Abstract
Pronounced circadian rhythms in numbers of circulating T cells reflect a systemic control of adaptive immunity whose mechanisms are obscure. Here, we show that circadian variations in T cell subpopulations in human blood are differentially regulated via release of cortisol and catecholamines. Within the CD4+ and CD8+ T cell subsets, naive cells show pronounced circadian rhythms with a daytime nadir, whereas (terminally differentiated) effector CD8+ T cell counts peak during daytime. Naive T cells were negatively correlated with cortisol rhythms, decreased after low-dose cortisol infusion, and showed highest expression of CXCR4, which was up-regulated by cortisol. Effector CD8+ T cells were positively correlated with epinephrine rhythms, increased after low-dose epinephrine infusion, and showed highest expression of β-adrenergic and fractalkine receptors (CX3CR1). Daytime increases in cortisol via CXCR4 probably act to redistribute naive T cells to bone marrow, whereas daytime increases in catecholamines via β-adrenoceptors and, possibly, a suppression of fractalkine signaling promote mobilization of effector CD8+ T cells from the marginal pool. Thus, activation of the major stress hormones during daytime favor immediate effector defense but diminish capabilities for initiating adaptive immune responses.
Introduction
Whereas recent research has provided some valuable insights into the complex processes of systems regulation underlying the organism's immunity, circadian rhythms have been barely investigated in this context, although many immune functions exhibit striking circadian rhythms.1-3 T cells are the major mediators of adaptive immunity. In humans, the number of circulating T cells during the circadian nadir (14:00 h) is decreased by approximately 40% compared with T cell counts during the circadian peak (2:00 h).1,4,5
The 2 major stress-systems, the sympathetic nervous system and the hypothalamo-pituitary-adrenal (HPA) system, play a key role in conveying circadian rhythms to the immune system controlling also the redistribution of T cells.2,6 Plasma concentrations of cortisol and catecholamines exhibit most distinct circadian variations. In humans, levels are minimal at night and rise to a maximum in the beginning of the day, with this rise considered to prepare the organism for the demands of the active period.4,7,8 Circadian variations in the global population of circulating T cells show a quite robust negative correlation with 24-hour changes in plasma cortisol,5,9 and administration of corticosteroids to humans and animals reduces blood lymphocyte counts, by redistributing these cells to the bone marrow.6,10,11 Opposite to corticosteroids, catecholamines generally increase cell numbers in blood. Infusion of catecholamines induces rapid and transient increases in CD8+ (cytotoxic) T cells and natural killer cells,12,13 and both populations are decreased when endogenous catecholamines are suppressed by stellate ganglion block.14
CD4+ and CD8+ T cells are, in itself, heterogeneous populations. Each can be divided into at least 4 subsets with different function and recirculating patterns: (1) CD45RA+ naive and (2) CD45RA− central memory T cells, which express CD62L and CCR7 molecules and therefore recirculate through secondary lymphoid organs, and (3) CD45RA− memory effector and (4) CD45RA+ (terminally differentiated) effector T cells, which lack CD62L and CCR7 expression and thus are excluded from lymph nodes but expected to migrate to peripheral nonlymphoid tissue.15-17 To what extent the redistribution of these subsets is differentially regulated by circadian release of corticosteroids and catecholamines is obscure.18,19
Here we demonstrate specific circadian rhythms for these CD4+ and CD8+ T cell subsets that differ in phase because of their primary regulation via glucocorticoid and catecholamine signaling, respectively. Naive CD4+ and CD8+ T cells displayed clear circadian rhythms that peaked during nighttime and were conveyed primarily via cortisol and CXCR4 signaling, whereas effector CD8+ T cells peaked during daytime and were modulated via catecholamine and fractalkine receptor (CX3CR1) signaling.
Methods
Subjects
Fourteen healthy men participated in the main experiment to assess circadian rhythms of T cell subpopulations (mean age, 25 years; range, 21-30 years). All persons were nonsmokers presenting with a normal nocturnal sleep pattern and did not take any medication at the time of the experiments. Acute and chronic illness was excluded by medical history, physical examination, and routine laboratory investigation.
The men were synchronized by daily activities and nocturnal rest. They had a regular sleep-wake rhythm for at least 6 weeks before the experiments and no signs of sleep disturbances, including apnea and nocturnal myoclonus. All subjects were adjusted to the experimental setting by spending at least one adaptation night in the laboratory. The study was approved by the Ethics Committee of the University of Lübeck (Lübeck, Germany). All men gave written informed consent in accordance with the Declaration of Helsinki.
Experimental design
Each participant was examined during two 24-hour periods starting each at 20:00 h, one including a regular sleep-wake cycle whereas during the other period the subject remained awake continuously (24-hour continuous wakefulness). Both conditions for a subject were separated by at least 4 weeks, and the order of conditions was balanced across subjects.
On experimental days, subjects arrived at the laboratory at 18:00 h for preparing blood sampling and, in the regular sleep-wake cycle condition, for standard polysomnographic recordings. Sleep was allowed between 23:00 h (lights off) and 07:00 h in the morning. In the condition of 24-hour wakefulness, subjects stayed awake in bed during this period in a half-supine position, watching TV, reading, listening to music, and talking to the experimenter at normal room light (∼ 300 lx). During daytime (between 07:00 and 20:00 h), subjects stayed in the laboratory in both conditions. Standardized meals were provided at appropriate times for breakfast (08:00 h), lunch (12:00 h), and dinner (18:00 h).
Blood was sampled first at 20:00 h, then every 1.5 hours between 23:00 and 08:00 h, and every 3 hours between 08:00 and 20:00 h the next day. During sleep (23:00-07:00 h), blood was sampled via an intravenous forearm catheter, which was connected to a long thin tube and enabled blood collection from an adjacent room without the subject's awareness (comparison of blood collected at 23:00 h in the absence and presence of the additional long tube used for sampling during sleep, did not provide any hint at a biasing effect of the tube on the blood measures of interest). To prevent clotting, approximately 700 mL of saline solution was infused throughout the 24-hour experimental period. The total volume of blood sampled during a 24-hour period was 250 mL. Blood samples were always processed immediately after sampling. Standard polysomnographic recordings assured normal nocturnal sleep in the sleep condition.
T cell subpopulations
Absolute counts of CD3+ total T cells, CD4+ T-helper cells, and CD8+ cytotoxic T cells were determined by a “lyse no-wash” flow cytometry procedure. Briefly, 50 μL of an undiluted blood sample was immunostained with anti-CD3/fluorescein isothiocyanate (FITC), anti-CD8/phycoerythrin (PE), anti-CD45/peridinin chlorophyll protein (PerCP), and anti-CD4/allophycocyanin (APC) in Trucount tubes (BD Biosciences, San Jose, CA). After 15 minutes of incubation at room temperature, 0.45 mL of fluorescence-activated cell sorting (FACS) lysing solution (BD Biosciences) was added followed by incubation for 15 minutes. Finally, samples were mixed gently, and at least 10 000 CD3+ cells were acquired on a FACSCalibur using CellQuest Software (BD Biosciences).
For detection of naive, central memory, effector memory, and effector T cells, whole blood was incubated with anti-CD4/FITC and anti-CD8/FITC (Diatec, Oslo, Norway), anti-CD62L/PE and anti-CD3/PerCP (BD Biosciences), and anti-CD45RA/APC (Invitrogen, Carlsbad, CA). Cells were lysed with FACS lysing solution (BD Biosciences), washed, resuspended, and at least 10 000 CD4+ or CD8+ T cells were acquired on the FACSCalibur using CellQuest Software (BD Biosciences). Absolute counts of T cell subsets were calculated based on the proportion of the respective CD4+ and CD8+ T cell subpopulation and on absolute counts obtained by the “lyse no-wash” procedure.
Hormone assays
Samples for measuring hormone concentrations were kept frozen at −70°C until assay. Cortisol was measured in serum using a commercial assay (Immulite, DPC-Biermann, Bad Nauheim, Germany). Epinephrine and norepinephrine were measured in plasma by standard high-performance liquid chromatography. Sensitivity and intraassay and interassay coefficients of variation were as follows: cortisol 0.2 μg/dL, less than 10%; epinephrine 2.0 pg/mL, less than 5.6%; norepinephrine 5.0 pg/mL, less than 6.1%.
Administration of cortisol and epinephrine
Supplementary experiments were performed in 10 healthy men who were intravenously infused for 30 minutes with sodium chloride (placebo), cortisol (8 μg/kg per minute, Hydrocortison 100; Rotexmedica, Trittau, Germany), or epinephrine (0.005 μg/kg per minute, Suprarenin; Sanofi-Aventis, Bridgewater, NJ) on 3 different occasions, according to a double-blind, within-subject crossover design. The doses were chosen to induce changes in blood concentration comparable with the nadir-to-peak difference in the circadian variation of the respective hormone. Infusions started always in the evening (21:00 h) when levels of endogenous cortisol are close to the circadian nadir. Subject characteristics were the same as in the main experiment. Subjects were prepared for blood sampling one hour before infusions started and remained in a supine position throughout the experimental session. Blood samples to determine hormone concentrations and immunologic parameters were drawn via a second catheter (inserted into the vein of the other arm) before (baseline), during (15, 30 minutes), and after (60-300 minutes) the start of the infusion. Heart rate and an electrocardiogram were continuously monitored to exclude any adverse effects. Hormones and T cell subpopulations were determined and analyzed in the same way as in the main experiments, except that cells were acquired on a FACSCanto II using FACSDiva Software (BD Biosciences).
Determination of glucocorticoid and adrenergic receptors
Whole blood was used for simultaneous immunophenotyping and intracellular glucocorticoid receptor (GR) determination in T cell subpopulations.20 The following panel of monoclonal antibodies was used: anti-GR/FITC and antimouse isotope control (AbD, Serotec, Oxford, United Kingdom); anti-CD62L/PE, anti-CD8/PerCP, anti-CD3/PE-Cy7, anti-CD45RA/APC, and anti-CD4/APC-Cy7 (all from BD Biosciences). The staining was performed by incubating 100 μL of undiluted blood sampled in the morning with the surface antibodies for 15 minutes. Cells were then fixed, permeabilized, and stained against intracellular GR according to the manufacturer's instructions using reagents from BD Biosciences. At least 1000 naive, central memory, effector memory, and effector CD4+ or CD8+ T cells were acquired and subsequently analyzed for expression of GR by a FACSCanto II using FACSDiva Software (BD Biosciences). The relative quantity of GR (mean GR fluorescence) expressed as mean fluorescence intensity (MFI) was calculated as the difference between mean values of GR and isotype control labeled samples.
To determine numbers of β-adrenergic receptors (β-AR) in T cell subpopulations, cell purification was first performed. Peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coat of 300 mL morning blood of healthy donors by Ficoll-Paque gradient centrifugation in Leucosep tubes (Greiner Bio-One, Frickenhausen, Germany) according to the manufacturer's instruction. Purified CD4+ and CD8+ T cells were separated from PBMCs by negative isolation using the magnetic-activated cell sorting (MACS) technology (Miltenyi Biotec, Auburn, CA). CD62L-positive and -negative CD4+ and CD8+ cells were then sorted using anti-CD62L/FITC antibody and anti-FITC MultiSort Kit (Miltenyi Biotec). Finally, the remaining microbeads of the positive populations were removed by releasing reagent. Effector memory and effector CD8+ T cells were additionally isolated from the CD62L− fraction by MoFlo Hi Speed Cell Sorter using anti-CD45RA antibody (Dako North America, Carpinteria, CA). The purity of the isolated T cell subpopulations was evaluated by flow cytometry and exceeded 90% in all experiments performed.
The number of β-AR on the purified T cell subpopulations was determined as described before.21 Briefly, aliquots of 3 × 105 cells were incubated for 60 minutes at 37°C with 5 different concentrations of I-cyanopindolol (125I-CYP; PerkinElmer Life and Analytical Sciences, Waltham, MA), ranging from 12.5 to 200 pM 125I-CYP. Radioactivity was determined in an LKB 1282 CompuGamma CS gamma counter (LKB Wallac, Turku, Finland). Unspecific binding of 125I-CYP was determined in parallel by incubating the T cell subpopulations in the presence of 1 μM propranolol, a competitive antagonist of 125I-CYP. Specific binding of 125I-CYP was determined by subtracting unspecific binding from the total binding capacity. The maximum number of 125I-CYP binding sites (Bmax), representing the number of β-AR, was calculated according to the method of Scatchard.
Detection of adhesion molecules and chemokine receptors
Whole blood from 6 healthy subjects sampled at 09:00 and 21:00 h on the same day was immediately labeled with anti-CD3, anti-CD4, anti-CD8, anti-CD45RA, anti-CD62L, anti-CD11a, anti-CD11b, anti-CD49d, anti-CCR5, anti-CCR7, anti-CXCR1, anti-CXCR3, anti-CXCR4 (all from BD Biosciences), and anti-CX3CR1 (MBL International, Woburn, MA). Samples were then lysed, acquired, and analyzed for the expression of adhesion molecules and chemokine receptors on T cell subsets using the FACSCanto II with FACSDiva Software (BD Biosciences).
To test the influence of cortisol on the expression of CXCR4 on T cell subsets, blood samples taken at 21:00 h were additionally incubated at 37°C in the absence or presence of 20 μg/dL cortisol for up to 6 hours. CXCR4 expression measured as MFI was analyzed before and every hour after the start of incubation.
Statistical analysis
Data are generally presented as mean plus or minus SEM. To identify circadian rhythms, cosinor analysis was performed separately for the conditions of a regular sleep-wake cycle and of 24-hour continuous wakefulness as well as collapsed across both conditions using Chronolab.22 (Cosinor analysis identifies circadian rhythm based on a least square fit of a cosine wave to the time series data.) To assess temporal relationships between circadian rhythms in T cell subpopulations of interest and diurnal variations in hormonal concentrations, cross-correlation functions were calculated. For these analyses, individual data were normalized (ie, values were ln-transformed and the individual 24-hour mean was subtracted). Then product-moment correlation coefficients and stepwise linear regression coefficients were calculated across all time points and subjects. Bonferroni correction was applied on resulting coefficients yielding a significance level of P = .002. Analyses of effects of intravenous cortisol and epinephrine infusions on T cell subsets were based on analysis of variance, including a substance factor (“Cort/Plac” and “Epi/Plac”), a “Population” factor representing the different T cell subsets, and a “Time” factor reflecting the different time points of measurement. Considering the different kinetics of hormone actions, 6 time points (60-300 minutes after start of infusion) were chosen for cortisol and 2 time points (15 and 30 minutes after start of infusion) were chosen for epinephrine. In case of significant substance effects, differences between effects of placebo and, respectively, cortisol and epinephrine, were analyzed at single time points using paired t test. Analysis of variance and subsequent t tests were also used to analyze differences in GR, β-AR, and adhesion molecule/chemokine receptor expression among T cell subsets, including the factors “Population” and “Time” (for daytime differences in molecule expression and to confirm CXCR4 up-regulation after in vitro incubation with cortisol). Degrees of freedom were adjusted according to the Greenhouse-Geisser procedure where appropriate. A P value less than .05 was considered significant.
Results
Undifferentiated total T cell populations show strong circadian rhythms
Total numbers of CD3+, CD4+, and CD8+ T cells showed the expected pronounced circadian rhythm, with maximum values (acrophase) at approximately 02:00 h at night and minimum values (nadir) at approximately 14:00 h (P < .001, derived from cosinor analysis; Table 1). Circadian rhythms of these cell populations were remarkably similar during regular sleep-wake conditions and 24-hour continuous wakefulness, except for a slight but consistent decrease in cell counts when subjects slept during nighttime (P < .05, comparison of nighttime levels4,5 ). Because overall the effects of sleep remained small and did not essentially alter circadian rhythm characteristics in the cell counts of interest, we focused our further analyses on data collapsed across both the regular sleep-wake condition and the condition of 24-hour continuous wakefulness.
Cosinor data for circulating T cell subsets and hormone concentrations
| . | Mesor . | Amplitude . | Acrophase . | Significance . |
|---|---|---|---|---|
| CD3+ T cells/μL | 1902.08 ± 85.00 | 387.91 ± 38.10 | 02:10 h ± 20 min | < .01 |
| CD4+ T-helper cells/μL | 1120.42 ± 70.29 | 243.99 ± 25.4 | 02:17 h ± 22 min | < .01 |
| Naive/μL | 510.20 ± 43.13 | 131.65 ± 16.00 | 02:41 h ± 23 min | < .01 |
| Central memory/μL | 349.87 ± 18.15 | 74.58 ± 6.60 | 02:02 h ± 20 min | < .01 |
| Effector memory/μL | 221.36 ± 15.40 | 42.31 ± 5.20 | 01:31 h ± 23 min | < .01 |
| Effector/μL | — | — | — | NS |
| CD8+ cytotoxic T cells/μL | 658.71 ± 30.08 | 140.01 ± 13.80 | 02:03 h ± 19 min | < .01 |
| Naive/μL | 326.49 ± 22.27 | 96.22 ± 9.90 | 02:09 h ± 20 min | < .01 |
| Central memory/μL | 86.52 ± 6.76 | 15.33 ± 2.00 | 02:16 h ± 26 min | < .01 |
| Effector memory/μL | 188.87 ± 12.37 | 24.72 ± 4.50 | 01:56 h ± 29 min | < .01 |
| Effector/μL | 66.69 ± 11.64 | 6.96 ± 3.4 | 15:34 h ± 116 min | < .05 |
| Cortisol, μg/dL | 7.68 ± 0.22 | 4.64 ± 0.20 | 10:40 h ± 19 min | < .01 |
| Epinephrine, pg/mL | 25.55 ± 3.03 | 6.69 ± 1.70 | 16:10 h ± 54 min | < .01 |
| Norepinephrine, pg/mL | 239.28 ± 17.18 | 71.41 ± 10.80 | 14:05 h ± 16 min | < .01 |
| . | Mesor . | Amplitude . | Acrophase . | Significance . |
|---|---|---|---|---|
| CD3+ T cells/μL | 1902.08 ± 85.00 | 387.91 ± 38.10 | 02:10 h ± 20 min | < .01 |
| CD4+ T-helper cells/μL | 1120.42 ± 70.29 | 243.99 ± 25.4 | 02:17 h ± 22 min | < .01 |
| Naive/μL | 510.20 ± 43.13 | 131.65 ± 16.00 | 02:41 h ± 23 min | < .01 |
| Central memory/μL | 349.87 ± 18.15 | 74.58 ± 6.60 | 02:02 h ± 20 min | < .01 |
| Effector memory/μL | 221.36 ± 15.40 | 42.31 ± 5.20 | 01:31 h ± 23 min | < .01 |
| Effector/μL | — | — | — | NS |
| CD8+ cytotoxic T cells/μL | 658.71 ± 30.08 | 140.01 ± 13.80 | 02:03 h ± 19 min | < .01 |
| Naive/μL | 326.49 ± 22.27 | 96.22 ± 9.90 | 02:09 h ± 20 min | < .01 |
| Central memory/μL | 86.52 ± 6.76 | 15.33 ± 2.00 | 02:16 h ± 26 min | < .01 |
| Effector memory/μL | 188.87 ± 12.37 | 24.72 ± 4.50 | 01:56 h ± 29 min | < .01 |
| Effector/μL | 66.69 ± 11.64 | 6.96 ± 3.4 | 15:34 h ± 116 min | < .05 |
| Cortisol, μg/dL | 7.68 ± 0.22 | 4.64 ± 0.20 | 10:40 h ± 19 min | < .01 |
| Epinephrine, pg/mL | 25.55 ± 3.03 | 6.69 ± 1.70 | 16:10 h ± 54 min | < .01 |
| Norepinephrine, pg/mL | 239.28 ± 17.18 | 71.41 ± 10.80 | 14:05 h ± 16 min | < .01 |
T cell counts and hormone concentrations were collapsed across conditions of a regular sleep-wake cycle and 24-hour continuous wakefulness and analyzed by the cosinor method. SEM values are presented as minutes.
Mesor indicates average value during a 24-hour period; Amplitude, difference between estimated maximum/minimum and Mesor (peak/nadir values can be calculated by adding the amplitude to/subtracting the amplitude from Mesor values, respectively); Acrophase, time of day of the maximum; Significance, P value of the zero amplitude test; and —, not applicable.
Opposing rhythms for T cell subpopulations
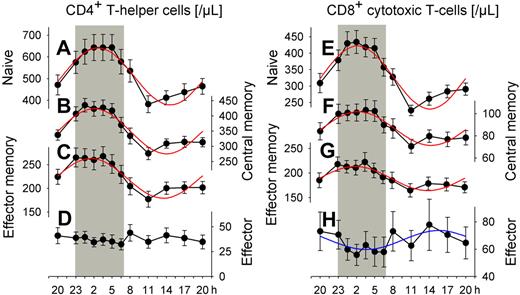
We classified CD4+ and CD8+ T cells into 4 distinct subpopulations: naive, central memory, effector memory, and effector T cells. Cosinor analysis revealed distinct and significant (P < .05) circadian rhythms in absolute cell counts for all subpopulations of interest, except for effector CD4+ T cells (Figure 1, Table 1). The identified rhythms differed remarkably with respect to their peak times and relative amplitudes. Acrophases during the night were evident for naive, central memory, and effector memory CD4+ and CD8+ T cells (between 01:31 and 02:41 h). In contrast, effector CD8+ T cell counts peaked during daytime, in the afternoon hours (15:34 h). Rhythms were most pronounced for naive CD4+ and CD8+ T cells. With reference to peak counts, cell counts during nadir times were reduced, respectively, by 41% and 46%, in these 2 populations (Table 2).
Rhythms of circulating T cell subsets. Mean (± SEM) of (A, E) naive (CD45RA+CD62L+), (B, F) central memory (CD45RA−CD62L+), (C, G) effector memory (CD45RA−CD62L−), and (D, H) effector (CD45RA+CD62L−) CD4+ (left) and CD8+ (right) T cells; n = 14. Rhythms with nocturnal peaks are more pronounced for naive T cells than for central memory and effector memory T cells. In contrast, effector CD8+ T cells peak during the day. Cosinor analysis of circadian rhythms was performed on data collapsed across conditions of a regular sleep-wake cycle and 24-hour continuous wakefulness (Table 1, rhythm characteristics). Adapted cosine curves are indicated for confirmed circadian rhythm. Shaded area represents bedtime.
Rhythms of circulating T cell subsets. Mean (± SEM) of (A, E) naive (CD45RA+CD62L+), (B, F) central memory (CD45RA−CD62L+), (C, G) effector memory (CD45RA−CD62L−), and (D, H) effector (CD45RA+CD62L−) CD4+ (left) and CD8+ (right) T cells; n = 14. Rhythms with nocturnal peaks are more pronounced for naive T cells than for central memory and effector memory T cells. In contrast, effector CD8+ T cells peak during the day. Cosinor analysis of circadian rhythms was performed on data collapsed across conditions of a regular sleep-wake cycle and 24-hour continuous wakefulness (Table 1, rhythm characteristics). Adapted cosine curves are indicated for confirmed circadian rhythm. Shaded area represents bedtime.
T cell subset characteristics
| . | CD4+ T-helper cells . | CD8+ cytotoxic T cells . | ||||||
|---|---|---|---|---|---|---|---|---|
| Naive . | Central memory . | Effector memory . | Effector . | Naive . | Central memory . | Effector memory . | Effector . | |
| CD62L | + | + | − | − | + | + | − | − |
| CD45RA | + | − | − | + | + | − | − | + |
| Changes from night to day†, % | −41 | −35 | −32 | NS | −46 | −30 | −23 | 23 |
| Correlation with cortisol‡ | −0.65* | −0.62* | −0.54* | NS | −0.68* | −0.54* | −0.42* | NS |
| Changes after cortisol§, % | −42 | −38 | −35 | NS | −39 | −27 | −21 | NS |
| GR expression‖ | 1063 ± 31* | 1098 ± 34*** | 1167 ± 35 | 1181 ± 36 | 1168 ± 33 | 1228 ± 50* | 1280 ± 51** | 1367 ± 55 |
| Correlation with epinephrine‡ | NS | NS | NS | NS | NS | NS | NS | 0.26* |
| Changes after epinephrine§, % | NS | NS | NS | NS | NS | NS | NS | 30 |
| Naive and central memory | Effector memory and effector | Naive and central memory | Effector memory | Effector | ||||
| β-AR expression¶ | 197 ± 42* | 827 ± 239 | 727 ± 181** | 2045 ± 579 | 2112 ± 602 | |||
| . | CD4+ T-helper cells . | CD8+ cytotoxic T cells . | ||||||
|---|---|---|---|---|---|---|---|---|
| Naive . | Central memory . | Effector memory . | Effector . | Naive . | Central memory . | Effector memory . | Effector . | |
| CD62L | + | + | − | − | + | + | − | − |
| CD45RA | + | − | − | + | + | − | − | + |
| Changes from night to day†, % | −41 | −35 | −32 | NS | −46 | −30 | −23 | 23 |
| Correlation with cortisol‡ | −0.65* | −0.62* | −0.54* | NS | −0.68* | −0.54* | −0.42* | NS |
| Changes after cortisol§, % | −42 | −38 | −35 | NS | −39 | −27 | −21 | NS |
| GR expression‖ | 1063 ± 31* | 1098 ± 34*** | 1167 ± 35 | 1181 ± 36 | 1168 ± 33 | 1228 ± 50* | 1280 ± 51** | 1367 ± 55 |
| Correlation with epinephrine‡ | NS | NS | NS | NS | NS | NS | NS | 0.26* |
| Changes after epinephrine§, % | NS | NS | NS | NS | NS | NS | NS | 30 |
| Naive and central memory | Effector memory and effector | Naive and central memory | Effector memory | Effector | ||||
| β-AR expression¶ | 197 ± 42* | 827 ± 239 | 727 ± 181** | 2045 ± 579 | 2112 ± 602 | |||
Columns show data of naive (CD45RA+CD62L+), central memory (CD45RA−CD62L+), effector memory (CD45RA−CD62L−), and effector (CD45RA+CD62L−) CD4+ and CD8+ T cells.
NS indicates not significant.
Percentage changes comparing the two extremes of the circadian rhythm during nighttime and daytime.
Correlations assessed by stepwise linear regression analyses between circadian variation in cortisol/epinephrine and respective T cell subpopulation, measured with a delay (time lag) of 3 and 0 hours with reference to cortisol and epinephrine, respectively. *P < .001.
Percentage change in cell numbers 180 and 30 minutes after starting cortisol and epinephrine infusions, respectively, compared with placebo numbers (Figure 4I,J); n = 10.
Morning levels of GR expression in MFI, mean ± SEM; n = 8. *P < .05, **P < .01, ***P < .001, pairwise comparisons between naive versus central memory, central memory versus effector memory, and effector memory versus effector T cells, respectively.
Morning levels of β-AR expression measured by specific binding of 125I-CYP in isolated T cells; data of saturation curves (Figure 5) were analyzed using nonlinear regression plot, and the maximum number of 125I-CYP binding sites (Bmax) is presented as mean ± SEM; n = 6. *P < .05, **P < .01, pairwise comparisons between CD62L+ versus CD62L− T cells and effector memory versus effector CD8+ T cells, respectively.
Circadian rhythms of cortisol and catecholamines
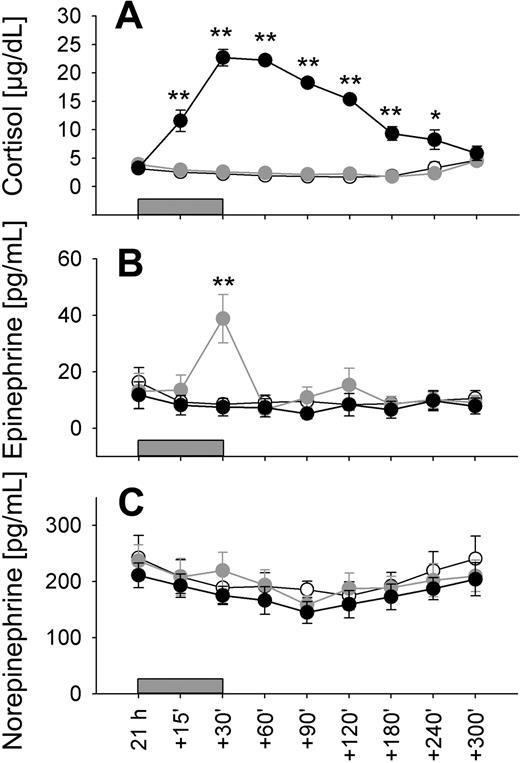
Plasma concentrations of both cortisol and catecholamines were characterized by distinct circadian rhythms, which were equally observed during conditions of regular sleep and 24-hour wakefulness (P < .01; Table 1 collapsed data). Cortisol concentrations showed a minimum approximately 00:30 h and a maximum around the time of morning awakening approximately 08:00 h (Figure 2A). Concentrations of both epinephrine and norepinephrine were decreased during nighttime hours (with a slightly more pronounced decrease for norepinephrine when subject slept; P < .05) and reached maximum levels in the morning between 08:00 and 11:00 h, with epinephrine showing a second increase in the evening hours (Figure 2B,C).
Circadian variations in stress hormone concentrations. Mean (± SEM) plasma concentrations of (A) cortisol, (B) epinephrine, and (C) norepinephrine during the 24-hour period collapsed across conditions of a regular sleep-wake cycle and 24-hour continuous wakefulness; n = 14. Shaded area represents bedtime.
Circadian variations in stress hormone concentrations. Mean (± SEM) plasma concentrations of (A) cortisol, (B) epinephrine, and (C) norepinephrine during the 24-hour period collapsed across conditions of a regular sleep-wake cycle and 24-hour continuous wakefulness; n = 14. Shaded area represents bedtime.
Temporal associations between T cell subset counts and hormone concentrations
There was a marked negative correlation between the rhythm of cortisol and those of total CD4+ and total CD8+ T cell counts. Correlations were maximal when CD4+ and CD8+ T cell counts were measured with a delay of 3 hours with reference to cortisol concentrations (r = −0.65 and −0.64, respectively, P < .001). Although less strong, epinephrine and norepinephrine concentrations also correlated negatively with total numbers of CD4+ and CD8+ T cells, maximally at a zero time lag (r = −0.27 to −0.40, P < .001).
T cell subset counts were also correlated to hormone concentrations (summarized in Table 2). Stepwise linear regression analyses indicated marked negative correlations of naive, central memory, and effector memory CD4+ and CD8+ T cells to cortisol concentrations. In contrast, effector CD8+ T cells counts were correlated positively with epinephrine levels. As for the total CD4+ and CD8+ T cell counts, correlations with cortisol were maximal for cell subset counts measured with a delay of 3 hours.
Influence of cortisol and epinephrine on the redistribution of T cell subpopulations
During infusion of cortisol and epinephrine, plasma concentrations of the respective hormone increased, reaching peak values after 30 minutes. Thereafter, concentrations declined, with a more gradual decrease for cortisol than epinephrine concentrations, reflecting the different half-lives of the hormones (Figure 3). Cortisol administration did not affect plasma epinephrine concentrations and, vice versa, epinephrine administration did not affect cortisol concentrations (P > .5 for all comparisons).
Low-dose cortisol and epinephrine infusions to mimic nadir-to-peak concentration difference in circadian rhythm. Mean (± SEM) plasma concentrations of (A) cortisol, (B) epinephrine, and (C) norepinephrine in healthy men before (21:00 h), during (15, 30 minutes), and after (60, 90, 120, 180, 240, and 300 minutes) 30-minute infusions ( ) of placebo (sodium chloride, ○), cortisol (8 μg/kg per minute, ●) and epinephrine (0.005 μg/kg per minute,
) of placebo (sodium chloride, ○), cortisol (8 μg/kg per minute, ●) and epinephrine (0.005 μg/kg per minute,  ); n = 10. * P < .05, ** P < .01, *** P < .001, pairwise comparisons between cortisol/placebo (A) and epinephrine/placebo conditions (B) at single time points.
); n = 10. * P < .05, ** P < .01, *** P < .001, pairwise comparisons between cortisol/placebo (A) and epinephrine/placebo conditions (B) at single time points.
Low-dose cortisol and epinephrine infusions to mimic nadir-to-peak concentration difference in circadian rhythm. Mean (± SEM) plasma concentrations of (A) cortisol, (B) epinephrine, and (C) norepinephrine in healthy men before (21:00 h), during (15, 30 minutes), and after (60, 90, 120, 180, 240, and 300 minutes) 30-minute infusions ( ) of placebo (sodium chloride, ○), cortisol (8 μg/kg per minute, ●) and epinephrine (0.005 μg/kg per minute,
) of placebo (sodium chloride, ○), cortisol (8 μg/kg per minute, ●) and epinephrine (0.005 μg/kg per minute,  ); n = 10. * P < .05, ** P < .01, *** P < .001, pairwise comparisons between cortisol/placebo (A) and epinephrine/placebo conditions (B) at single time points.
); n = 10. * P < .05, ** P < .01, *** P < .001, pairwise comparisons between cortisol/placebo (A) and epinephrine/placebo conditions (B) at single time points.
As expected, infusion of cortisol compared with placebo induced a clear decrease in both total CD4+ and CD8+ T cell counts, which started 90 minutes after infusion onset and reached minimum levels after 180 minutes (F(1,9) = 41.4 and 60.1, respectively, P < .001, for Cort/Plac). However, there were also systematic differences in the size of the suppression among the subpopulations of interest (F(7,63) = 24.5, P < .001, for Population × Cort/Plac). The suppression was strongest for naive T cells, intermediate for central memory T cells, weaker for effector memory T cells, and entirely absent for effector T cells (Figure 4I,J; Table 2, percentage changes compared with placebo 180 minutes after starting cortisol infusion). Of note, effector CD4+ and CD8+ T cells were the only subsets not influenced by cortisol (Figure 4D,H).
Cortisol and epinephrine differentially influence T cell subpopulations. Mean (± SEM) numbers of (A,E) naive (CD45RA+CD62L+), (B,F) central memory (CD45RA−CD62L+), (C,G) effector memory (CD45RA−CD62L−), and (D,H) effector (CD45RA+CD62L−) CD4+ (left) and CD8+ (right) T cells after a 30-minute intravenous infusion ( ) of placebo (sodium chloride, ○), cortisol (●), and epinephrine (
) of placebo (sodium chloride, ○), cortisol (●), and epinephrine ( ); n = 10, *P < .05, **P < .01, ***P < .001, pairwise comparison between cortisol/placebo conditions (A-C,E-G), and epinephrine/placebo conditions (H) at single time points. Bar charts represent percentage changes in (I) CD4+ and (J) CD8+ T cell subsets 180 minutes after cortisol (
); n = 10, *P < .05, **P < .01, ***P < .001, pairwise comparison between cortisol/placebo conditions (A-C,E-G), and epinephrine/placebo conditions (H) at single time points. Bar charts represent percentage changes in (I) CD4+ and (J) CD8+ T cell subsets 180 minutes after cortisol ( ) and 30 minutes after epinephrine infusion (
) and 30 minutes after epinephrine infusion ( ), respectively, compared with placebo (Table 2). *P < .05, **P < .01, ***P < .001, analysis of variance main effect (Cort/Plac, Epi/Plac) and for pairwise comparison of cortisol effects between naive vs central memory, central memory vs effector memory, and effector memory vs effector T cells.
), respectively, compared with placebo (Table 2). *P < .05, **P < .01, ***P < .001, analysis of variance main effect (Cort/Plac, Epi/Plac) and for pairwise comparison of cortisol effects between naive vs central memory, central memory vs effector memory, and effector memory vs effector T cells.
Cortisol and epinephrine differentially influence T cell subpopulations. Mean (± SEM) numbers of (A,E) naive (CD45RA+CD62L+), (B,F) central memory (CD45RA−CD62L+), (C,G) effector memory (CD45RA−CD62L−), and (D,H) effector (CD45RA+CD62L−) CD4+ (left) and CD8+ (right) T cells after a 30-minute intravenous infusion ( ) of placebo (sodium chloride, ○), cortisol (●), and epinephrine (
) of placebo (sodium chloride, ○), cortisol (●), and epinephrine ( ); n = 10, *P < .05, **P < .01, ***P < .001, pairwise comparison between cortisol/placebo conditions (A-C,E-G), and epinephrine/placebo conditions (H) at single time points. Bar charts represent percentage changes in (I) CD4+ and (J) CD8+ T cell subsets 180 minutes after cortisol (
); n = 10, *P < .05, **P < .01, ***P < .001, pairwise comparison between cortisol/placebo conditions (A-C,E-G), and epinephrine/placebo conditions (H) at single time points. Bar charts represent percentage changes in (I) CD4+ and (J) CD8+ T cell subsets 180 minutes after cortisol ( ) and 30 minutes after epinephrine infusion (
) and 30 minutes after epinephrine infusion ( ), respectively, compared with placebo (Table 2). *P < .05, **P < .01, ***P < .001, analysis of variance main effect (Cort/Plac, Epi/Plac) and for pairwise comparison of cortisol effects between naive vs central memory, central memory vs effector memory, and effector memory vs effector T cells.
), respectively, compared with placebo (Table 2). *P < .05, **P < .01, ***P < .001, analysis of variance main effect (Cort/Plac, Epi/Plac) and for pairwise comparison of cortisol effects between naive vs central memory, central memory vs effector memory, and effector memory vs effector T cells.
In striking contrast to the effects of cortisol, infusion of epinephrine induced a selective increase in numbers of effector CD8+ T cells only (F(1,9) = 7.0, P < .05, for Epi/Plac). The increase was short-lived and concentrated around the end of the infusion period when epinephrine levels were maximal (Figure 4H). All other T cell subsets remained unchanged by epinephrine infusion (P > .35). Comparable effects were revealed when subpopulations were categorized by CCR7 and CD45RA expression (data not shown).
Expression of glucocorticoid and adrenergic receptors on T cell subpopulations
Differences in GR expression between subpopulations were only subtle, although highly significant, with generally higher levels in CD8+ than CD4+ cells (F(7,49) = 37.1, P < .001; Table 2, pairwise comparisons between subpopulations). Surprisingly, however, cells (like CD4+ naive T cells) that were highly sensitive to the reducing effect of cortisol showed on average even slightly lower rather than higher GR expression.
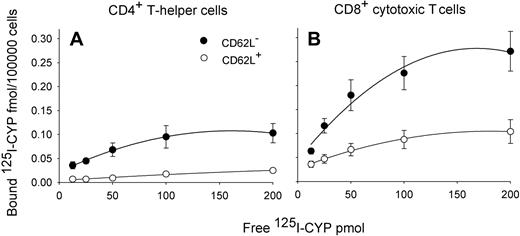
When we analyzed expression of β-AR on MACS-sorted T cell subsets, we found striking differences between different subpopulations in the expected direction (F(3,12) = 9.3, P < .05; Table 2, pairwise comparisons between subpopulations).23 β-AR levels were higher on CD8+ than CD4+ T cells and, within these cell populations, higher on CD62L− (effector memory and effector T cells) than on CD62L+ T cells (naive and central memory cells; Figure 5). However, the specific binding of 125I-CYP was comparable in effector memory and effector CD8+ T cells (P > .6).
Saturation curves indicating β-AR expression in T cell subsets. Mean (± SEM) specific binding of β-AR agonist 125I-cyanopindolol (125I-CYP) per 100 000 cells at different 125I-CYP concentrations; n = 6. β-AR expression is greater in CD62L− (●) than CD62L+ (○) T cells and in CD8+ (B) than CD4+ T cells (A). Values of specific binding were obtained by subtracting nonspecific binding from total binding. Table 2 contains Bmax values.
Saturation curves indicating β-AR expression in T cell subsets. Mean (± SEM) specific binding of β-AR agonist 125I-cyanopindolol (125I-CYP) per 100 000 cells at different 125I-CYP concentrations; n = 6. β-AR expression is greater in CD62L− (●) than CD62L+ (○) T cells and in CD8+ (B) than CD4+ T cells (A). Values of specific binding were obtained by subtracting nonspecific binding from total binding. Table 2 contains Bmax values.
Expression of adhesion molecules and chemokine receptors on T cell subpopulations
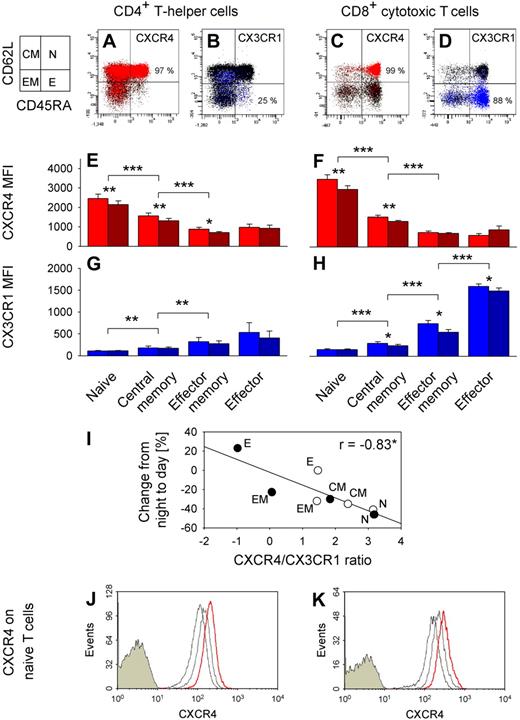
We revealed quite distinct expression patterns of adhesion molecules and chemokine receptors in the different T cell subpopulations (Figure 6). CXCR4 expression was highest in naive, lower in central memory, and lowest in effector memory and effector CD4+ and CD8+ T cells (F(7,35) = 94.2, P < .001; Figure 6E,F, pairwise comparison between subpopulations). By contrast, CX3CR1 was preferentially expressed on effector CD8+ T cells (F(7,35) = 50.2, P < .001; Figure 6G,H). Effector CD8+ T cells were also characterized by highest expression of CXCR1, CD11a, and CD11b, whereas CD49d and CCR5 density was lowest on naive CD4+ and CD8+ T cells. CXCR3 expression was not selective for any subset (data not shown). Of note, the ratio of CXCR4/CX3CR1 expression on T cell subsets strongly correlated with percentage changes of the respective subpopulation from nighttime to daytime (Figure 6I).
CXCR4 and CX3CR1 expression on T cell subsets. Dot plots from a representative subject of CXCR4+ (red, A,C) and CX3CR1+ (blue, B,D) naive (N, CD45RA+CD62L+), central memory (CM, CD45RA−CD62L+), effector memory (EM, CD45RA−CD62L−), and effector (E, CD45RA+CD62L−) CD4+ (left) and CD8+ (right) T cells. Chemokine receptor-postitive cells are shown as colored dots, and mean percentages indicate respective subpopulation with the highest proportion of chemokine receptor positive cells; n = 6. Bar charts show mean plus or minus SEM of (E,F) CXCR4 and (G,H) CX3CR1 expression indicated as MFI; n = 6. *P < .05, **P < .01, ***P < .001, pairwise comparison between morning (09:00 h, red and blue bars) and evening (21:00 h, dark red and dark blue bars) levels and pairwise comparisons between naive vs central memory, central memory vs effector memory, and effector memory vs effector T cell counts (collapsed across morning and evening samples). (I) Correlation of CXCR4/CX3CR1 ratio (ln(CXCR4 MFI/CX3CR1 MFI), mean of n = 6) and percentage changes from night to day (Table 2) for naive (N), central memory (CM), effector memory (EM), and effector (E) CD4+ (○) and CD8+ (●) T cells. *P < .05. A high ratio predicts a strong daytime decrease, whereas a low ratio predicts a daytime increase. In an additional experiment, whole blood was sampled at 21:00 h and cultured in the presence or absence of cortisol at normal daytime concentrations (20 μg/dL). CXCR4 expression in naive (CD45RA+CD62L+) (J) CD4+ and (K) CD8+ T cells is presented as fluorescence intensity. Cells were stained with control immunoglobulin (filled histograms) or anti-CXCR4 antibody (empty histograms). FACS profiles are shown (from a representative subject) for gated T cells before (left empty histogram, thin line) and after 3 hours of culture without (middle empty histogram, gray line) and with cortisol (right empty histogram, red line).
CXCR4 and CX3CR1 expression on T cell subsets. Dot plots from a representative subject of CXCR4+ (red, A,C) and CX3CR1+ (blue, B,D) naive (N, CD45RA+CD62L+), central memory (CM, CD45RA−CD62L+), effector memory (EM, CD45RA−CD62L−), and effector (E, CD45RA+CD62L−) CD4+ (left) and CD8+ (right) T cells. Chemokine receptor-postitive cells are shown as colored dots, and mean percentages indicate respective subpopulation with the highest proportion of chemokine receptor positive cells; n = 6. Bar charts show mean plus or minus SEM of (E,F) CXCR4 and (G,H) CX3CR1 expression indicated as MFI; n = 6. *P < .05, **P < .01, ***P < .001, pairwise comparison between morning (09:00 h, red and blue bars) and evening (21:00 h, dark red and dark blue bars) levels and pairwise comparisons between naive vs central memory, central memory vs effector memory, and effector memory vs effector T cell counts (collapsed across morning and evening samples). (I) Correlation of CXCR4/CX3CR1 ratio (ln(CXCR4 MFI/CX3CR1 MFI), mean of n = 6) and percentage changes from night to day (Table 2) for naive (N), central memory (CM), effector memory (EM), and effector (E) CD4+ (○) and CD8+ (●) T cells. *P < .05. A high ratio predicts a strong daytime decrease, whereas a low ratio predicts a daytime increase. In an additional experiment, whole blood was sampled at 21:00 h and cultured in the presence or absence of cortisol at normal daytime concentrations (20 μg/dL). CXCR4 expression in naive (CD45RA+CD62L+) (J) CD4+ and (K) CD8+ T cells is presented as fluorescence intensity. Cells were stained with control immunoglobulin (filled histograms) or anti-CXCR4 antibody (empty histograms). FACS profiles are shown (from a representative subject) for gated T cells before (left empty histogram, thin line) and after 3 hours of culture without (middle empty histogram, gray line) and with cortisol (right empty histogram, red line).
We assessed these markers additionally in blood samples collected in the evening (21:00 h) when cortisol concentrations are low compared with morning levels. Interestingly, expression was higher in the morning than evening for CD62L, CD49d, CXCR1, CXCR4, and CX3CR1 (all P < .05), whereas CCR7 expression did not differ between the time points. Daytime differences in CD62L and CXCR4 expression were most pronounced for naive and central memory CD4+ and CD8+ T cells, whereas daytime differences in CX3CR1 expression were most pronounced in effector memory and effector T cells (Figure 6E-H, CXCR4 and CX3CR1).
We incubated T cells drawn at 21:00 h in the evening (when endogenous cortisol is low, < 5 μg/dL) with cortisol at concentrations mimicking those in the morning (20 μg/dL). Cortisol induced a distinct linear increase in CXCR4 expression in both naive and central memory CD4+ and CD8+ T cells (P < .01; Figure 6J,K, naive T cells). The increase was detectable as early as one hour after the start of incubation and continued throughout the 6-hour monitoring period. It was comparable in all subpopulations of interest.
Discussion
We show here in humans a fine-tuned systems regulation of circadian rhythms in circulating T cell subpopulations that is controlled by the brain's major stress-hormone systems, that is, by release of glucocorticoids via the HPA system and release of catecholamines via the sympathetic nervous system. Distinct rhythms with nocturnal peaks were evident for naive and less pronounced for central memory and effector memory CD4+ and CD8+ T cells. In contrast, the rhythm of (terminally differentiated) effector CD8+ T cells peaked during daytime. The rhythms were not substantially affected by sleep (compared with a condition of 24-hour continuous wakefulness) and showed robust temporal associations to the circadian variations in plasma cortisol and epinephrine. T cell subsets with nocturnal peak rhythms were negatively correlated with cortisol, whereas effector CD8+ T cells were positively correlated with epinephrine. Administration of cortisol and epinephrine, at low doses mimicking in size endogenous increases in blood concentrations of these hormones, produced strikingly different effects on the T cell subpopulations. Cortisol with a delay of 3 hours decreased to the greatest extent naive T cells and to a lesser extent central memory and effector memory T cells, but left effector T cells unaffected. In contrast, epinephrine induced an immediate and highly selective increase in effector CD8+ T cells but did not affect any other subpopulation of interest. Greater sensitivity to suppressing influences of cortisol on T cell subset counts was associated with increased expression of CXCR4, a candidate molecule mediating effects of cortisol on T cell migration. Epinephrine-induced increases in effector CD8+ T cells on the other hand are probably conveyed by an increased expression of β-AR and CX3CR1 on these cells.
The pronounced global circadian variation in total numbers of circulating T cells, peaking during nighttime (at ∼ 02:00 h), is well documented.4,5,9 However, because T cells constitute a functionally highly heterogeneous population, assessment of total T cell counts masks the specific rhythms characterizing the various CD4+ and CD8+ T cell subpopulations. Rhythms of naive, central memory, and effector memory T cells were critically controlled by cortisol; that is, the greater the sensitivity of the respective subset to the reducing influence of cortisol, the higher was the rhythm amplitude in this subpopulation. Cortisol infusion at concentrations mimicking morning increases in cortisol under natural conditions reduced naive T cell counts by approximately 40%, an effect size remarkably similar to the peak-to-nadir difference in the circadian rhythm of this subpopulation. Previous studies showed that glucocorticoid administration and exercise-induced endogenous cortisol release suppress CD45RA+ and CD62L+ T cells to a greater extent than their CD45RA− and CD62L− counterparts.18,24 Thus, coexpression of both surface markers on naive T cells identifies this subpopulation as the one most sensitive to the reducing effects of cortisol. In combination, the data strongly support the notion that the marked circadian rhythm in naive T cells peaking during nighttime when cortisol reaches nadir levels is controlled by the brain via HPA glucocorticoid release.2 Surprisingly, however, graded sensitivity to cortisol among the T cell subpopulations did not relate to differences in glucocorticoid receptor expression, although cortisol is known to influence T cell migration through this receptor.25
In contrast to naive T cells, effector CD8+ T cells increased during daytime. The increase in cell numbers of this subset synchronized with the daytime increase in epinephrine reflects a particular sensitivity of these cells that are CD62L− to the influence of catecholamines.26 Accordingly, infusion of epinephrine at concentrations that mimicked normal day values produced an increase only in circulating effector CD8+ T cells but had no appreciable effect on the other T cell subsets. This finding concurs with previous studies in which exercise, mental stress, and administration of sympathomimetics selectively mobilized CD62L− and CCR7− T cells via β2-AR.19,24,27,28 As a rule, the mobilization of these CD62L− or CCR7− subsets is much more pronounced for CD45RA+ effector than CD45RA− effector memory T cells27 and also for CD8+ than CD4+ cells.19,24 In our experiments, β-AR density partly reflected the differences in catecholamine sensitivity with higher receptor numbers in CD8+ than CD4+ T cells and higher numbers in CD62L− than CD62L+ T cells. However, expression of β-AR in effector CD8+ T cells was comparable with that in effector memory CD8+ T cells, which were not affected by epinephrine injection. Therefore, the effect of epinephrine selectively increasing numbers of effector CD8+ T cells is not sufficiently explained by β-AR expression alone.
Fast recovery of T cell numbers in blood after concentrations of cortisol or epinephrine had normalized (after infusion of the hormones) suggests that the changes induced by these hormones represent a redistribution, rather than effects on production rate or apoptosis of the respective T cell subpopulation.10,12,29,30 Therefore, and because expression of neither GR nor β-AR could fully account for the differential responses to cortisol and epinephrine in the T cell subsets of interest, we concentrated on an analysis of adhesion molecule and chemokine receptor profiles regulating migratory behavior of these cells. Cortisol- and epinephrine-responsive T cell subpopulations greatly differ in their recirculating pattern and function with 2 extremes: Whereas naive T cells are critically involved in initiating adaptive immune responses in secondary lymphatic tissues, effector CD8+ T cells exert immediate, mainly cytotoxic effector functions in the periphery.16,17,31 Cortisol depletes T cells from peripheral blood and redistributes them to the bone marrow,11 whereas epinephrine mobilizes T cells from the so-called marginal pool, cells that roll along the endothelial layer, by demargination.12,13,32 Both facets of cell migration essentially depend on different selectins, integrins, chemokines, and respective receptors (ie, molecules regulating the attraction and adhesion of the cells to the vascular endothelium).33,34
Bone marrow homing of T cells is mainly regulated by the expression of CD62L, CD49d, and CXCR4.35 Of these molecules, expression of CXCR4 showed the strongest association with the cells' sensitivity to the redistributing effects of cortisol, with highest CXCR4 expression in naive T cells, intermediate in central memory, and low expression in effector memory and effector T cells.36 In functional studies, high CXCR4 expression on naive and central memory CD8+ T cells was revealed to be critical for the facilitated homing of these cells to bone marrow compared with effector subpopulations.35 Glucocorticoids enhance CXCR4 expression and signaling in T cells in vitro.37,38 Here we extend this observation by showing increased CXCR4 expression in the different T cell subsets of interest after incubation with low, physiologic concentrations of cortisol. This analysis, together with comparisons of blood sampled in the morning vs evening, points to a most pronounced cortisol-induced up-regulation of CXCR4 in naive and central memory CD4+ and CD8+ T cell subsets. Interestingly, not only CXCR4 but also bone marrow production of CXCL12, the ligand of CXCR4, follow a strong circadian rhythm with a peak during the active period, although influences of cortisol on this rhythm have not yet been elucidated.39 Overall data provide good evidence that up-regulation of CXCR4 substantially contributes to the cortisol-dependent control of circadian rhythms, especially in circulating naive and central memory T cells, leading to enhanced redistribution of the cells to bone marrow in the morning hours.
Catecholamines, in contrast to cortisol, act by recruiting immune cells with high expression of β2-AR from the marginal pool to the circulation, by reducing the adhesive properties of the cells.12 This process is fast occurring within minutes,12,40 probably through conformational changes of adhesion molecules. Of the CD8+ T cells with high β-AR density, effector CD8+ T cells were hallmarked by a profile comprising high expression of CD11a, CD11b, CXCR1, and especially of CX3CR1. Stress, exercise, and catecholamine injections are known to increase CD11a+, CD11b+,19,24,41 CXCR1+, and CX3CR1+ cells (S.D., J.B., and T.L., unpublished data, March 7, 2009). One probable candidate contributing to margination of effector T cells and their demargination by catecholamines appears to be CX3CR1: This molecule replaces function of selectin as adhesion molecule on CD62L− cells,31,34,42 it regulates conformational changes of integrins such as CD11a,33,42 and, like β-AR, it is a G protein-coupled receptor, enabling immediate suppressing influences of catecholamines on CX3CR1 signaling, eg, via cyclic adenosine monophosphate.43,44
In conclusion, our results point to a coordinate system regulation of circadian rhythms in circulating T cell subpopulations (Figure 7). This regulation presumably originates from the brain's major circadian oscillators (in the nucleus suprachiasmaticus and associated hypothalamic structures) and synchronizes immune functions via 2 separate pathways, the HPA axis and the sympathetic nervous system, leading eventually to rhythms differing in phase. Naive, central memory, and, less pronounced, effector memory T cells show clear circadian rhythms peaking during nighttime. The higher the rhythm's amplitude, the stronger in these subsets is the expression of CXCR4, making these cells sensitive to the daily rhythm in cortisol. In contrast, effector CD8+ T cells show a rhythm peaking during daytime. These cells with a CD62L− phenotype show high expression of β-AR, CD11a, and CD11b and, unlike the other T cell subsets, of CXCR1 and CX3CR1, making these cells particularly sensitive to the immediate demarginating influences of catecholamines. Given that circadian blood T cell counts run in parallel with content of the cells in lymph nodes,45,46 the release of naive T cells from bone marrow during nighttime might allow a facilitated homing of the cells to secondary lymphatic tissues, thus supporting the initiation of adaptive immune responses. On the other hand, the increase in circulating effector CD8+ T cells during daytime represents a circadian feature that is shared with other cytotoxic cell populations sensitive to catecholamines, like natural killer cells.5,26 It presumably acts to strengthen effector immune defense against tissue damage and infection encountered during the active phase.12,47,48 The temporally disparate activation and distribution of T cell subsets and associated immune functions by glucocorticoids and catecholamines may form part of a more basic mechanism optimizing the organism's immunologic adaptation not only to the changing demands of the circadian rest-activity cycle but also to stress in general.
Synopsis. Numbers of circulating T cell subpopulations show opposing rhythms with a nighttime peak (represented by naive CD4+ T cells; red cosine curve) or daytime peak (effector CD8+ T cells; blue cosine curve). Circadian rhythms peaking at night are controlled through the release of cortisol, which strongly increases during the early hours of daytime and via GR activation (with a delay of 3 hours) redistributes T cells from the circulation to bone marrow. Rhythms peaking during daytime are controlled by release of catecholamines, which is increased during daytime, and via β-AR activation leads to an immediate demargination of the cells from the vascular endothelium. Cortisol-sensitive T cells are characterized by high CXCR4 expression, whereas epinephrine-sensitive cells show highest CX3CR1 expression. The 2 chemokine receptors mark 2 distinct T cell populations with only few double-positive cells (conture plots for CXCR4 and CX3CR1 expression on CD4+ [top] and CD8+ T cells [bottom]). CXCR4 mediates homing of T cells to bone marrow with cortisol supporting this traffic by up-regulating CXCR4. Available data on CX3CR1 tempt to speculate that this molecule facilitates margination of T cells to vascular endothelium, with epinephrine inducing demargination by suppressing adhesive fractalkine signaling.
Synopsis. Numbers of circulating T cell subpopulations show opposing rhythms with a nighttime peak (represented by naive CD4+ T cells; red cosine curve) or daytime peak (effector CD8+ T cells; blue cosine curve). Circadian rhythms peaking at night are controlled through the release of cortisol, which strongly increases during the early hours of daytime and via GR activation (with a delay of 3 hours) redistributes T cells from the circulation to bone marrow. Rhythms peaking during daytime are controlled by release of catecholamines, which is increased during daytime, and via β-AR activation leads to an immediate demargination of the cells from the vascular endothelium. Cortisol-sensitive T cells are characterized by high CXCR4 expression, whereas epinephrine-sensitive cells show highest CX3CR1 expression. The 2 chemokine receptors mark 2 distinct T cell populations with only few double-positive cells (conture plots for CXCR4 and CX3CR1 expression on CD4+ [top] and CD8+ T cells [bottom]). CXCR4 mediates homing of T cells to bone marrow with cortisol supporting this traffic by up-regulating CXCR4. Available data on CX3CR1 tempt to speculate that this molecule facilitates margination of T cells to vascular endothelium, with epinephrine inducing demargination by suppressing adhesive fractalkine signaling.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank C. Otten, A. Otterbein, T. Kriesen, and E. Böschen for technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (grant SFB 654: Plasticity and Sleep) who had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: S.D., C.B., D.H., J.B., and T.L. designed the study; S.D., C.B., D.H., and T.L. performed experiments and collected data; S.D., J.B., and T.L. analyzed and interpreted data and performed statistical analyses; and S.D., C.B., J.W., J.B., and T.L. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tanja Lange, Department of Neuroendocrinology, University of Lübeck, Ratzeburger Allee 160, Haus 23a, 23538 Lübeck, Germany; e-mail: lange@kfg.uni-luebeck.de.



![Figure 7. Synopsis. Numbers of circulating T cell subpopulations show opposing rhythms with a nighttime peak (represented by naive CD4+ T cells; red cosine curve) or daytime peak (effector CD8+ T cells; blue cosine curve). Circadian rhythms peaking at night are controlled through the release of cortisol, which strongly increases during the early hours of daytime and via GR activation (with a delay of 3 hours) redistributes T cells from the circulation to bone marrow. Rhythms peaking during daytime are controlled by release of catecholamines, which is increased during daytime, and via β-AR activation leads to an immediate demargination of the cells from the vascular endothelium. Cortisol-sensitive T cells are characterized by high CXCR4 expression, whereas epinephrine-sensitive cells show highest CX3CR1 expression. The 2 chemokine receptors mark 2 distinct T cell populations with only few double-positive cells (conture plots for CXCR4 and CX3CR1 expression on CD4+ [top] and CD8+ T cells [bottom]). CXCR4 mediates homing of T cells to bone marrow with cortisol supporting this traffic by up-regulating CXCR4. Available data on CX3CR1 tempt to speculate that this molecule facilitates margination of T cells to vascular endothelium, with epinephrine inducing demargination by suppressing adhesive fractalkine signaling.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/21/10.1182_blood-2008-11-190769/6/m_zh89990935940007.jpeg?Expires=1767722507&Signature=JVT9TVCSgiRem~pZY0-yY~GojjAzumCxbgVYBkZEfU22wOlzCBkvOLYjOFptj7FcqVhhtjSAlHbMoZ7DUY3cVpk~nmpLMu3kQ2--Pc2RVjM4swSAAqCOEXTr4RRsAwVcraD-dhZSwC2IBppYSKvdGxxNnQU7odMLNT-NHqRYW9qcnt-3F5H9dQxXpicc01xbPuYsXuhQzvA8JXg6~zIGRAw0X4hIP1ILcJXLGdnQvB5RV24oQRhB2Z33QZtNIy22WBR8WNXwi8-GB42xw5JlTeYhEC79Ih3FxH2lOP1AYvAwepprWDGzPReovMDDYmQfbT4lo5BmxP99oaONyEbyGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal