Abstract
The use of dendritic cells (DCs) as anticancer vaccines holds promise for therapy but requires optimization. We have explored the potential of costimulatory ligand CD70 to boost the capacity of DCs to evoke effective CD8+ T-cell immunity. We show that immature conventional DCs, when endowed with CD70 expression by transgenesis, are converted from a tolerogenic state into an immunogenic state. Adoptively transferred CD70-expressing immature DCs could prime CD8+ T cells, by CD27, to become tumor-eradicating cytolytic effectors and memory cells with a capacity for robust secondary expansion. The CD8+ T-cell response, including memory programming, was independent of CD4+ T-cell help, because the transferred immature DCs were loaded with major histocompatibility complex class I–restricted peptide only. Without CD70 expression, the DCs generated abortive clonal expansion, dysfunctional antitumor responses, and no CD8+ T-cell memory. CD70-expressing CD8+ DCs were the primary subset responsible for CD8+ T-cell priming and performed comparably to fully matured DCs. These data highlight the importance of CD27/CD70 interactions at the T-cell/DC interface and indicate that CD70 should be considered in the design of DC vaccination strategies.
Introduction
The potency of dendritic cells (DCs) in inducing adaptive immune responses has led to their application in immunotherapy of cancer. DC vaccination studies in murine models and patients have provided proof of principle, although the efficacy of current approaches needs to be improved.1 Immunostimulatory properties of DCs are linked to their maturation state, with steady state immature DCs maintaining T-cell tolerance to self-antigens and mature DCs stimulating responses to nonself antigens.1 To direct the T-cell response in DC vaccination, it is important to identify key molecules on mature DCs that determine the fate of primed T cells. On maturation, DCs up-regulate the costimulatory ligands CD80 and CD86 that bind to CD28 on primed T cells and thus promote clonal expansion.2 However, they also gain expression of various tumor necrosis factor (TNF)–related costimulatory ligands that, by triggering their respective receptors on the T cell, promote cell survival and thereby additionally determine size and quality of effector and memory T-cell pools.3
TNF family members OX40 ligand (L) and 4-1BBL have been explored in DC therapy. This work was prompted by early studies showing that agonistic monoclonal antibodies directed to their receptors OX40 (CD134) and 4-1BB (CD137) promoted T-cell responses to weakly immunogenic tumors.4,5 Phase 1 and 2 clinical trials with humanized anti–4-1BB antibodies are currently ongoing in patients with metastasized solid tumors. Subsequent preclinical studies have explored the use of adoptively transferred DCs, loaded with tumor antigens, in combination with agonistic antibodies to OX40 or 4-1BB.6-9 In addition, adoptively transferred DCs have been endowed with DNA or mRNA encoding these molecules and tested for their immunostimulatory activities.10-14 In all cases, deliberate engagement of OX40 or 4-1BB improved the antitumor effect of DCs, which was attributed to their capacity to sustain ongoing T-cell responses.
Compared with OX40L and 4-1BBL, their close relative CD70 has, until recently,15 received only modest attention concerning its application in immunotherapy of cancer despite the promising data collected on its function.16 Murine conventional (c)DCs acquire CD70 expression on their activation, with the highest levels reached after combined Toll-like receptor and CD40 stimulation.17 Conditions that allow for CD70 induction on human monocyte-derived DCs have also been described.18,19 It is emerging that CD70 induction is a critical event in CD4+ T cell–mediated DC “licensing,” that is, endowing DCs with the capacity to induce a CD8+ T-cell response.20-22 In contrast to OX40 and 4-1BB, the receptor for CD70, CD27, is already expressed on naive CD4+ and CD8+ T cells both in mice and humans, indicating an important role at the DC interface during early T-cell priming.3,16 This is underlined by a unique intracellular localization of CD70 in activated DCs that allows its polarized delivery to the immune synapse.23
CD27/CD70 interactions promote clonal expansion of primed CD4+ and CD8+ T cells and their accumulation at effector sites, to a large extent by supporting T-cell survival.24-26 The CD27 signal acts complementary to the CD28 signal in this respect, as shown for CD8+ T cells in infection, transplantation, and DC vaccination models.20,24,27 In addition, together with OX40 and 4-1BB, CD27 delivers signals to CD8+ T cells that determine the size of the CD8+ memory T-cell pool and improve the capacity of these memory cells to undergo secondary expansion.28 CD27 input does not affect the cytolytic effector program of CD8+ T cells,29 but it enhances IFN-γ production on a per cell basis.30 For CD4+ T cells in both human and mouse, CD27/CD70 interactions can promote Th1-type effector differentiation, although this may depend on the cellular environment.26,31,32 Interestingly, CD4+ T cells also require CD27 input to become competent for CD8+ memory T-cell programming.31
We have recently shown that expression of CD70 on steady state conventional cDCs can overrule peripheral CD8+ T-cell tolerance, including that imposed by CTLA-4 and PD-1, giving rise to effective antiviral and antitumor immunity instead.33 This striking capacity of CD70-expressing immature cDCs has lead us to explore their potential in a vaccination setting, in which immature DCs can inhibit effector T-cell responses,34 whereas DCs matured by certain inflammatory cytokines can generate suppressive regulatory T cells.35 Here, we show that adoptively transferred immature cDCs, when engineered to express CD70 and loaded with major histocompatibility complex (MHC) class I–restricted peptide, induce robust effector and memory CD8+ T-cell responses in the absence of CD4+ T-cell help. This potential is amenable to exploitation in DC therapy.
Methods
Mice
The murine CD70 cDNA was introduced into a DNA cassette, allowing its DC-specific expression under control of the CD11c promoter. CD11c-CD70 transgenic (tg) mice were made by injection of this construct into fertilized oocytes of C57BL/6 mice. A description of these transgenic mice has been published.33 CD11c-CD70tg mice were maintained on a CD27−/− background,29 thus ruling out constitutive CD27/CD70 interaction and effects on lymphocyte homeostasis.33 These CD11c-CD70tg;CD27−/− mice were used as DC donors, and CD27−/− mice were used as matching control donors. OT-I mice36 that are transgenic for Vα2/Vβ5 TCR specific for the OVA257-264 (SIINFEKL) peptide presented in the context of H-2Kb were used as T-cell donors. Wild-type (WT), CD27−/−, CD11c-CD70tg;CD27−/−, OT-I, and OT-I;CD27−/− mice, all on a C57BL/6 background, were used for experiments at 7 to 12 weeks of age.
Flow cytometry
Tetrameric complexes of biotinylated murine MHC class I H-2Kb heavy chain, β2 microglobulin, and relevant peptide were prepared according to standard procedures, labeled with PE- or APC-conjugated streptavidin and used in combination with anti-CD8α mAb 53-6.7. Incubations were performed with indicated antibodies or MHC tetramers diluted in PBS, 1% BSA, 0.01% sodium azide on ice (or at room temperature for tetramer staining). Monoclonal antibodies (mAbs) used were anti-CD4 (GK1.5), anti-CD8α (53-6.7), anti-CD8β (53-5.8), anti-CD11b (M1/70), anti-CD11c (HL3), anti-CD40 (3/23), anti-CD45R/B220 (RA3-6B2), anti-CD70 (FR70), anti-CD24 (M1/69), anti-MHC class II I-Ab (AF6-120.1), anti-CD86 (GL1), and anti–IFN-γ (XMG1.2). These antibodies were obtained from BD Biosciences (San Jose, CA), eBioscience (San Diego, CA), or prepared as purified Ig from available hybridomas. Propidium iodide–stained dead cells were excluded from analysis. For intracellular IFN-γ staining, splenocytes were incubated for 6 hours at 37°C with or without 10−6 M of OVA257-264 peptide in the presence of 5 μg/mL brefeldin A. Cells were surface stained with anti-CD8α mAb and stained intracellularly with anti–IFN-γ Ab. Cells were analyzed by flow cytometry with FACSCalibur (Becton Dickinson, Franklin Lakes, NJ) and FlowJo analysis software (TreeStar, Ashland, OR).
Mouse treatments
To generate large numbers of immature DCs for adoptive transfer, donor mice were injected subcutaneously with 4 × 106 Fms-like tyrosine kinase 3 ligand (Flt3L)–secreting B16 tumor cells.37 Purified OT-I T cells at 104 cells per recipient and OVA peptide-loaded DCs at 2 × 106 cells per recipient were injected intravenously (retro-orbitally) in Hanks balanced salt solution (HBSS). The murine melanoma-derived B16 cell line expressing a carboxy-terminal fragment of ovalbumin (OVA) encompassing amino acids 161 to 38538 was harvested at the exponential growth phase, washed to remove serum components, and implanted subcutaneously at the indicated number in 200 μL HBSS on the flank of recipient mice. Tumors were measured by caliper, and mice were killed when tumors reached a diameter of 15 mm or caused discomfort. For recall, LPS (Escherichia coli O111:B4) was injected at 5 μg/mouse and OVA peptide at 20 μg/mouse, both intravenously. All mouse experiments were done in accordance with national guidelines, and experiments were approved by the Experimental Animal Committee of The Netherlands Cancer Institute (Amsterdam, The Netherlands).
DC and T-cell isolation
At 10 to 14 days after B16-Flt3L tumor cell implantation, DCs were isolated from donor mice as described.39 Briefly, chopped spleen pieces were vigorously resuspended with a mixture of collagenase type 3 at 1 mg/mL (Worthington Biochemicals, Freehold, NJ) and 0.01% DNAase I (Roche, Basel, Switzerland) for 20 minutes at room temperature. Light-density cells (5% of the total nucleated spleen cells) were selected by centrifugation in a medium containing Nycoprep 1.077 (Axis-Shield, Dundee, United Kingdom). DCs were further purified by magnetic-activated cell sorting (MACS) using anti-CD11c (N418)–conjugated MACS beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. All procedures (beside enzymatic digestion) were performed on ice to prevent spontaneous DC maturation. Purity and viability of DCs were checked by flow cytometry. For DC subtype isolation, DCs were stained with mAbs to CD11c, B220, CD24, and CD11b. CD8+ cDCs were sorted as CD11c+B220−CD24hiCD11blo cells, and CD8− cDCs were sorted as CD11c+B220−CD24loCD11bhi cells.39 OVA257-264 peptide was synthesized with standard 9-fluorenylmethoxycarbonyl chemistry at the Netherlands Cancer Institute. Before adoptive transfer, DCs were incubated for 3 hours at 37°C with OVA257-264 peptide at 10 μg/mL in RPMI 1640 medium with 5% fetal calf serum. In case of ex vivo DC maturation with LPS (E coli O111:B4; Sigma Chemical, Poole, United Kingdom), this was added at 1 μg/mL as specified in the figure legends. Naive CD8+ T cells specific for the OVA peptide/H-2Kb complex were purified to 95% to 98% homogeneity from spleens of OT-I mice36 with a T-cell enrichment kit (BD Biosciences).
Results
Generation and characterization of CD70tg DCs
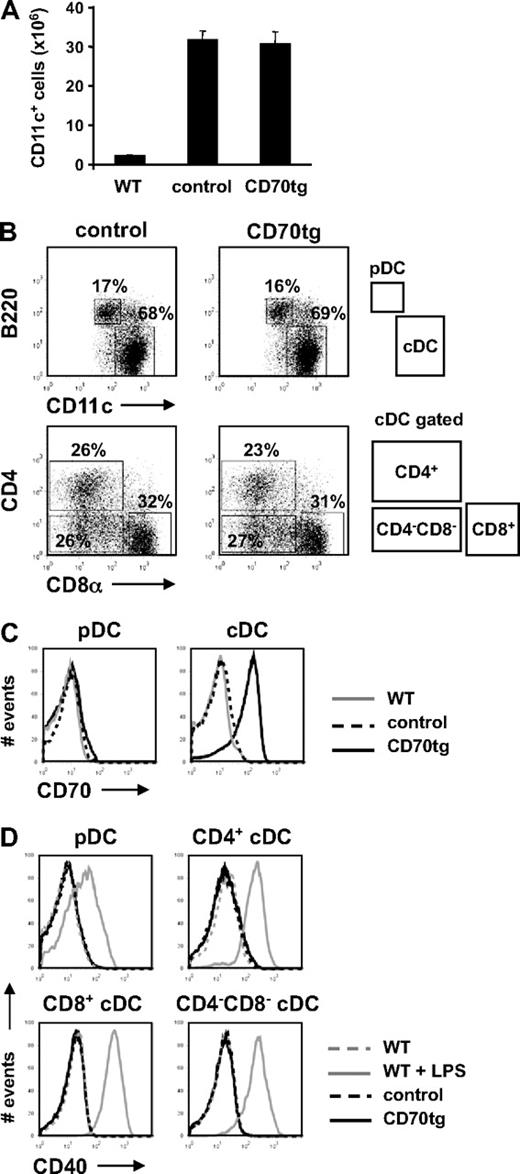
Mature DCs express CD70 but also other costimulatory ligands.17 To delineate the contribution of CD70 on DCs to CD8+ T-cell priming and programming, we generated DCs that expressed CD70 constitutively at steady state, before TLR and/or CD40 stimulation. This was done by constructing transgenic mice that expressed the CD70 cDNA under control of the DC-specific CD11c promoter (CD11c-CD70tg, hereafter called CD70tg).33 These mice were maintained on a CD27−/− background29 to avoid effects of constitutive CD27/CD70 interaction. Expansion of steady state DCs in donor mice was achieved by systemic administration of Flt3L, which drives development of all major DC subtypes37,39 (Figure 1A). Flt3L-amplified DC populations of donor mice were examined for subset distribution. Plasmacytoid (p) and conventional (c) DC populations, as well as CD4+, CD8+, and CD4−CD8− cDC subsets were comparable in size between CD70tg;CD27−/− mice and their CD27−/− littermates (Figure 1B). The CD70 transgene gave rise to uniformly high CD70 expression on cDCs, but not on pDCs (Figure 1C), in agreement with the differential activity of the CD11c promoter in these populations.40 CD70 expression was absent on steady state WT DCs, as described.17,23
Characterization of CD70tg DCs. CD27−/− (control) or CD70tg;CD27−/− (CD70tg) donor mice were injected subcutaneously with B16 melanoma cells that secrete Flt3L, and analysis was performed 2 weeks later. (A) Mean numbers + SEM of CD11c+ cells in spleens of Flt3L-treated control and CD70tg mice and untreated WT mice (n = 5). (B) DC subset distribution. DCs were isolated from spleen by density gradient centrifugation; stained with directly conjugated antibodies to CD11c, CD45R/B220, CD4, CD8α, CD70, and CD40; and analyzed by flow cytometry. Gating strategy to identify pDC and cDC subsets and their percentage are indicated. (C) Expression of CD70 at the cell surface of pDCs and cDCs isolated from untreated WT and Flt3L-treated control and CD70tg mice, as determined by flow cytometry on gated populations as indicated above. (D) CD40 expression on unstimulated or LPS-stimulated DCs from untreated WT mice and on DCs from Flt3L-treated control and CD70tg mice. Data are representative of 2 independent experiments.
Characterization of CD70tg DCs. CD27−/− (control) or CD70tg;CD27−/− (CD70tg) donor mice were injected subcutaneously with B16 melanoma cells that secrete Flt3L, and analysis was performed 2 weeks later. (A) Mean numbers + SEM of CD11c+ cells in spleens of Flt3L-treated control and CD70tg mice and untreated WT mice (n = 5). (B) DC subset distribution. DCs were isolated from spleen by density gradient centrifugation; stained with directly conjugated antibodies to CD11c, CD45R/B220, CD4, CD8α, CD70, and CD40; and analyzed by flow cytometry. Gating strategy to identify pDC and cDC subsets and their percentage are indicated. (C) Expression of CD70 at the cell surface of pDCs and cDCs isolated from untreated WT and Flt3L-treated control and CD70tg mice, as determined by flow cytometry on gated populations as indicated above. (D) CD40 expression on unstimulated or LPS-stimulated DCs from untreated WT mice and on DCs from Flt3L-treated control and CD70tg mice. Data are representative of 2 independent experiments.
To minimize the risk of inducing DC maturation by handling the cells, DCs were continuously kept at 0°C to 4°C during the isolation procedure, apart from a short incubation step at room temperature. Importantly, both control (CD27−/−) and CD70tg (CD70tg;CD27−/−) DC populations remained immature as verified by low-level CD40 (Figure 1D) and MHC class II expression (data not shown). This setting allowed us to compare side by side the potential of adoptively transferred control and CD70tg DCs to generate a CD8+ T-cell response.
Immature CD8α+ cDC subset elicits CD8+ T-cell expansion when endowed with CD70 expression
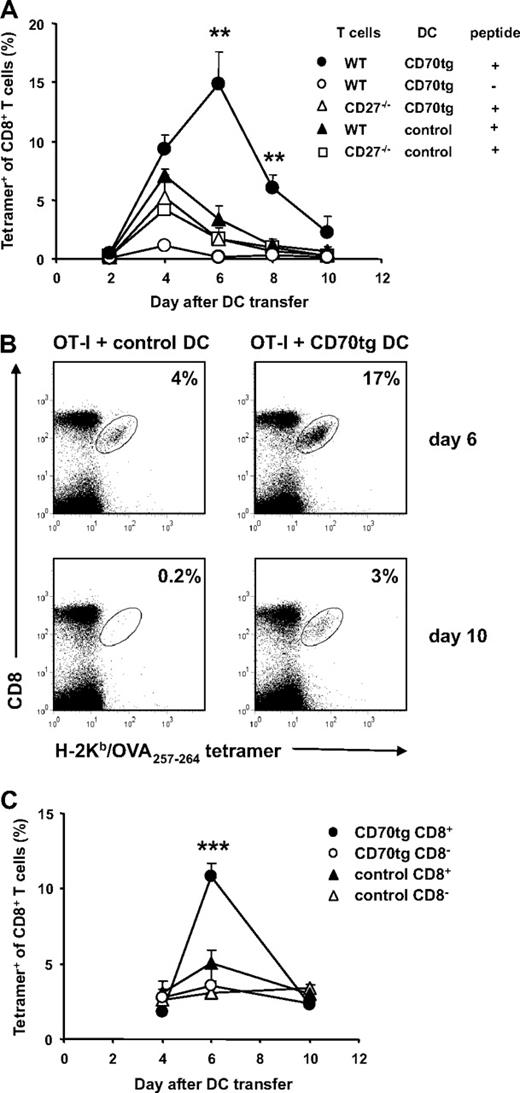
To follow the CD8+ T-cell response induced by transferred DCs, we used OT-I TCR transgenic T cells that recognize a complex of H-2Kb and the OVA257-264 peptide.36 OT-I T cells and OVA257-264 peptide-loaded DCs were transferred into CD27−/− recipient mice, to allow for the exclusive read-out of CD27/CD70 interactions between the adoptively transferred cells. The intravenous route was used, because such delivery results in rapid distribution of immature DCs throughout the recipient, including recruitment of all DC populations to the spleen.41 In recipients of CD70tg DCs, CD8+ T cells expanded in response to H-2Kb–presented OVA257-264 peptide to a significantly greater extent than in recipients of control DCs (Figure 2A,B). OVA-specific CD8+ T-cell numbers peaked on day 6 when they constituted approximately 15% of total CD8+ T cells. In recipients of control DCs, OVA-specific CD8+ T-cell numbers peaked earlier and amounted at maximum to 7% of total CD8+ T cells (Figure 2A,B). The responsiveness to CD70tg DCs was fully dependent on OVA peptide loading. The OVA-specific CD8+ T-cell response to CD70tg and control DCs relied to the same extent on interaction with CD27, as shown by the reduced accumulation of CD27−/− OT-I T cells (Figure 2A). T-cell responsiveness was higher in the absence of CD27 than in the absence of peptide, indicating that adoptively transferred DCs delivered a certain degree of costimulation, presumably by CD80 or CD86 or both.
Antigen-specific T-cell responses after DC transfer. CD27−/− recipients received WT or CD27−/− OT-I T cells, and 1 day later they were injected intravenously with Flt3L-induced, OVA257-264 peptide-loaded or unloaded total DCs (A,B) or flow cytometrically sorted DC subsets (C) derived from CD27−/− (control) or CD70tg;CD27−/− (CD70tg) donor mice. (A) Percentages of H-2Kb/OVA257-264 tetramer-positive cells among CD8+ T cells in blood of recipients at the indicated days after DC transfer. (B) Representative flow cytometric data of the same experiment as in panel A depicting tetramer+ CD8+ T cells in blood of DC recipients at day 6 (peak of the response in CD70tg recipients) and day 10 (contraction phase). Numbers indicate percentages of H-2Kb/OVA257-264 tetramer-positive cells within the CD8+ T-cell population. (C) Expansion of WT OT-I T cells in response to OVA257-264 peptide-loaded CD8+ or CD8− cDC subsets derived from control or CD70tg donor mice. Data in panels A and C represent mean values (+ SEM) for 4 to 5 mice per group and are representative of 2 or more independent experiments. Asterisks indicate statistical significance between the groups that received control or CD70tg peptide-loaded DCs and WT T cells according to Student t test for *P < .05, **P < .01, and ***P < .001.
Antigen-specific T-cell responses after DC transfer. CD27−/− recipients received WT or CD27−/− OT-I T cells, and 1 day later they were injected intravenously with Flt3L-induced, OVA257-264 peptide-loaded or unloaded total DCs (A,B) or flow cytometrically sorted DC subsets (C) derived from CD27−/− (control) or CD70tg;CD27−/− (CD70tg) donor mice. (A) Percentages of H-2Kb/OVA257-264 tetramer-positive cells among CD8+ T cells in blood of recipients at the indicated days after DC transfer. (B) Representative flow cytometric data of the same experiment as in panel A depicting tetramer+ CD8+ T cells in blood of DC recipients at day 6 (peak of the response in CD70tg recipients) and day 10 (contraction phase). Numbers indicate percentages of H-2Kb/OVA257-264 tetramer-positive cells within the CD8+ T-cell population. (C) Expansion of WT OT-I T cells in response to OVA257-264 peptide-loaded CD8+ or CD8− cDC subsets derived from control or CD70tg donor mice. Data in panels A and C represent mean values (+ SEM) for 4 to 5 mice per group and are representative of 2 or more independent experiments. Asterisks indicate statistical significance between the groups that received control or CD70tg peptide-loaded DCs and WT T cells according to Student t test for *P < .05, **P < .01, and ***P < .001.
To examine which cDC population was responsible for CD8+ T-cell priming, we isolated CD8α+ and CD8α− cDC subsets39 by flow cytometry and used them in the same experimental setting as outlined for Figure 2A and B. Strikingly, CD70 transgenesis enabled CD8α+ cDCs, but not CD8α− cDCs, to prime an OT-I T-cell response (Figure 2C). As before, CD70-expressing DCs were significantly more potent than control DCs in eliciting OT-I T-cell expansion. However, also in the control situation, CD8α+ cDCs performed significantly better than CD8α− DCs (P = .03 on day 6; Figure 2C). We conclude that the deliberate expression of CD70 on immature DCs enables the CD8α+ cDC subset to elicit dramatic clonal expansion of CD8+ T cells in response to peptide presentation in MHC class I.
CD70+ immature cDCs elicit helper-independent CD8+ T-cell memory
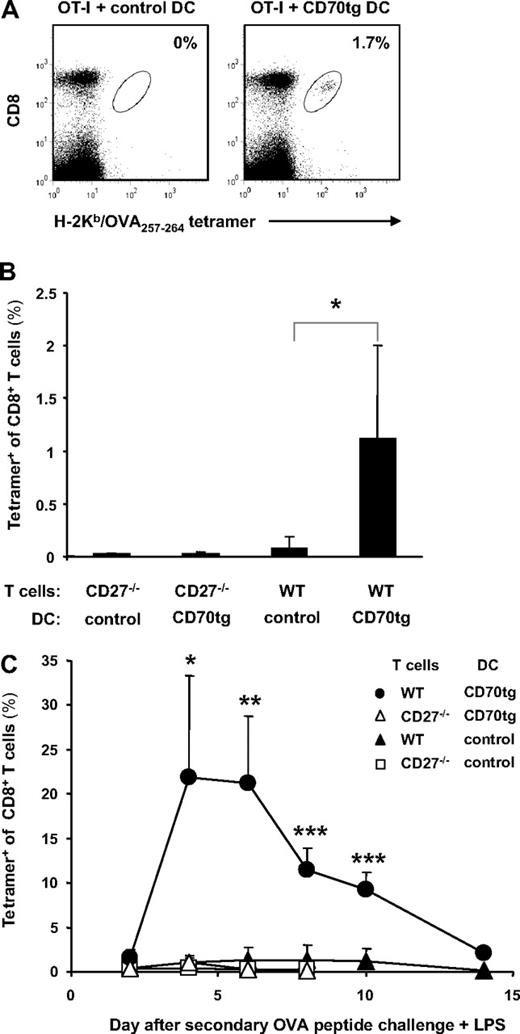
Successful DC-based cancer immunotherapy should elicit a primary CD8+ effector T-cell response, as well as immunologic memory. OVA-specific CD8+ T cells primed by CD70tg DCs not only displayed increased accumulation but also delayed contraction, compared with cells primed by control DCs (Figure 2A,B). This is in agreement with reduced CD8+ effector T-cell contraction in transgenic mice that express CD70 constitutively on B cells42 and with decreased CD8+ memory T-cell formation in CD27−/− mice.28 To assess the formation of CD8+ T-cell memory in the adoptive transfer setting, OVA-specific CD8+ T cells were enumerated in blood at 10 weeks after injection of peptide-loaded DCs and OT-I T cells. A clear population was detected in mice that had received WT OT-I T cells and CD70tg DCs, whereas such a population was hardly detectable in mice that had received control DCs (Figure 3A). Analysis of 4 mice per group underlined that transgenic CD70 expression on the transferred DCs significantly increased CD8+ memory T-cell formation (Figure 3B). Generation of this memory CD8+ T-cell pool was dependent on CD27, because priming of CD27−/− T cells did not result in detectable memory formation (Figure 3B). We conclude that endowing immature cDCs with CD70 enables them to increment the size of CD8+ T-cell effector and memory pools by triggering of CD27 on the T cell.
Memory formation and secondary responsiveness after DC transfer. Experimental set-up as described for Figure 2 with CD27−/− recipients of OT-I T cells and DCs. (A) Representative flow cytometric profiles of blood cells at 10 weeks after DC transfer. Numbers indicate percentages of H-2Kb/OVA257-264 tetramer-positive cells within the CD8+ T-cell population as marked by the oval. (B) Quantification of the steady state memory T-cell pool in blood at 10 weeks after adoptive transfer of WT or CD27−/− OT-I T cells and CD27−/− (control) or CD70tg;CD27−/− (CD70tg) OVA257-264 peptide-loaded DCs. (C) At 10 weeks after primary challenge, recipients were injected intravenously with OVA257-264 peptide in combination with LPS and percentages of H-2Kb/OVA257-264 tetramer-positive cells among CD8+ T cells in blood were determined on successive days. Data in panels B and C represent mean values (+SEM) for 4 mice per group and are representative of 2 independent experiments. Asterisks indicate statistical significance between the groups that received control or CD70tg peptide-loaded DCs and WT T cells according to Student t test for *P < .05, **P < .01, and ***P < .001.
Memory formation and secondary responsiveness after DC transfer. Experimental set-up as described for Figure 2 with CD27−/− recipients of OT-I T cells and DCs. (A) Representative flow cytometric profiles of blood cells at 10 weeks after DC transfer. Numbers indicate percentages of H-2Kb/OVA257-264 tetramer-positive cells within the CD8+ T-cell population as marked by the oval. (B) Quantification of the steady state memory T-cell pool in blood at 10 weeks after adoptive transfer of WT or CD27−/− OT-I T cells and CD27−/− (control) or CD70tg;CD27−/− (CD70tg) OVA257-264 peptide-loaded DCs. (C) At 10 weeks after primary challenge, recipients were injected intravenously with OVA257-264 peptide in combination with LPS and percentages of H-2Kb/OVA257-264 tetramer-positive cells among CD8+ T cells in blood were determined on successive days. Data in panels B and C represent mean values (+SEM) for 4 mice per group and are representative of 2 independent experiments. Asterisks indicate statistical significance between the groups that received control or CD70tg peptide-loaded DCs and WT T cells according to Student t test for *P < .05, **P < .01, and ***P < .001.
Memory T cells should have the capacity to effectively expand on renewed antigenic challenge. To test this ability in the recipient mice, secondary challenge was performed by intravenous administration of OVA257-264 peptide together with bacterial LPS. This results in antigen loading as well as maturation of DCs (and B cells), allowing them to present antigen and provide optimal T-cell costimulation. In recipients of control DCs, a very modest secondary response of WT OVA-specific CD8+ T cells ensued, which was lower than the primary and barely detectable above the response of CD27−/− OVA-specific CD8+ T cells (Figure 2C). This showed that the expansion in the primary response was abortive and resulted in functional deletion of the CD8+ responder T cells, either by cell death or anergy induction.43 This is an example of peripheral T-cell tolerance and confirms that the transferred DCs remained immature under the experimental conditions. In contrast, OVA-specific CD8+ T cells that had been primed by CD70tg DCs expanded to a significantly greater extent on secondary challenge than those primed by control DCs. This memory response was greater in magnitude (22% vs 15% of CD8+ T cells) and faster (peak at day 4 vs day 6) than the primary response and showed delayed and decreased contraction. Clearly, provision of immature cDCs with CD70 sufficed to make these cells strongly immunogenic and allowed them to generate robust CD8+ T-cell memory. Importantly, CD4+ T cells did not participate in this setting, because we challenged with MHC class I–restricted peptide only. Adoptively transferred DCs clearly affected CD8+ T-cell fate in the long term, in agreement with the concept that CD8+ T cells can be programmed for secondary expansion during the priming phase.44 We conclude that deliberate CD27/CD70 costimulation at the CD8+ T-cell/DC interface during priming can bypass the documented requirement for CD4+ T-cell help in programming CD8+ T cells for secondary expansion.
CD70+ immature cDCs generate CD8+ effector T cells that can eradicate established tumors
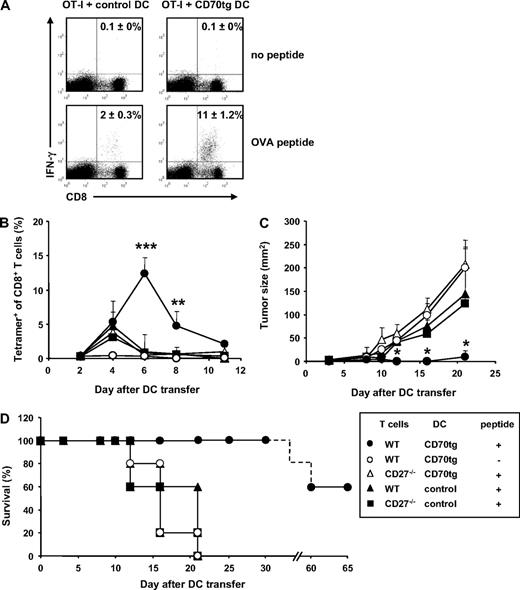
Because CD8+ T-cell priming in a tolerance-inducing setting not only produces abortive expansion, but also defective effector T-cell differentiation,43 we tested whether CD70-expressing immature cDCs could induce effector functions in primed CD8+ T cells. IFN-γ production by OT-I T cells was determined at day 5 of the response, in the experiment outlined in Figure 2. In recipients of control DCs, IFN-γ–producing CD8+ T cells represented 2% of total CD8+ T cells. By contrast, this population represented approximately 11% of CD8+ T cells in recipients of CD70tg DCs (Figure 4A). IFN-γ production in both test situations was elicited specifically by OVA257-264 peptide stimulation.
CTL effector functions after DC transfer. (A) Experimental set-up as outlined for Figure 2. CD8+ T cells harvested from spleens at day 5 after DC transfer were incubated in vitro for 5 hours in the presence or absence of OVA257-264 peptide and stained intracellularly for IFN-γ. Numbers in quadrants indicate the percentages of IFN-γ+ cells within the CD8+ T-cell population as means (±SEM) derived from 2 independent experiments with 2 mice per group. Flow cytometric plots are representative. (B-D) CD27−/− recipients were challenged subcutaneously with 105 B16-OVA tumor cells. Three days later, these mice received WT or CD27−/− OT-I T cells and the subsequent day OVA peptide-loaded or unloaded DCs derived from CD27−/− (control) or CD70tg;CD27−/− (CD70tg) donor mice. Test groups are indicated in the box. (B) Accumulation of OVA-specific CD8+ T cells, measured as outlined for Figure 2A. (C) Tumor size measured by caliper. Data represent mean values (+SEM) for 5 mice per group. (D) Survival data for the indicated groups of recipient tumor bearing mice. Asterisks indicate statistical significance between the groups that received control or CD70tg peptide-loaded DCs and WT T cells according to Student t test for *P < .05, **P < .01, and ***P < .001.
CTL effector functions after DC transfer. (A) Experimental set-up as outlined for Figure 2. CD8+ T cells harvested from spleens at day 5 after DC transfer were incubated in vitro for 5 hours in the presence or absence of OVA257-264 peptide and stained intracellularly for IFN-γ. Numbers in quadrants indicate the percentages of IFN-γ+ cells within the CD8+ T-cell population as means (±SEM) derived from 2 independent experiments with 2 mice per group. Flow cytometric plots are representative. (B-D) CD27−/− recipients were challenged subcutaneously with 105 B16-OVA tumor cells. Three days later, these mice received WT or CD27−/− OT-I T cells and the subsequent day OVA peptide-loaded or unloaded DCs derived from CD27−/− (control) or CD70tg;CD27−/− (CD70tg) donor mice. Test groups are indicated in the box. (B) Accumulation of OVA-specific CD8+ T cells, measured as outlined for Figure 2A. (C) Tumor size measured by caliper. Data represent mean values (+SEM) for 5 mice per group. (D) Survival data for the indicated groups of recipient tumor bearing mice. Asterisks indicate statistical significance between the groups that received control or CD70tg peptide-loaded DCs and WT T cells according to Student t test for *P < .05, **P < .01, and ***P < .001.
To test whether T cells primed by immature CD70tg DCs could migrate into target tissues and display cytolytic activity, we evaluated tumor rejection. B16 melanoma cells, engineered to express a fragment of the OVA protein,38 were injected subcutaneously into CD27−/− recipients and allowed to grow for 3 days before transfer of WT or CD27−/− OT-I cells. One day later, immature CD70tg or control DCs were pulsed with OVA257-264 peptide and transferred into the recipients. As seen before in tumor-free mice, peptide-loaded CD70-expressing DCs induced a significantly greater OVA-specific CD8+ T-cell response than control DCs. The response was OVA specific and largely dependent on CD27 or CD8+ T cells (Figure 4B). The tumor reached a palpable size while T-cell expansion was ongoing, allowing us to evaluate tumor rejection. Tumor outgrowth was significantly reduced in recipients of CD70tg DCs, compared with recipients of control DCs (Figure 4C). Tumor control relied on interaction between CD27 on the OT-I T cells and CD70 on the DCs and was OVA peptide dependent. In 60% of recipients, the WT OT-I T-cell response to peptide-loaded CD70tg DCs resulted in complete tumor rejection and long-term survival (Figure 4D). In the other test groups, tumor growth progressed, and mice had to be killed within 3 weeks after tumor inoculation.
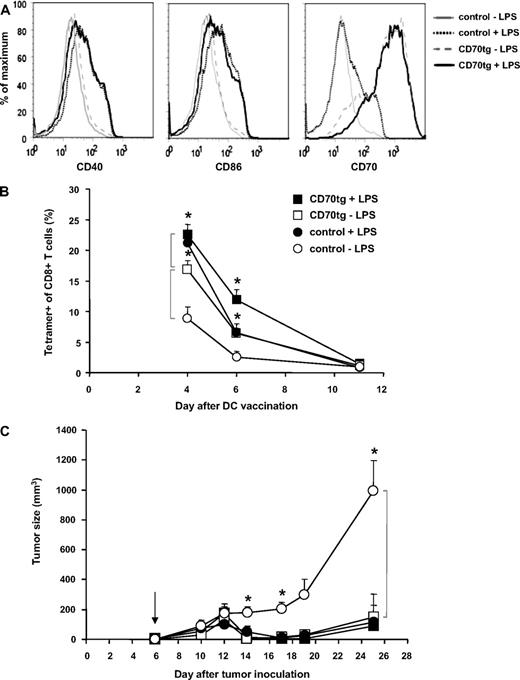
Next, we evaluated the effect of DC maturation on the comparative performance of control and CD70tg DCs. After their isolation from spleens of Flt3L tumor-bearing control or CD70tg donor mice, DCs were loaded for 3 hours with OVA peptide as before, but in this case split in 2 groups that were either kept on ice or cultured with LPS overnight. The next day, DCs were examined for viability, representation of the CD8+ subset, and expression of maturation markers. Although overnight LPS treatment compromised viability of the DCs to a certain extent (from 90% to 60%; data not shown), we chose this setting to ensure demonstrable maturation, as hallmarked by up-regulation of CD40, CD86, and CD70 (Figure 5A). The number of DCs injected was corrected to result in delivery of 2 × 106 live DCs in all cases. The CD8+ subset was somewhat underrepresented in the LPS-treated CD70tg group but equally represented in the other groups (data not shown). In this experimental set-up, the kinetics of the OT-I T-cell response was slightly altered (peak at or before day 4, rather than day 6), but again a significant difference was found between the response to control or CD70tg immature DCs (Figure 5B). Maturation of control DCs significantly improved the OT-I T-cell response (P = .003 on day 4), making it comparable with that found in recipients of CD70tg DCs. Maturation of CD70tg DCs also improved the T-cell response to a significant degree (P = .03 on day 4). In terms of tumor control, however, immature and mature CD70tg DCs, as well as mature control DCs, all performed similarly, giving rise to significant reduction in tumor outgrowth (Figure 5C). Only in recipients of immature control DCs, tumor growth was not impeded. We conclude that CD70 expression on immature cDCs that present MHC class I–restricted peptide overcomes tolerance induction and allows for formation of a tumor-eradicating CTL pool. In fact, the CTL response was comparable with that primed by LPS-matured DCs.
Comparison of immature and mature DCs in tumor rejection. CD27−/− recipients were challenged subcutaneously with 4 × 105 B16-OVA tumor cells on the shaved flank. Five days later, those mice in which tumor take was palpable were injected with WT OT-I cells, and the subsequent day (arrow, C) the same mice were injected with CD27−/− (control) or CD70tg;CD27−/− (CD70tg) DCs. These DCs had been loaded with OVA peptide for 3 hours and subsequently kept overnight either on ice (−LPS) or under culture conditions with LPS (+LPS). Maturation status of DCs was examined before transfer by flow cytometric analysis of cell surface expression of CD40, CD86, and CD70 within the PI−B220−CD11c+ gate (live cDCs) (A), and the equivalent of 2 × 106 viable DCs was injected per recipient. (B) Accumulation of OVA-specific CD8+ T cells, measured as outlined for Figure 2A. (C) Tumor size, as measured by caliper. Data represent mean values (+SEM) for 4 to 6 mice per group. Asterisks indicate statistical significance between the groups indicated by brackets according to Student t test for *P < .05, **P < .01, and ***P < .001.
Comparison of immature and mature DCs in tumor rejection. CD27−/− recipients were challenged subcutaneously with 4 × 105 B16-OVA tumor cells on the shaved flank. Five days later, those mice in which tumor take was palpable were injected with WT OT-I cells, and the subsequent day (arrow, C) the same mice were injected with CD27−/− (control) or CD70tg;CD27−/− (CD70tg) DCs. These DCs had been loaded with OVA peptide for 3 hours and subsequently kept overnight either on ice (−LPS) or under culture conditions with LPS (+LPS). Maturation status of DCs was examined before transfer by flow cytometric analysis of cell surface expression of CD40, CD86, and CD70 within the PI−B220−CD11c+ gate (live cDCs) (A), and the equivalent of 2 × 106 viable DCs was injected per recipient. (B) Accumulation of OVA-specific CD8+ T cells, measured as outlined for Figure 2A. (C) Tumor size, as measured by caliper. Data represent mean values (+SEM) for 4 to 6 mice per group. Asterisks indicate statistical significance between the groups indicated by brackets according to Student t test for *P < .05, **P < .01, and ***P < .001.
Discussion
Cancer therapy requires large numbers of CTLs that can migrate into the tumor, kill the tumor cells, and then persist to prevent recurrence of the disease. To enable these functions, appropriate signals must be delivered to CTL precursors. Evidence is accumulating that optimal CTL priming occurs in a ménage a trois between a CD8+ T cell, an activated DC, and a CD4+ helper T cell. The CD4+ T cell will express CD40L on recognition of MHC class II/peptide complexes on the DC and in turn trigger CD40 on the DC. This signal “licenses” the DC for priming of an appropriate CD8+ T-cell response in which the CD8+ T cell gains effector functions and the capacity for secondary expansion. In the case of an optimal DC stimulation by Toll-like receptors, CD4+ T-cell help can be redundant for the primary CD8+ response, but it is important for optimal CD8+ T-cell memory function.44 The requirement for CD8+ T-cell memory programming of is of great interest for vaccine development. Given the documented requirement for CD4+ T-cell help in generating effective and long-lasting CD8+ T-cell responses, vaccines now include defined or suspected helper T-cell epitopes next to a CTL epitope.
We report here that vaccination with immature CD70-expressing cDCs, loaded with MHC class I–restricted peptide, generates effective primary and memory CD8+ T-cell responses, apparently bypassing the requirement for CD4+ T-cell help. Our findings indicate that the interaction between CD70 on the DC and CD27 on the CD8+ T cell lies downstream from CD4+ T cell–induced DC licensing. This is in full agreement with the finding that anti-CD70 mAb blocks CD40-mediated CD8+ T-cell priming.20-22 Interestingly, this mechanism does not only pertain to CD4+ T-cell help, because it was recently reported that invariant natural killer T cells can also deliver CD40-dependent help for the CD8+ T-cell response by CD70 induction on DCs.45
Vaccines are designed to include DC maturation stimuli, because immature DCs are tolerogenic. Likewise, adoptive therapy approaches use DCs that are matured ex vivo or genetically modified to express molecules that are acquired on DC maturation. We performed our vaccination studies with DCs that are equivalent to those found at steady state in vivo. Such DCs maintain peripheral tolerance at least in part by engaging the coinhibitory receptors PD-1 and CTLA-4 on passing T cells.46 Signaling by PD-1 is a particularly important pathway, because its ligand, PD-L1, is broadly expressed on multiple tissues and acts as a molecular shield to protect from autoimmunity. In this way, however, it can also impede antitumor immunity.47 In our recent analysis of CD11c-CD70tg mice, we have shown by a genetic approach that deliberate CD70 expression on steady state cDCs breaks PD-1 and CTLA-4–imposed tolerance to the lymphocytic choriomeningitis virus. We also found that vaccination of CD11c-CD70tg mice with MHC class I–restricted peptide in PBS could elicit a CTL response to endogenous tumor antigens of B16 melanoma.33
In the present study, we have translated these findings to a DC vaccination setting. We generated large numbers of DCs by a previously established method of systemic administration of Flt3L that drives development of all major DC subtypes.37,39 DCs were immature at the time of transfer as determined by flow cytometric analysis and confirmed by the induction of T-cell tolerance by control DCs, which was apparent on recall (Figure 3C). Our experiments show that CD27 triggering on the CD8+ T cell during priming is required and, in this case, even sufficient for appropriate memory programming. It has been shown before that CD27 stimulation can strongly promote the CD8+ T-cell response, using soluble recombinant CD7025 or agonistic anti-CD27 antibody.48 However, we have translated this effect to a DC adoptive therapy approach. Our data further indicate that offering CD70 in the context of a DC is important for bypassing CD4+ T-cell help in memory CD8+ T-cell programming, because constitutive expression of CD70 on B cells could not instill memory function into CD8+ T cells.33 The potency of CD70 to promote CD8+ T-cell responses in the absence of other costimulatory input will undoubtedly depend on the strength of the TCR signal, because our previous work has indicated that CD27-mediated survival signals lower the threshold for T-cell activation.16 In our case of OT-I T cells with a high-affinity TCR, maturation of CD70-expressing DCs did not significantly enhance tumor control, but this may well be the case when other TCRs play a role in the antitumor repertoire.
Even though all cDCs can process antigens and present them to T cells for priming, in general CD8α+ DCs are more efficient in priming naive CD8+ T cells than the CD8α− DCs are.48 We confirm here this superior capacity of CD8α+ DCs, but we find that this can be unrelated to the ability of CD8α+ cDCs to cross-present exogenous antigen, because DCs were directly loaded with OVA peptide restricted to MHC class I. In this setting, therefore, some feature distinct from antigen processing by CD8α+ DC must be responsible for their CD8+ T-cell priming capacity. It is interesting in this light that CD8α+ DCs, but not CD8α− DCs, were found to induce IFN-γ production by CD4+ T cells in an IL-12–independent but CD70-dependent manner.32
One caveat of a vaccination strategy based on enhanced CD27/CD70 interaction is its potential effect on regulatory T cells. The effects of CD27 signaling on this population are not yet fully understood, but it was recently found that CD70+ non-Hodgkin lymphoma B cells can support activity of intratumoral regulatory T cells by sustaining their Foxp3 expression in a CD27-dependent manner.50 However, we have shown that DCs loaded with MHC class I–restricted peptide only can induce CD4+ helper-independent CD8+ T-cell responses. This is a attractive strategy, because it is not expected to engage CD4+ regulatory T cells.
At present, DC vaccination is used clinically, and the technology to endow DCs with molecules of interest is in place. Clearly, if preclinical studies indicate that a single molecule, CD70, can convert tolerogenic DCs into immunogenic DCs, this provides a guideline for optimizing DC vaccination strategies. Previous work has shown that mouse bone marrow–derived and GM-CSF/IL-4–driven DCs transfected with mRNA encoding OX40L augmented the Th1 CD4+ T-cell response, but they had no effect on CD8+ T-cell responses.11 DCs generated by the same protocol, when endowed with 4-1BBL by adenoviral transduction, strongly enhanced primary and memory CD8+ T-cell responses.12 Our previous work has indicated that in the mouse CD27, 4-1BB and OX40 act in concert to promote primary and secondary responses of the same antigen-specific CD8+ T-cell pool.28 A recent report documented that ligands for all 3 receptors, namely CD70, 4-1BBL. and OX40L, can be induced on human monocyte-derived DCs that are generated in a standard GM-CSF/IL-4 protocol, provided that prostaglandin E2 is added.19 Thus, a protocol is available for CD70 induction. CD70 can also be induced on human monocyte-derived DCs by culture in GM-CSF and TNF.18 Perhaps more straightforward is purposely endowing DCs with CD70 by mRNA transfection, which has been shown to improve their priming capacity.15 If a choice has to be made between CD70, 4-1BBL, and OX40L in a DC vaccination trial, it is important to consider that OX40 and 4-1BB are induced after T-cell priming, whereas CD27 is expressed already on naive T cells, allowing CD27 signaling to occur immediately on DC/T-cell contact. Modulation of signaling at this cellular interface may be the optimal way to dictate CD8+ T-cell fate. We therefore propose that the CD27/CD70 costimulatory axis, as a potent inducer of robust antitumor immunity, should be incorporated in the design of new DC-based immunotherapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank I. Verbrugge, G. van der Horst, A. Kaiser, M. de Witte, A. Jorritsma, F. van Diepen, A. Pfauth, and the personnel of the experimental animal facility of the Netherlands Cancer Institute for expert help and advice.
This work was supported by the Dutch Cancer Society (KWF; grant NKI 2003-2859).
Authorship
Contribution: A.M.K. designed and performed research, analyzed data, and wrote the manuscript; Y.X., V.P., and S.H.N. performed research and analyzed data; and J.B. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jannie Borst, Division of Immunology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; e-mail: j.borst@nki.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal