of these stimuli results in changes in the survival times and rates of homeostatic proliferation of naive T cells.29,30 Previous modeling studies addressing naive T-cell and TREC dynamics have drawn on this idea of resource competition and assume that rates of proliferation or loss depend only on total naive T-cell numbers or densities,5,8,16,17,30,31 usually with an associated fixed homeostatic set point or “carrying capacity.”
These ecologic models of T-cell population dynamics can describe how peripheral T-cell numbers change over short timescales after loss or addition of cells, for example, in the context of antiretroviral treatment of HIV infection. However, it is not clear that they are appropriate tools for understanding how peripheral T-cell numbers and rates of turnover respond to gradual changes in physiology over timescales of years. In this paper, we deal with this latter process; we are concerned with the steady-state average rates of division and loss in replete naive T-cell pools and how these rates change with age. Our results show that both rates of proliferation and loss (on a per-cell basis) fall with age and increasing pool size. These observations are difficult to explain using simple ecologic competition models. As persons grow, their naive T-cell pools grow too, along with (presumably) the total availability of homeostatic resources such as interleukin-7 (IL-7) or self-peptide–MHCII complexes. Thus, ecologic models of naive T-cell competition and homeostasis with a fixed carrying capacity are probably age-specific, and operate in the context of slow changes in the lymphocytes' environment; alone, they cannot provide a complete description of naive T-cell dynamics between birth and adulthood.
Scaling of immune system size with body mass
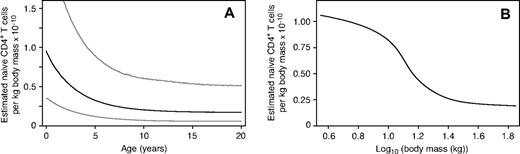
Elegant theoretical studies have proposed scaling laws that relate total body lymphocyte numbers to body mass or blood volume. Langman and Cohn32 proposed that organisms with immune systems require a threshold of lymphocyte density to provide protection from pathogens, and use this to suggest that the number of B cells per unit blood volume ought to be roughly constant across persons and species. An alternative argument was proposed by Wiegel and Perelson,33 who drew on allometric biologic scaling laws34,–36 (principles that relate quantities such as life span, heart rate, and metabolic rates to body mass, through the branching structure of the circulatory system) to predict that both B- and T-cell densities (cells/kg) should scale roughly with the logarithm of body mass. In contrast, our analysis shows that the estimated number of naive CD4+ T cells per unit body mass declines almost 4-fold between birth and adulthood and stabilizes soon after puberty, and that there is an inverse correlation between naive CD4 T-cell density and the logarithm of body mass in this age group (Figure 6).
Scaling of naive T-cell densities with age. (A) Estimated mean naive CD4+ T cells per kilogram of body mass as a function of age (black line), with 2.5 and 97.5 percentiles at the population level (gray lines). (B) Estimated dependence of mean naive CD4+ T-cell density (cells/kg) on log10 body mass.
Scaling of naive T-cell densities with age. (A) Estimated mean naive CD4+ T cells per kilogram of body mass as a function of age (black line), with 2.5 and 97.5 percentiles at the population level (gray lines). (B) Estimated dependence of mean naive CD4+ T-cell density (cells/kg) on log10 body mass.
If these scaling laws are to hold for CD4+ T cells, memory CD4+ T cells should accumulate rapidly in the very young to compensate. This is intuitively reasonable; we might expect that the per-cell rate of exit from the naive compartment through exposure to cognate antigen, and the corresponding net rate of accumulation of memory phenotype cells, will be higher in immunologically inexperienced young persons than in adults with diverse memory repertoires. This is consistent with the decline in the naive cell loss rate with age that we demonstrate here.
Phenotypic heterogeneity and clonal diversity in the naive T-cell pool
Despite the slow increase in naive T-cell numbers over the timescale of years, over short timescales (days or weeks), the pool is in a state of quasi-homeostasis, in which the number of cells lost or displaced from the naive pool each day is approximately equal to thymic production and peripheral division combined. However, our model tells us nothing about whether this displacement occurs randomly or whether some naive subpopulations have a selective advantage.
The combination of our estimates of naive cell loss rates and the results of deuterium labeling studies shows that there may be heterogeneity in cells' expected survival or residence times with respect to the time since the last division. Vrisekoop et al25 performed deuterated water incorporation assays in healthy young adult volunteers and found that newly produced cells (ie, cells that had taken up label, either through division in the periphery or through division in the thymus and export soon after) were lost at a very low rate, much lower than the rate of successful division. This implies that recently divided naive cells have a survival advantage over the average cell in the pool as a whole. Thus, one would predict that as mean division rates fall with age, loss rates would increase, not decrease, as our analysis indicates. To resolve this apparent inconsistency requires that the per-cell loss rate of naive T cells depends both on time since last division and also on a person's age, either explicitly or indirectly through (for example) population or clone size. This suggests a 2-layered mechanism for maintaining clonal diversity in the naive T-cell pool; the aim being (1) to provide recent thymic emigrants, which are costly to produce and help to increase our T-cell receptor repertoire, with a survival advantage and (2) to increase naive cell longevity as thymic output wanes. Precisely how this regulation of cell loss changes with age remains an open question. Perhaps the simplest explanation is that naive T cells are intrinsically long-lived and, as we have shown, the per-cell rate of recruitment of naive cells into effector cell status declines as we become more immunologically experienced and develop a diverse memory T-cell repertoire. This slowing of recruitment will increase the expected residence time of naive T cells as we age.
Together, these results suggest a more complex, structured picture of naive T-cell dynamics that has perhaps previously been considered. Per-cell division rates may vary as a function of age, total naive T-cell numbers, or cell density, through resource competition. They may also vary as a function of time since leaving the thymus and entering the periphery. Further, survival may depend on the time since last division as well as resource availability. How then do the signals T cells receive from their environment translate into survival or proliferative benefits? Because IL-7 is required for both naive T-cell survival and division, cells that have accumulated sufficient signal to divide may well have the additional benefit of a survival advantage through (for example) accumulation of an antiapoptotic factors. Interestingly, memory T-cell populations appear to follow different rules; in the CD4+CD45RO+ population, recently divided cells appear to be more prone to apoptosis than quiescent cells.4
As other authors have pointed out,5 mathematical models are enormously valuable for aiding our interpretation of lymphocyte turnover assays. With more cross-talk between experts in both areas, we can begin to uncover the potentially quite simple rules that underlie the complex dynamics of our naive and memory T-cell repertoires.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Judith Mandl and Rob de Boer for critical readings of the manuscript. Computation was performed using Mathematica39 and R40 .
I.B. was supported by a Bogue Research Fellowship from University College London. A.J.Y. and R.A. were supported by the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: I.B. and A.J.Y. performed the analyses and wrote the paper; and I.B., R.A., R.C., and A.J.Y. designed the study and contributed to the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew J. Yates, Emory University, Department of Biology, 1510 Clifton Rd, Atlanta, GA 30322; e-mail: ayates2@emory.edu.
Appendix
Calculating the distribution of total body naive CD4+ T cells with age
Linderkamp et al11 reported the linear relationship
where the SE on the slope is 0.05. Average body weight as a function of age is determined using standard growth charts for healthy children published by the Centers for Disease Control and Prevention.10 To estimate the distribution of total CD4+ T-cell numbers across the population at a given age t, we repeat the following:
- Draw the logarithm of body weight, log(W(t)), from a normal distribution with mean equal to log(W50(t)) and estimated SD. where Wi(t) is the ith percentile of body weight at age t; W50(i), W2.5(t), and W95 are shown in Figure 2A.
Calculate a blood volume V(t) using the body weight logW(t) generated in the previous step; V(t) is drawn from a log-normal distribution with mean given by Equation A1 and SD calculated from the SE on the slope of the regression line. The distribution of total blood volume across persons by age, obtained by combining steps 1 and 2, is shown in Figure 2B.
- Calculate the naive (CD45RA+) CD4+ T-cell density per microliter of blood, ν(t), from the fitted relationship in Huenecke et al7 (Figure 2C): with the 95% confidence interval defined as (ν(t)/3, 2ν(t)). Draw values for ν(t) from a log-normal distribution with parameters μ = log ν̄(t)−0.1 and σ = 0.45.
Total body naive CD4+ T-cell numbers are then estimated from their blood density using N(t) = 50 ν(t)V(t), with the factor 50 accounting for the estimated 2% of naive cells in the blood at any time.
From the empirical distribution generated by repeating steps 1 to 4, we estimate the mean values for total body naive CD4+ T-cell numbers at age t, along with the estimated 2.5 and 97.5 percentiles. We repeat this procedure for ages in the range of 0 to 20 years (Figure 2D).
Modeling the age-dependent distribution of naive T cells between blood and lymphoid organs
An assumption of our study is that the proportion of the naive T-cell pool found in the blood is independent of age. However, Watanabe et al41 showed that the ratio of spleen volume to body weight declines slightly from the age of 0 to 20 years. If we assume that lymphoid tissues grow proportionally with the spleen, then their results suggest that the percentage of lymphocytes found in the blood increases linearly from approximately 1% at birth to 2% at the age of 20 years.
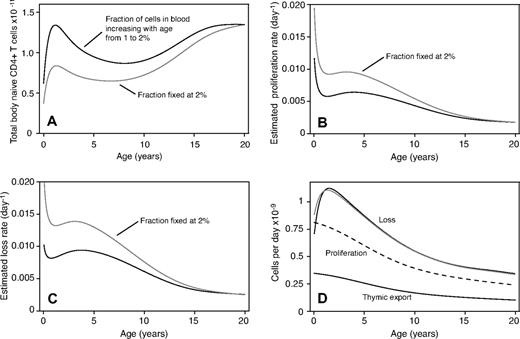
If we include this shift in lymphocyte distribution in our analysis, we see a more rapid expansion of total body cell numbers early in life (Figure 7A) and predicted rates of proliferation and loss are lower (Figure 7B,C), although the general decline in both rates with age remains. The predicted total number of cells produced each day by proliferation does not change (Figure 7D dashed line) as this is related to thymic export and observed TREC frequencies (Equation 6). The total rate of loss from the naive pool is marginally lower between the ages of 0 to 5 (Figure 7D dotted and gray lines) to account for the more rapid population growth in this age group. Thus, although estimated proliferation and loss rates are sensitive to the distribution of naive T cells between blood and lymphoid organs, the total and relative contributions of thymic export, proliferation, and loss are robust to it.
Assessing the sensitivity of our analysis to the assumption that 2% of naive cells are found in blood. (A) Total body naive population as a function of age. (B) Predicted rate of proliferation. (C) Predicted rate of loss. (D) Predicted absolute number of cells exported by thymus (solid line), produced in periphery (dashed line), and lost from naive pool (gray line, fixed 2% for all ages; dotted line, proportion of cells in the blood increases linearly from 1% at birth to 2% at age 20).
Assessing the sensitivity of our analysis to the assumption that 2% of naive cells are found in blood. (A) Total body naive population as a function of age. (B) Predicted rate of proliferation. (C) Predicted rate of loss. (D) Predicted absolute number of cells exported by thymus (solid line), produced in periphery (dashed line), and lost from naive pool (gray line, fixed 2% for all ages; dotted line, proportion of cells in the blood increases linearly from 1% at birth to 2% at age 20).
Modeling constant TREC densities
The observation that the TREC content per naive CD4+ T cell, T(t)/N(t), is a constant, τ, means
Substituting the expressions for dN/dt and dT/dt from Equations 1 and 2, respectively, we obtain Equation 4.

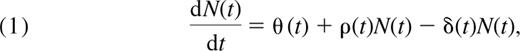
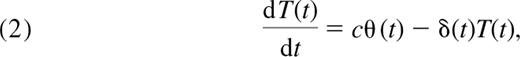
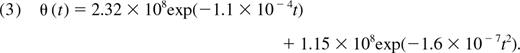
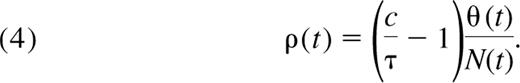

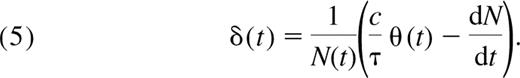
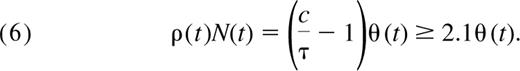
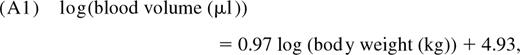
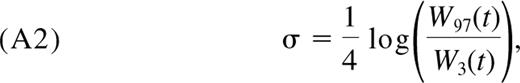
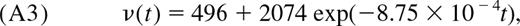
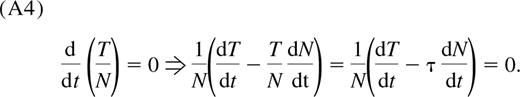
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal