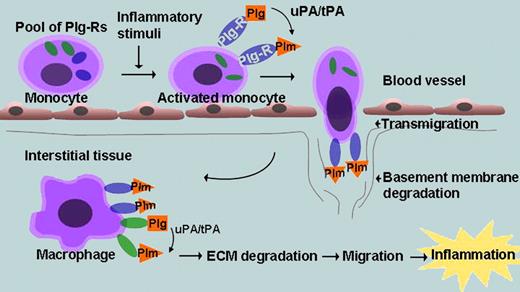
Over the past decade, studies conducted in plasminogen-deficient mice have created a compelling case for involvement of plasminogen and plasmin in inflammatory cell recruitment.1 This role of plasmin(ogen) appears distinct from its function, as the primary fibrinolytic enzyme and depends on its ability to facilitate leukocyte migration across extracellular matrices, through either its direct degradation of matrix proteins or its activation of matrix metalloproteinases, which degrade collagens.2 Such plasmin-dependent pericellular proteolysis is optimized when plasmin(ogen) is tethered to the cell surface, an interaction mediated by a heterogeneous group of plasminogen receptors (Plg-Rs). Many, but not all, Plg-Rs have a lysine at their C-terminus, which interacts with the certain kringles of plasmin(ogen). When engaged in this fashion, plasminogen is more readily activated to plasmin, and plasmin is protected from inactivation by its natural inhibitors. When monocytoid cells are stimulated by various agonists or differentiated into macrophages, their plasminogen binding capacity increases,3 presumably a reflection of changes in the number or repertoire of Plg-Rs. A model depicting how plasminogen and Plg-Rs might contribute to and be regulated on the surface of monocytes is shown in the figure.
At least 6 different Plg-Rs have been identified on cells of the monocytoid lineage. Enolase-1 was the first described Plg-R and has lysine as its C-terminal amino acid.4 Although primarily an intracellular glycolytic enzyme, its expression on the cell surface was clearly detected on monocytoid cells and subsequently on many other cell types. Indeed, the enhanced binding and activation of plasminogen by neoplastic cells was attributed to enhanced enolase-1 expression.5 However, evidence from monocytoid cells suggested that enolase-1 was only one of several Plg-Rs and its contribution to plasminogen binding was modest in the models tested.6,7 More recent studies have emphasized the importance of the annexin 2 heterotetramer (both the annexin 2 and p11 subunits are capable of binding plasminogen) and histone H2B as Plg-Rs. With both in vitro and in vivo studies supporting the significance of annexin-2 and H2B in plasminogen binding, the role of enolase-1 as a functional Plg-R fell into obscurity.
In their article, Wygrecka et al resurrect the role of enolase-1 as a Plg-R and make several notable observations that attest to its importance in monocyte recruitment into injured lungs.8 First, using a monocytoid cell line and isolated blood monocytes, cell-surface expression of enolase-1 was shown to increase dramatically in as little as 6 hours after lipopolysaccharide (LPS) stimulation. Interestingly, new protein synthesis was not required for LPS-induced exteriorization of intracellular enolase-1. As enolase-1 as well as the aforementioned Plg-Rs all lack signal sequences and transmembrane domains, as yet undetermined nonconventional pathway(s) must regulate their surface expression. Second, plasmin generation and plasminogen dependent migration of LPS-stimulated monocytoid cells through membranes, monolayers of epithelial cells or matrigel toward MCP-1 was abrogated by an antibody to enolase-1 that blocked its ability to bind plasminogen. These observations were corroborated with cells over-expressing enolase-1 or a mutant form (ENO-1ΔPLG) that lacked the C-terminal lysine and could not bind plasminogen; wild-type enolase-1 expression enhanced plasminogen related functions whereas ENO-1ΔPLG did not. Third, in an LPS-induced lung inflammation model in mice, monocytes overexpressing wild-type enolase-1 accumulated in the bronchoalveolar lavage while monocytes overexpressing ENO-1ΔPLG did not. Fourth, blood and alveolar monocytes from pneumonia patients showed intense expression of enolase-1, whereas monocytes from healthy individuals did not. Collectively, these results establish a central role of enolase-1 as a Plg-R in monocyte recruitment in inflammatory lung disease.
In a previous study from our laboratory, antibodies to histone H2B were shown to block macrophage recruitment in thioglycollate-induced peritonitis by approximately 50%.9 In these experiments, anti–enolase-1 served as a control and reduced recruitment by only approximately 20%. The basis for these differences is uncertain but raises some interesting prospects. One possibility is that the contribution of specific Plg-Rs to monocyte recruitment may be organ specific (lung vs peritoneum) or may be stimulus specific (LPS vs thioglycollate). Also, the contribution of individual Plg-Rs to monocyte recruitment may change over time (enolase-1 early vs H2B later). These suggestions imply that different populations of Plg-Rs could be targeted to regulate inflammatory cell recruitment in a temporal or site-specific manner. The resurrection of enolase-1 as a Plg-R by the Wygrecka et al provides impetus for such comparative studies.
Role of plasminogen receptors (Plg-Rs) during monocyte/macrophage recruitment. Monocyte activation leads to expression of cytosolic pools of Plg-Rs at the cell surface. Plasminogen (Plg) binds to the Plg-R and is converted to plasmin (Plm) by Plg activators (uPA/tPA). The bound Plm facilitates transmigration and basement membrane degradation. As monocytes differentiate into macrophages, additional Plg-Rs support additional Plg binding, which aids in ECM degradation and macrophage recruitment. Enolase-1 functions as a Plg-R for monocyte recruitment into lung tissue.
Role of plasminogen receptors (Plg-Rs) during monocyte/macrophage recruitment. Monocyte activation leads to expression of cytosolic pools of Plg-Rs at the cell surface. Plasminogen (Plg) binds to the Plg-R and is converted to plasmin (Plm) by Plg activators (uPA/tPA). The bound Plm facilitates transmigration and basement membrane degradation. As monocytes differentiate into macrophages, additional Plg-Rs support additional Plg binding, which aids in ECM degradation and macrophage recruitment. Enolase-1 functions as a Plg-R for monocyte recruitment into lung tissue.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal