Abstract
There have been no prior large population-based studies focusing on cutaneous lymphomas (CL) in the United States. Using the Surveillance, Epidemiology and End Results (SEER) program data, we analyzed age-adjusted CL incidence rates (IRs) and survival rates by sex and race/ethnicity. There were 3884 CLs diagnosed during 2001-2005. Cutaneous T-cell lymphomas (CTCLs) accounted for 71% (age-adjusted incidence rate [IR] = 7.7/1 000 000 person-years), whereas cutaneous B-cell lymphomas(CBCLs) accounted for 29% (IR = 3.1/1 000 000 person-years). Males had a statistically significant higher IR of CL than females (14.0 vs 8.2/1 000 000 person-years, respectively; male-female IR ratio [M/F IRR] = 1.72; P < .001). CL IRs were highest among blacks and non-Hispanic whites (both 11.5/1 000 000 person-years), followed by Hispanic whites (7.9) and Asian/Pacific Islanders (7.1). The CTCL IR was highest among blacks (10.0/1 000 000 person-years), whereas the CBCL IR was highest among non-Hispanic whites (3.5). Over the past 25 years, the CL IR increased from 5.0/1 000 000 person-years during 1980-1982 to 14.3 during 2001-2003. During 2004-2005, the CL IR was 12.7. This recent apparent change could be incomplete case ascertainment or potential leveling off of IRs. CLs rates vary markedly by race and sex, supporting the notion that they represent distinct disease entities.
Introduction
Primary cutaneous lymphomas (CL) represent 19% of extranodal non-Hodgkin lymphomas (NHLs) and are a diverse group of lymphoid neoplasms manifesting heterogeneous clinical, histologic, immunophenotypic, cytogenetic, and molecular features. Given their rarity and heterogeneity, CLs present diagnostic and therapeutic challenges. CLs have unique clinical features. Some lymphomas such as mycosis fungoides (MF) present only in the skin and are never primary in the lymph nodes or other extranodal sites. In contrast, some primary CLs histologically resemble their counterparts in the lymph nodes, but differ in terms of phenotype, clinical behavior, and prognosis, suggesting that they represent distinct entities.1 Because of their unique features and fundamental differences with noncutaneous sites, it is important to study the epidemiology of CLs. Although the epidemiology of MF has been reported in multiple case series, only a few population-based studies have been conducted to date.2-4 Furthermore, the epidemiology of other cutaneous natural killer (NK)/T-cell and B-cell lymphomas overall has been very limited or unknown.5-7
The classification of primary CLs has evolved over the past 50 years, and in 2005 they were categorized according to the World Health Organization (WHO)–European Organization for Research and Treatment of Cancer (EORTC) joint classification.1,6 The emphasis of this classification is on the definition of disease entities, and cell lineage is the starting point in the classification. This consensus classification takes into account clinical behavior as well as the distinct histologic and molecular genetic features compared with nodal counterparts,8 and it includes 3 main categories based on the cell of origin: cutaneous mature T-cell and/or NK-cell lymphoma (CTCL), cutaneous B-cell lymphoma (CBCL), and immature hematologic malignancies.2
CTCLs are the most common CL, and they include mycosis fungoides (MF), Sézary syndrome (SS), cutaneous CD30+ T-cell lymphoproliferative disorders, and primary cutaneous peripheral T-cell lymphoma. MF is the most common type and is characterized by a proliferation of small to medium-sized T lymphocytes with cerebriform nuclei.1 Risk factors for MF include advanced age, black race, and male sex.4 Although infectious agents9-11 and environmental exposures12 have been studied, the etiology of MF remains unknown. The CD30+ lymphoproliferative disorders are characterized by expression of the cell-surface receptor CD30+, which is a marker of activated T cells and a member of the tumor necrosis factor superfamily.13 Cutaneous CD30+ lymphoproliferative disorders include both lymphomatoid papulosis, which is a chronic recurrent lymphoproliferative skin disease, and primary cutaneous anaplastic large-cell lymphoma, which is a low-grade malignancy. Cutaneous peripheral T-cell lymphoma represents a heterogeneous group of lymphomas that do not fit into any of the better defined CTCL subtypes.1,6
CBCLs are much rarer than CTCLs. In the 2005 WHO-EORTC classification, there are 3 main subtypes of primary cutaneous B-cell lymphomas: marginal zone B-cell lymphoma, follicle center lymphoma, and diffuse large B-cell lymphoma.1,6 Primary cutaneous diffuse large B-cell lymphoma (DLBCL)–leg type typically presents with tumors mostly restricted to the legs and has a poorer prognosis (5-year survival rate of 70%) compared with patients with other types of CBCLs.14
Literature on the CLs has been limited because of the rarity and inability to study large numbers of patients. Therefore, the overall epidemiology of CL subtypes has not been well investigated using population-based data. Because the etiology of CL subtypes remains largely unknown, comparison of incidence rates and patterns for specific subtypes may elucidate important clues for future studies. In this study, we conducted a comprehensive analysis of CL incidence rates and relative survival rates in the US population-based Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute (NCI) according to patient demographic characteristics and histologic types, using the 2005 WHO-EORTC classification.1,6
Methods
We analyzed incidence and survival data for cutaneous lymphoma cases diagnosed among residents of 16 SEER program registries during 2001-2005.15 The 16 registries include 8 states (Connecticut, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, and Utah), greater California, rural Georgia, and 6 metropolitan areas (Atlanta, Detroit, Los Angeles, San Francisco–Oakland, San Jose–Monterrey, and Seattle–Puget Sound). These registries represent approximately 26% of the US population, including 25% of whites, 23% of blacks, 53% of Asian/Pacific Islanders 15 . Data for American Indians/Alaska Natives, the Alaska registry, and cases with race coded as “unknown” were excluded.
The SEER cancer registry personnel include highly trained abstractors and coders who review medical records, including pathology reports. Quality control efforts include review of case-finding, reabstracting, and recoding. Registry data are submitted electronically without personal identifiers to the NCI twice per year. SEER records the primary site of the tumor and does not collect data regarding metastatic sites.
Cutaneous lymphoma cases were identified using the World Health Organization's International Classification of Diseases for Oncology, 3rd edition (ICD-O-3), codes for primary cutaneous (anatomic site codes C44.0-44.9) non-Hodgkin (morphology codes 9670-9728 and 9827, excluding 9675) lymphomas.16 Because several lymphoma codes were added to the ICD-O in the third edition, which SEER first used for cases diagnosed during 2001, we included cases diagnosed during 2001-2005. We categorized individual 4-digit histology codes into major histologic groups according to the criteria specified in the latest 2005 WHO-EORTC Classification of cutaneous lymphomas.1,6 The specific morphology codes that were used are shown in Table 1. Tumors classified as malignant lymphoma not otherwise specified (NOS) ICD-O-3 (9590), non-Hodgkin NOS (9591), mixed small and large cell, diffuse lymphoma (9675), and precursor T-cell lymphoblastic lymphoma (9729) were excluded (n = 237, 5.5% of cases). CTCLs classified as B-cell immunophenotype, CBCLs classified as T-cell immunophenotype, and all tumors classified as null cell immunophenotype were also excluded (n = 180, 4.2% of cases). Anatomic sites were tabulated according to ICD-O-3 topography codes: head and neck (skin of the lip [C44.0], eyelid [C44.1], external ear [C44.2], unspecified parts of the face [C44.3], and scalp and neck [44.4]); trunk (C44.5); upper limb and shoulder (C44.6); lower limb and hip (C44.7); multisite tumors (C44.8); and tumors classified as “not otherwise specified” (C44.9). The category primary cutaneous diffuse large B-cell lymphoma, leg (pcDLBCL-leg) was based on the specific histologic codes (9680, 9684) and the topography code for skin of the lower limbs (C44.7).
Cutaneous lymphomas diagnosed during 2001-2005 in the 16 SEER program registries by histologic type and sex
| . | ICD-O-3*codes . | Total . | Frequency by . | Male . | Female . | M/FIRR . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases . | Frequency, % . | Cell type, % . | Rate† . | Cases . | Rate . | Cases . | Rate . | ||||
| Total | 9670-9728, 9827 excluding 9675, 9721 | 3884 | 100.0 | 10.7 | 2285 | 14.0 | 1599 | 8.2 | 1.72 | < .001 | |
| Mature T-cell and NK-cell neoplasms (CTCLs) | 2769 | 71.3 | 100.0 | 7.7 | 1626 | 10.0 | 1143 | 5.9 | 1.70 | < .001 | |
| Mycosis fungoides (MF) | 9700 | 1487 | 38.3 | 53.7 | 4.1 | 868 | 5.3 | 619 | 3.2 | 1.66 | < .001 |
| Sézary syndrome (SS) | 9701 | 33 | 0.8 | 1.2 | 0.1 | 20 | 0.1 | 13 | 0.1 | 2.11 | .05 |
| CD30+ T-cell lymphoproliferative disorders of the skin (CD30+ LPD) | 9714, 9718 | 396 | 10.2 | 14.3 | 1.1 | 233 | 1.5 | 163 | 0.8 | 1.73 | < .001 |
| Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) | 9708 | 23 | 0.6 | 0.8 | 0.1 | 6 | ∼‡ | 17 | 0.1 | ∼ | ∼ |
| Primary cutaneous peripheral T-cell lymphoma (pcPTL) | 9702, 9709 | 809 | 20.8 | 29.2 | 2.2 | 485 | 3.0 | 324 | 1.7 | 1.82 | < .001 |
| Extranodal NK/T-cell lymphoma, nasal type | 9719 | 12 | 0.3 | 0.4 | 0.0 | 9 | ∼ | 3 | ∼ | ∼ | ∼ |
| Adult T-cell leukemia/lymphoma (HTLV-1 pos) | 9827 | 2 | 0.1 | 0.1 | ∼ | 2 | ∼ | 0 | ∼ | ∼ | ∼ |
| Angioimmunoblastic T-cell lymphoma | 9705 | 7 | 0.2 | 0.3 | ∼ | 3 | ∼ | 4 | ∼ | ∼ | ∼ |
| Mature B-cell neoplasms | 1105 | 28.5 | 100.0 | 3.1 | 651 | 4.0 | 454 | 2.3 | 1.76 | < .001 | |
| Cutaneous marginal zone B-cell lymphoma (pcMZL) | 9699 | 274 | 7.1 | 24.8 | 0.8 | 161 | 1.0 | 113 | 0.6 | 1.28 | .001 |
| Cutaneous follicle center lymphoma (pcFCL) | 9690, 9691, 9695, 9698 | 331 | 8.5 | 30.0 | 0.9 | 200 | 1.2 | 131 | 0.7 | 1.84 | < .001 |
| Cutaneous diffuse large B-cell lymphoma (pcDLBCL) | 9680, 9684 | 443 | 11.4 | 40.1 | 1.2 | 252 | 1.6 | 191 | 0.9 | 1.70 | < .001 |
| pcDLBCL-leg | 9680, 9684 | 101 | 2.6 | 9.1 | 0.3 | 45 | 0.3 | 56 | 0.3 | 1.19 | .44 |
| pcDLBCL-other | 9680, 9684 | 342 | 8.8 | 31.0 | 1.0 | 207 | 1.3 | 135 | 0.7 | 1.90 | < .001 |
| Extracutaneous lymphomas involving the skin | 9670, 9671, 9673, 9687 | 57 | 1.5 | 5.2 | 0.2 | 38 | 0.2 | 19 | 0.1 | 2.55 | .001 |
| Small lymphocytic lymphoma | 9670 | 32 | 0.8 | 2.9 | 0.1 | 21 | 0.1 | 11 | 0.1 | 2.44 | .024 |
| Lymphoplasmacytic lymphoma | 9671 | 9 | 0.2 | 0.8 | ∼ | 4 | ∼ | 5 | ∼ | ∼ | ∼ |
| Mantle cell lymphoma | 9673 | 12 | 0.3 | 1.1 | 0.0 | 10 | 0.1 | 2 | ∼ | ∼ | ∼ |
| Burkitt lymphoma | 9687 | 4 | 0.1 | 0.4 | ∼ | 3 | ∼ | 1 | ∼ | ∼ | ∼ |
| Immature hematologic neoplasms | 9727, 9728 | 10 | 0.3 | 100.0 | 0.0 | 8 | ∼ | 2 | ∼ | ∼ | ∼ |
| Blastic NK-cell lymphoma | 9727 | 6 | 0.2 | 60.0 | ∼ | 6 | ∼ | 0 | ∼ | ∼ | ∼ |
| Precursor B-cell lymphoblastic lymphoma | 9728 | 4 | 0.1 | 40.0 | ∼ | 2 | ∼ | 2 | ∼ | ∼ | ∼ |
| . | ICD-O-3*codes . | Total . | Frequency by . | Male . | Female . | M/FIRR . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases . | Frequency, % . | Cell type, % . | Rate† . | Cases . | Rate . | Cases . | Rate . | ||||
| Total | 9670-9728, 9827 excluding 9675, 9721 | 3884 | 100.0 | 10.7 | 2285 | 14.0 | 1599 | 8.2 | 1.72 | < .001 | |
| Mature T-cell and NK-cell neoplasms (CTCLs) | 2769 | 71.3 | 100.0 | 7.7 | 1626 | 10.0 | 1143 | 5.9 | 1.70 | < .001 | |
| Mycosis fungoides (MF) | 9700 | 1487 | 38.3 | 53.7 | 4.1 | 868 | 5.3 | 619 | 3.2 | 1.66 | < .001 |
| Sézary syndrome (SS) | 9701 | 33 | 0.8 | 1.2 | 0.1 | 20 | 0.1 | 13 | 0.1 | 2.11 | .05 |
| CD30+ T-cell lymphoproliferative disorders of the skin (CD30+ LPD) | 9714, 9718 | 396 | 10.2 | 14.3 | 1.1 | 233 | 1.5 | 163 | 0.8 | 1.73 | < .001 |
| Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) | 9708 | 23 | 0.6 | 0.8 | 0.1 | 6 | ∼‡ | 17 | 0.1 | ∼ | ∼ |
| Primary cutaneous peripheral T-cell lymphoma (pcPTL) | 9702, 9709 | 809 | 20.8 | 29.2 | 2.2 | 485 | 3.0 | 324 | 1.7 | 1.82 | < .001 |
| Extranodal NK/T-cell lymphoma, nasal type | 9719 | 12 | 0.3 | 0.4 | 0.0 | 9 | ∼ | 3 | ∼ | ∼ | ∼ |
| Adult T-cell leukemia/lymphoma (HTLV-1 pos) | 9827 | 2 | 0.1 | 0.1 | ∼ | 2 | ∼ | 0 | ∼ | ∼ | ∼ |
| Angioimmunoblastic T-cell lymphoma | 9705 | 7 | 0.2 | 0.3 | ∼ | 3 | ∼ | 4 | ∼ | ∼ | ∼ |
| Mature B-cell neoplasms | 1105 | 28.5 | 100.0 | 3.1 | 651 | 4.0 | 454 | 2.3 | 1.76 | < .001 | |
| Cutaneous marginal zone B-cell lymphoma (pcMZL) | 9699 | 274 | 7.1 | 24.8 | 0.8 | 161 | 1.0 | 113 | 0.6 | 1.28 | .001 |
| Cutaneous follicle center lymphoma (pcFCL) | 9690, 9691, 9695, 9698 | 331 | 8.5 | 30.0 | 0.9 | 200 | 1.2 | 131 | 0.7 | 1.84 | < .001 |
| Cutaneous diffuse large B-cell lymphoma (pcDLBCL) | 9680, 9684 | 443 | 11.4 | 40.1 | 1.2 | 252 | 1.6 | 191 | 0.9 | 1.70 | < .001 |
| pcDLBCL-leg | 9680, 9684 | 101 | 2.6 | 9.1 | 0.3 | 45 | 0.3 | 56 | 0.3 | 1.19 | .44 |
| pcDLBCL-other | 9680, 9684 | 342 | 8.8 | 31.0 | 1.0 | 207 | 1.3 | 135 | 0.7 | 1.90 | < .001 |
| Extracutaneous lymphomas involving the skin | 9670, 9671, 9673, 9687 | 57 | 1.5 | 5.2 | 0.2 | 38 | 0.2 | 19 | 0.1 | 2.55 | .001 |
| Small lymphocytic lymphoma | 9670 | 32 | 0.8 | 2.9 | 0.1 | 21 | 0.1 | 11 | 0.1 | 2.44 | .024 |
| Lymphoplasmacytic lymphoma | 9671 | 9 | 0.2 | 0.8 | ∼ | 4 | ∼ | 5 | ∼ | ∼ | ∼ |
| Mantle cell lymphoma | 9673 | 12 | 0.3 | 1.1 | 0.0 | 10 | 0.1 | 2 | ∼ | ∼ | ∼ |
| Burkitt lymphoma | 9687 | 4 | 0.1 | 0.4 | ∼ | 3 | ∼ | 1 | ∼ | ∼ | ∼ |
| Immature hematologic neoplasms | 9727, 9728 | 10 | 0.3 | 100.0 | 0.0 | 8 | ∼ | 2 | ∼ | ∼ | ∼ |
| Blastic NK-cell lymphoma | 9727 | 6 | 0.2 | 60.0 | ∼ | 6 | ∼ | 0 | ∼ | ∼ | ∼ |
| Precursor B-cell lymphoblastic lymphoma | 9728 | 4 | 0.1 | 40.0 | ∼ | 2 | ∼ | 2 | ∼ | ∼ | ∼ |
pc indicates primary cutaneous.
ICD-O-3 CODES 9699, 9708, 9718, 9719, 9727, and 9728 are new and were not included in ICD-O-2.
Rates are per 1 000 000 person-years and age-adjusted to the 2000 US standard population (19 age groups; Census P25-1130).
Statistic not shown, based on fewer than 10 cases.
Age-adjusted (2000 US standard) incidence rates (IRs) were calculated using the SEER*Stat software public use program version 6.4.4.17 Incidence rates were expressed as new cases per 1 000 000 person-years and were analyzed by age, sex, race, ethnicity, and year of diagnosis. Long-term temporal trend analyses were based on 1980-2005 data from the SEER 9 registries, and short-term trends are based on 1992-2005 data from the SEER 12 registries. Temporal trends and age-specific rates were plotted using a semilog scale, with a y-axis/x-axis ratio of 1 log cycle = 40 years, such that an angle of 10 degrees portrayed a change of 1% per year.18 We aggregated over years to derive more stable rate estimates. Our study represents a descriptive exploratory analysis. Should the reader wish to compare rates, the variance of an incidence rate can be approximated by dividing the rate (number of cases/1 000 000 person-years) squared by the number of cases on which the rate was based. Differences in rates and ratios of rates can be tested by calculating approximate confidence intervals according to Miettinen and Nurminen.19
Population-based age distribution at diagnosis (or density plots) were stratified by cutaneous lymphoma subtype and sex, as previously described.20 Density plots showed smoothed age distributions at diagnosis using a nonparametric (model-free) approach. The area under each density plot represented 100% of cutaneous lymphoma cases, where density × 100 = percentage.
Five-year relative survival rates for cases diagnosed during 1992-1999 in the 12 SEER registries and during 2000 in the 16 SEER registries combined were calculated using the actuarial method in SEER*Stat 6.4.4.17 Relative survival is defined as the ratio of the proportion of observed survivors in a cohort of patients to the proportion of expected survivors in a comparable cohort of the general population (http://srab.cancer.gov/survival/measures.html), thus representing survival in the absence of other causes of death. Only cases with a cutaneous lymphoma as the first primary cancer were included in the survival analysis. Patients with CL as a second cancer as well as cases with a subsequent cancer were excluded. Relative survival rates were available only for whites and blacks and were not yet available for Hispanics and Asian/Pacific Islanders. The period of survival was from the date of diagnosis to the date of last contact, death, or December 31, 2005.
Results
The number of cases, percentage distribution, and IR of CLs are shown according to histologic type in Table 1. In total, 3884 cases (IR 10.7/1 000 000 person-years) of CLs were diagnosed among residents of the 16 SEER registries during 2001-2005. Examination of extranodal lymphomas by primary site revealed that CLs accounted for 19% of cases and were the second most common form of extranodal NHL, after the GI lymphomas (27%). This trend was observed during the past 3 decades 1976-1985 through 1996-2005 in the SEER 9 registries (data not shown).
CTCL was the most common CL subtype accounting for 2769 or 71% of cases (IR = 7.7/1 000 000 person-years; Table 1). MF was the most common CTCL subtype, comprising 54% of the CTCLs (IR 4.1/1 000 000 person-years), followed by cutaneous peripheral T-cell lymphoma (29%) and cutaneous CD30+ T-cell lymphoproliferative disorders (14%). Together, these 3 histologic types represented 97% of all CTCL cases. Other CTCLs included 23 cases of subcutaneous panniculitis-like T-cell lymphoma and 12 cases of NK/T-cell lymphoma-nasal type, both new disease entities included in the 2001 WHO classification. In contrast to CTCLs, CBCLs accounted for 1105 cases or 29% of all CLs (IR 3.1/1 000 000 person-years). The most common CBCL subtypes were (40%) primary cutaneous diffuse large-B cell lymphoma (pcDLBCL; IR 1.2/1 000 000 person-years) and cutaneous follicle center lymphoma (30%; IR 0.9/1 000 000 person-years), followed by cutaneous marginal zone B-cell lymphoma (25%; IR 0.8/1 000 000 person-years).
Overall, males had a statistically significant higher IR of CLs than females (14.0 vs 8.2/1 000 000 person-years, respectively; male-female IR ratio [M/F IRR] = 1.72; P < .001; Table 1). The M/F IRRs ranged between 1.28 to 2.55 among the various T-cell and B-cell lymphoma subtypes and were significantly elevated except for Sézary syndrome (M/F IRR = 2.11, P = .05, based on small numbers) and pcDLBCL-leg type (M/F IRR = 1.19, P = .44).
Blacks and non-Hispanic whites had the highest IR for CL overall (11.5/1 000 000 person-years), followed by Hispanic whites (7.9) and Asian/Pacific Islanders (7.1; Table 2). The latter 2 IRs were statistically significantly lower than the rate among non-Hispanic whites. CL IR among Asian/Pacific Islanders was lower than the other racial groups across all SEER registries, ranging from 4.8/1 000 000 person-years (95% CI = 2.2-9.0; n = 12) in New Jersey to 10.0 (95% CI = 7.2-13.4; n = 45) in San Francisco–Oakland.
Cutaneous lymphomas diagnosed during 2001-2005 in the 16 SEER program registries by histologic type and race/ethnicity
| . | Total . | Rate* . | Non-Hispanic white . | Black . | Hispanic white . | Asian/PacificIslander . | B:NHW . | HW:NHW . | A/PI:NHW . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases . | Rate . | Cases . | Rate . | Cases . | Rate . | Cases . | Rate . | IRR . | P . | IRR . | P . | IRR . | P . | |||
| Total | 3874§ | 10.7 | 2919 | 11.5 | 400 | 11.5 | 328 | 7.9 | 227 | 7.1 | 0.99 | .507 | 0.69 | < .001 | 0.61 | < .0001 |
| Mature T-cell and NK-cell neoplasms (CTCLs) | 2769 | 7.7 | 2032 | 8.1 | 349 | 10.0 | 219 | 5.1 | 169 | 5.1 | 1.24 | .001 | 0.64 | < .001 | 0.64 | < .0001 |
| Mycosis fungoides (MF) | 1487 | 4.1 | 1033 | 4.1 | 212 | 5.9 | 132 | 2.9 | 110 | 3.3 | 1.44 | < .001 | 0.71 | .001 | 0.80 | .0251 |
| Sézary syndrome (SS) | 33 | 0.1 | 28 | 0.1 | 1 | ∼‖ | 4 | ∼ | 0 | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ |
| CD30+ T-cell lymphoproliferative disorders of the skin (CD30+ LPD) | 396 | 1.1 | 315 | 1.3 | 31 | 0.9 | 29 | 0.7 | 21 | 0.7 | 0.71 | .076 | 0.57 | .004 | 0.52 | .002 |
| Subcutaneous panniculitis-like T-cell lymphoma (SCPTL) | 23 | 0.1 | 11 | 0.0 | 6 | ∼ | 0 | ∼ | 6 | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ |
| Primary cutaneous peripheral T-cell lymphoma (pcPTL) | 809 | 2.2 | 634 | 2.5 | 96 | 2.8 | 50 | 1.3 | 29 | 1.0 | 1.14 | .285 | 0.52 | < .001 | 0.38 | < .0001 |
| Other‡ | 21 | 0.1 | 11 | 0.0 | 3 | ∼ | 5 | ∼ | 2 | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ |
| Mature B-cell neoplasms (CBCLs) | 1105 | 3.1 | 887 | 3.5 | 51 | 1.5 | 109 | 2.8 | 58 | 1.9 | 0.43 | < .001 | 0.80 | .038 | 0.55 | < .0001 |
| Cutaneous marginal zone B-cell lymphoma (pcMZL) | 274 | 0.8 | 219 | 0.9 | 12 | 0.3 | 31 | 0.7 | 12 | 0.4 | 0.37 | .001 | 0.83 | .381 | 0.42 | .0016 |
| Cutaneous follicle center lymphoma (pcFCL) | 331 | 0.9 | 281 | 1.1 | 11 | 0.4 | 29 | 0.7 | 10 | 0.3 | 0.33 | < .001 | 0.62 | .015 | 0.29 | < .0001 |
| Cutaneous diffuse large B-cell lymphoma (pcDLBCL) | 443 | 1.2 | 347 | 1.3 | 23 | 0.7 | 42 | 1.2 | 31 | 1.1 | 0.50 | .001 | 0.89 | .525 | 0.80 | .2579 |
| pcDLBCL-leg | 101 | 0.3 | 70 | 0.3 | 4 | ∼ | 12 | 0.4 | 15 | 0.5 | 0.49 | .186 | 1.62 | .194 | 2.10 | .0263 |
| pcDLBCL-other | 342 | 1.0 | 277 | 1.1 | 19 | 0.5 | 30 | 0.8 | 16 | 0.5 | 0.51 | .003 | 0.71 | .103 | 0.49 | .0029 |
| Extracutaneous lymphomas involving the skin§ | 57 | 0.2 | 40 | 0.2 | 5 | ∼ | 7 | ∼ | 5 | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ |
| . | Total . | Rate* . | Non-Hispanic white . | Black . | Hispanic white . | Asian/PacificIslander . | B:NHW . | HW:NHW . | A/PI:NHW . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases . | Rate . | Cases . | Rate . | Cases . | Rate . | Cases . | Rate . | IRR . | P . | IRR . | P . | IRR . | P . | |||
| Total | 3874§ | 10.7 | 2919 | 11.5 | 400 | 11.5 | 328 | 7.9 | 227 | 7.1 | 0.99 | .507 | 0.69 | < .001 | 0.61 | < .0001 |
| Mature T-cell and NK-cell neoplasms (CTCLs) | 2769 | 7.7 | 2032 | 8.1 | 349 | 10.0 | 219 | 5.1 | 169 | 5.1 | 1.24 | .001 | 0.64 | < .001 | 0.64 | < .0001 |
| Mycosis fungoides (MF) | 1487 | 4.1 | 1033 | 4.1 | 212 | 5.9 | 132 | 2.9 | 110 | 3.3 | 1.44 | < .001 | 0.71 | .001 | 0.80 | .0251 |
| Sézary syndrome (SS) | 33 | 0.1 | 28 | 0.1 | 1 | ∼‖ | 4 | ∼ | 0 | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ |
| CD30+ T-cell lymphoproliferative disorders of the skin (CD30+ LPD) | 396 | 1.1 | 315 | 1.3 | 31 | 0.9 | 29 | 0.7 | 21 | 0.7 | 0.71 | .076 | 0.57 | .004 | 0.52 | .002 |
| Subcutaneous panniculitis-like T-cell lymphoma (SCPTL) | 23 | 0.1 | 11 | 0.0 | 6 | ∼ | 0 | ∼ | 6 | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ |
| Primary cutaneous peripheral T-cell lymphoma (pcPTL) | 809 | 2.2 | 634 | 2.5 | 96 | 2.8 | 50 | 1.3 | 29 | 1.0 | 1.14 | .285 | 0.52 | < .001 | 0.38 | < .0001 |
| Other‡ | 21 | 0.1 | 11 | 0.0 | 3 | ∼ | 5 | ∼ | 2 | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ |
| Mature B-cell neoplasms (CBCLs) | 1105 | 3.1 | 887 | 3.5 | 51 | 1.5 | 109 | 2.8 | 58 | 1.9 | 0.43 | < .001 | 0.80 | .038 | 0.55 | < .0001 |
| Cutaneous marginal zone B-cell lymphoma (pcMZL) | 274 | 0.8 | 219 | 0.9 | 12 | 0.3 | 31 | 0.7 | 12 | 0.4 | 0.37 | .001 | 0.83 | .381 | 0.42 | .0016 |
| Cutaneous follicle center lymphoma (pcFCL) | 331 | 0.9 | 281 | 1.1 | 11 | 0.4 | 29 | 0.7 | 10 | 0.3 | 0.33 | < .001 | 0.62 | .015 | 0.29 | < .0001 |
| Cutaneous diffuse large B-cell lymphoma (pcDLBCL) | 443 | 1.2 | 347 | 1.3 | 23 | 0.7 | 42 | 1.2 | 31 | 1.1 | 0.50 | .001 | 0.89 | .525 | 0.80 | .2579 |
| pcDLBCL-leg | 101 | 0.3 | 70 | 0.3 | 4 | ∼ | 12 | 0.4 | 15 | 0.5 | 0.49 | .186 | 1.62 | .194 | 2.10 | .0263 |
| pcDLBCL-other | 342 | 1.0 | 277 | 1.1 | 19 | 0.5 | 30 | 0.8 | 16 | 0.5 | 0.51 | .003 | 0.71 | .103 | 0.49 | .0029 |
| Extracutaneous lymphomas involving the skin§ | 57 | 0.2 | 40 | 0.2 | 5 | ∼ | 7 | ∼ | 5 | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ |
IRR indicates incidence rate ratio; and pc, primary cutaneous.
Rates are per 1 000 000 and age-adjusted to the 2000 US standard population (19 age groups; Census P25-1130).
Other includes extranodal NK/T-cell lymphoma, adult T-cell leukemia/lymphoma, and angioimmunoblastic T-cell lymphoma.
This group includes small lymphocytic lymphoma, lymphoplasmacytic lymphoma, mantle cell lymphoma, and Burkitt lymphoma. Immature hematologic neoplasms were excluded (n=10) due to small numbers.
Statistic not shown, based on fewer than 10 cases.
The highest CTCL IR was among blacks (IR = 10.0/1 000 000 person-years) followed by non-Hispanic whites (8.1), and Asian/Pacific Islanders and Hispanic whites (both 5.1; Table 2). The M/F rate for mycosis fungoides among blacks was 1.44 times the IR among non-Hispanic whites (P < .001), whereas the cutaneous peripheral T-cell lymphoma IRs among blacks and non-Hispanic whites were similar (2.8 and 2.5/1 000 000 person-years, respectively). In contrast to other CTCL subtypes, the IR for cutaneous CD30+ T-cell lymphoproliferative disorders was highest among non-Hispanic whites (1.3/1 000 000 person-years). The IRs of MF, cutaneous CD30+ T-cell lymphoproliferative disorders, and cutaneous peripheral T-cell lymphoma were each significantly lower among Hispanic whites and Asian/Pacific Islanders than non-Hispanic whites.
In contrast to CTCLs, non-Hispanic whites had the highest IR for CBCLs (3.5/1 000 000 person-years), followed by Hispanic whites (2.8), Asian/Pacific Islanders (1.9), and blacks, who had the lowest IR (1.5; Table 2). Hispanic and non-Hispanic whites had similar IRs of cutaneous marginal B-cell lymphoma (0.7 and 0.9/1 000 000 person-years, respectively, P = .38). The IRs for CBCL and most subtypes among blacks were half those among non-Hispanic whites.
We found that the IR of CL in metropolitan counties (11.5/1 000 000 person-years) was higher than nonmetropolitan counties (8.9/1 000 000 person-years, P < .001; data not shown). The IR of CTCL was also higher in metropolitan counties (8.3/1 000 000 person-years) than nonmetropolitan counties (6.1/1 000 000 person-years; P < .001). However, IRs of CBCL were similar in metropolitan (3.2/1 000 000 person-years) and nonmetropolitan counties (2.8/1 000 000 person-years; P = .222).
Age-specific incidence and age distribution at diagnosis
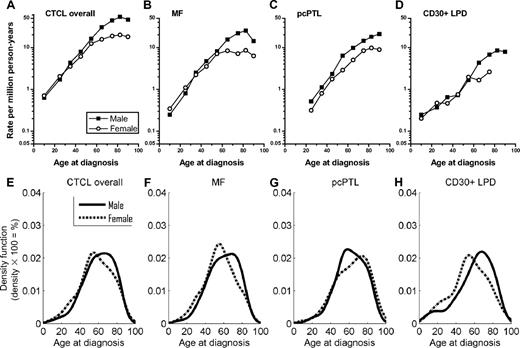
CTCL rates overall increased exponentially with age peaking around 80 years (Figure 1A); this pattern was generally apparent for MF, cutaneous peripheral T-cell lymphoma, and cutaneous CD30+ T-cell lymphoproliferative disorders (Figure 1B-D). Rates for CTCL overall, MF, cutaneous CD30+ T-cell lymphoproliferative disorders, and cutaneous peripheral T-cell lymphoma were similar among males and females at ages younger than 30 years, but notably higher among males than females at older ages; and by age 60 years, the IR among males was double that among females (Figure 1A-D). In addition, M/F IRRs increased from 0.95 in CTCL patients younger than 20 years to 2.66 in those older than 85 years (data not shown). Age-specific rates for CTCL reflected nonparametric age distributions at diagnosis (Figure 1E-H), with early- and late-onset peak frequencies near ages 50 to 60 years and 70 to 80 years, respectively. With the exception of cutaneous peripheral T-cell lymphoma, women demonstrated predominant early-onset cancer populations, whereas men had more prominent late-onset disease. In cutaneous peripheral T-cell lymphoma, men demonstrated predominant early-onset cancer populations and women had prominent late-onset disease.
Age-specific cutaneous T-cell lymphoma incidence rates and age distributions at diagnosis from 2001-2005 in the 16 SEER registries by sex. (A-D) Rates per 1 million person-years are shown for (A) all cutaneous T-cell lymphomas (CTCL) combined, (B) mycosis fungoides (MF), (C) primary cutaneous peripheral T-cell lymphoma (pcPTL), and (D) cutaneous CD30+ lymphoproliferative disorders (CD30+ LPD). (E-H) Density plots are shown for (E) all CTCL combined, (F) MF, (G) pcPTL, and (H) CD30+ LPD. Area under the curve includes 100% of cases. The vertical axis for each density plot represents smoothed estimates of the density of patients (density × 100 = %).
Age-specific cutaneous T-cell lymphoma incidence rates and age distributions at diagnosis from 2001-2005 in the 16 SEER registries by sex. (A-D) Rates per 1 million person-years are shown for (A) all cutaneous T-cell lymphomas (CTCL) combined, (B) mycosis fungoides (MF), (C) primary cutaneous peripheral T-cell lymphoma (pcPTL), and (D) cutaneous CD30+ lymphoproliferative disorders (CD30+ LPD). (E-H) Density plots are shown for (E) all CTCL combined, (F) MF, (G) pcPTL, and (H) CD30+ LPD. Area under the curve includes 100% of cases. The vertical axis for each density plot represents smoothed estimates of the density of patients (density × 100 = %).
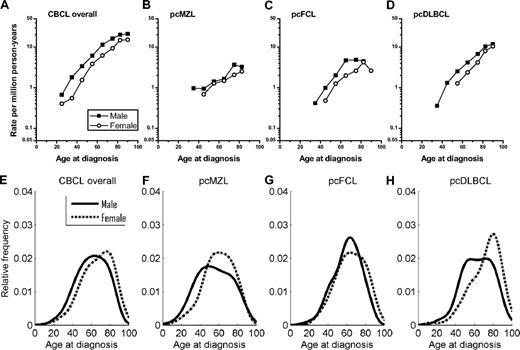
Age-specific rates for CBCL overall and the subtypes increased exponentially among both males and females, with males consistently having higher IR than females (Figure 2A-D). The male predominance decreased with age for CBCL overall and for pcDLBCL, in contrast to increasing male to female differences for the CTCLs (Figure 1A-D). M/F IRRs decreased from 1.66 in CBCL patients 20 to 29 years to 1.39 in patients older than 85 years (data not shown). Indeed, in contrast to CTCLs, CBCL density plots among women generally demonstrated dominant late-onset cutaneous lymphomas, whereas men had prominent early-onset disease (Figure 2E-H).
Age-specific cutaneous B-cell lymphoma incidence rates and age distributions at diagnosis from 2001-2005 in the 16 SEER registries by sex. (A-D) Rates per 1 million person-years are shown for (A) all cutaneous B-cell lymphomas (CBCL) combined, (B) primary cutaneous marginal zone lymphoma (pcMZL), (C) primary cutaneous follicle center lymphoma (pcFCL), and (D) primary cutaneous diffuse large B-cell lymphoma (pcDLBCL). (E-H) Density plots are shown for (E) all CBCL combined, (F) pcMZL, (G) pcFCL, and (H) pcDLBCL. Area under the curve includes 100% of cases. The vertical axis for each density plot represents smoothed estimates of the density of patients (density × 100 = %).
Age-specific cutaneous B-cell lymphoma incidence rates and age distributions at diagnosis from 2001-2005 in the 16 SEER registries by sex. (A-D) Rates per 1 million person-years are shown for (A) all cutaneous B-cell lymphomas (CBCL) combined, (B) primary cutaneous marginal zone lymphoma (pcMZL), (C) primary cutaneous follicle center lymphoma (pcFCL), and (D) primary cutaneous diffuse large B-cell lymphoma (pcDLBCL). (E-H) Density plots are shown for (E) all CBCL combined, (F) pcMZL, (G) pcFCL, and (H) pcDLBCL. Area under the curve includes 100% of cases. The vertical axis for each density plot represents smoothed estimates of the density of patients (density × 100 = %).
Temporal trends
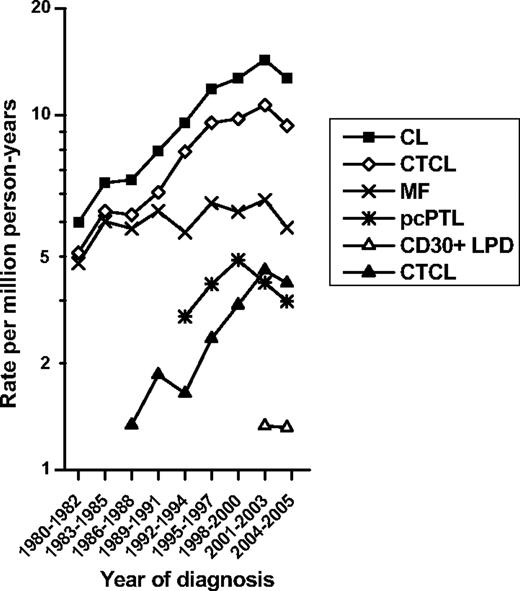
During 1980-2005 in the original 9 SEER areas, 5908 cases of CL were diagnosed. Over the past 25 years, CL IRs increased from 5.0/1 000 000 person-years during 1980-1982 to 14.3 during 2001-2003. However, during the period of 2004-2005 the IR of CL was 12.7/1 000 000 (Figure 3). This finding was inconsistent with the previous trend. The IRs for CTCL and CBCL rose before peaking during 2001-2003. In contrast, IRs for the major subtypes did not change greatly.
Cutaneous lymphoma temporal trends during 1980-1982 through 2004-2005. Data are shown by year of diagnosis in the 9 SEER program registries for all CL, CTCL, MF, pcPTL, and CD30+ LPD, and CBCL.
Cutaneous lymphoma temporal trends during 1980-1982 through 2004-2005. Data are shown by year of diagnosis in the 9 SEER program registries for all CL, CTCL, MF, pcPTL, and CD30+ LPD, and CBCL.
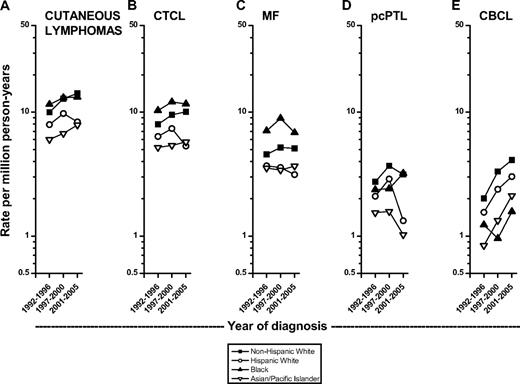
Generally, IRs for CL rose in each racial/ethnic group but IRs were lower among Hispanic whites and Asian/Pacific Islanders than among non-Hispanic whites (Figure 4A-E). During 1992- 1996, blacks had the highest IR for CL, but during 2001-2005 it was highest among non-Hispanic whites (Figure 4A). CTCL rates among Hispanic whites declined in the most recent time period (Figure 4B). IRs were clearly higher among blacks than whites for CTCL overall and MF (Figure 4B,C), but highest among non-Hispanic whites for cutaneous peripheral T-cell lymphoma (Figure 4D). During 1992-1996 to 2001-2005, IRs increased more rapidly for CBCL than CTCL (Figure 4B,E). CBCL IRs were highest among non-Hispanic whites and recent IRs were lowest among blacks (Figure 4E).
Age-adjusted cutaneous lymphoma incidence rates during 1992-1996 through 2001-2005 in the 12 SEER registries by year of diagnosis and race. (A) All cutaneous lymphomas; (B) all cutaneous T-cell lymphomas (CTCL); (C) mycosis fungoides (MF); (D) primary cutaneous peripheral T-cell lymphomas (pcPTL); and (E) cutaneous B-cell lymphomas (CBCL).
Age-adjusted cutaneous lymphoma incidence rates during 1992-1996 through 2001-2005 in the 12 SEER registries by year of diagnosis and race. (A) All cutaneous lymphomas; (B) all cutaneous T-cell lymphomas (CTCL); (C) mycosis fungoides (MF); (D) primary cutaneous peripheral T-cell lymphomas (pcPTL); and (E) cutaneous B-cell lymphomas (CBCL).
Anatomic distribution
The anatomic distribution of 39% of CTCLs was classified as “not otherwise specified” (NOS), especially MF (46%; Table 3). In contrast to CTCL, the head and neck was the most common anatomic site (50%) for CBCL, and only 10% were NOS. Most (70%) cutaneous follicle center lymphomas arose on the head and neck. The most frequently affected anatomic site of cutaneous DLBCL and cutaneous marginal B-cell lymphoma was also the head and neck.
Cutaneous lymphomas diagnosed during 2001-2005 in the 16 SEER program registries by histologic type and anatomic location
| . | Totalcases* . | Head and neck . | Trunk . | Upper limb† . | Lower limb‡ . | Multisite . | NOS . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases . | % . | Cases . | % . | Cases . | % . | Cases . | % . | Cases . | % . | Cases . | % . | ||
| Total | 3874 | 816 | 21.1 | 803 | 20.7 | 444 | 11.5 | 567 | 14.6 | 73 | 1.9 | 1171 | 30.2 |
| Mature T-cell and NK-cell neoplasms (CTCLs) | 2769 | 269 | 9.7 | 610 | 22.0 | 313 | 11.3 | 442 | 16.0 | 69 | 2.5 | 1066 | 38.5 |
| Mycosis fungoides (MF) | 1487 | 42 | 2.8 | 371 | 25.0 | 126 | 8.5 | 231 | 15.5 | 40 | 2.7 | 677 | 45.5 |
| Sézary syndrome (SS) | 33 | 0 | 0.0 | 2 | 6.1 | 0 | 0.0 | 1 | 3.0 | 2 | 6.1 | 28 | 84.9 |
| CD30+ T-cell lymphoproliferative disorders of the skin (CD30+ LPD) | 396 | 73 | 18.4 | 60 | 15.2 | 81 | 20.5 | 82 | 20.7 | 5 | 1.3 | 95 | 24.0 |
| Subcutaneous panniculitis-like T-cell lymphoma (SCPTL) | 23 | 1 | 4.4 | 1 | 4.4 | 4 | 17.4 | 3 | 13.0 | 1 | 4.4 | 13 | 56.5 |
| Primary cutaneous peripheral T-cell Lymphoma (pcPTL) | 809 | 150 | 18.5 | 172 | 21.3 | 98 | 12.1 | 121 | 15.0 | 20 | 2.5 | 248 | 30.7 |
| Extranodal NK/T-cell lymphoma, nasal type | 12 | 3 | 25.0 | 3 | 25.00 | 1 | 8.3 | 2 | 16.7 | 0 | 0.0 | 3 | 25.0 |
| Adult T-cell leukemia/lymphoma (HTLV-1 pos) | 2 | 0 | 0.0 | 0 | 0.0 | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 |
| Angioimmunoblastic T-cell lymphoma | 7 | 0 | 0.0 | 1 | 14.3 | 2 | 28.6 | 1 | 14.3 | 1 | 14.3 | 2 | 28.6 |
| Mature B-cell neoplasms (CBCLs) | 1105 | 547 | 49.5 | 193 | 17.5 | 131 | 11.9 | 125 | 11.3 | 4 | 0.4 | 105 | 9.5 |
| Cutaneous marginal zone B-cell lymphoma (pcMZL) | 274 | 98 | 35.8 | 62 | 22.6 | 59 | 21.5 | 14 | 5.1 | 2 | 0.7 | 39 | 14.2 |
| Cutaneous follicle center lymphoma (pcFCL) | 331 | 231 | 69.8 | 45 | 13.6 | 18 | 5.4 | 4 | 1.2 | 0 | 0.0 | 33 | 10.0 |
| Cutaneous diffuse large B-cell lymphoma (pcDLBCL) | 443 | 188 | 42.4 | 80 | 18.1 | 44 | 9.9 | 101 | 22.8 | 2 | 0.5 | 28 | 6.3 |
| Extracutaneous lymphomas involving the skin§ | 57 | 30 | 52.6 | 6 | 10.5 | 10 | 17.5 | 6 | 10.5 | 0 | 0.0 | 5 | 8.8 |
| . | Totalcases* . | Head and neck . | Trunk . | Upper limb† . | Lower limb‡ . | Multisite . | NOS . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases . | % . | Cases . | % . | Cases . | % . | Cases . | % . | Cases . | % . | Cases . | % . | ||
| Total | 3874 | 816 | 21.1 | 803 | 20.7 | 444 | 11.5 | 567 | 14.6 | 73 | 1.9 | 1171 | 30.2 |
| Mature T-cell and NK-cell neoplasms (CTCLs) | 2769 | 269 | 9.7 | 610 | 22.0 | 313 | 11.3 | 442 | 16.0 | 69 | 2.5 | 1066 | 38.5 |
| Mycosis fungoides (MF) | 1487 | 42 | 2.8 | 371 | 25.0 | 126 | 8.5 | 231 | 15.5 | 40 | 2.7 | 677 | 45.5 |
| Sézary syndrome (SS) | 33 | 0 | 0.0 | 2 | 6.1 | 0 | 0.0 | 1 | 3.0 | 2 | 6.1 | 28 | 84.9 |
| CD30+ T-cell lymphoproliferative disorders of the skin (CD30+ LPD) | 396 | 73 | 18.4 | 60 | 15.2 | 81 | 20.5 | 82 | 20.7 | 5 | 1.3 | 95 | 24.0 |
| Subcutaneous panniculitis-like T-cell lymphoma (SCPTL) | 23 | 1 | 4.4 | 1 | 4.4 | 4 | 17.4 | 3 | 13.0 | 1 | 4.4 | 13 | 56.5 |
| Primary cutaneous peripheral T-cell Lymphoma (pcPTL) | 809 | 150 | 18.5 | 172 | 21.3 | 98 | 12.1 | 121 | 15.0 | 20 | 2.5 | 248 | 30.7 |
| Extranodal NK/T-cell lymphoma, nasal type | 12 | 3 | 25.0 | 3 | 25.00 | 1 | 8.3 | 2 | 16.7 | 0 | 0.0 | 3 | 25.0 |
| Adult T-cell leukemia/lymphoma (HTLV-1 pos) | 2 | 0 | 0.0 | 0 | 0.0 | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 |
| Angioimmunoblastic T-cell lymphoma | 7 | 0 | 0.0 | 1 | 14.3 | 2 | 28.6 | 1 | 14.3 | 1 | 14.3 | 2 | 28.6 |
| Mature B-cell neoplasms (CBCLs) | 1105 | 547 | 49.5 | 193 | 17.5 | 131 | 11.9 | 125 | 11.3 | 4 | 0.4 | 105 | 9.5 |
| Cutaneous marginal zone B-cell lymphoma (pcMZL) | 274 | 98 | 35.8 | 62 | 22.6 | 59 | 21.5 | 14 | 5.1 | 2 | 0.7 | 39 | 14.2 |
| Cutaneous follicle center lymphoma (pcFCL) | 331 | 231 | 69.8 | 45 | 13.6 | 18 | 5.4 | 4 | 1.2 | 0 | 0.0 | 33 | 10.0 |
| Cutaneous diffuse large B-cell lymphoma (pcDLBCL) | 443 | 188 | 42.4 | 80 | 18.1 | 44 | 9.9 | 101 | 22.8 | 2 | 0.5 | 28 | 6.3 |
| Extracutaneous lymphomas involving the skin§ | 57 | 30 | 52.6 | 6 | 10.5 | 10 | 17.5 | 6 | 10.5 | 0 | 0.0 | 5 | 8.8 |
NOS indicates not otherwise specified; and pc, primary cutaneous.
Immature hematologic neoplasms had a total of 10 cases (5 on the head and neck, 2 on the trunk, 1 on the upper limb, and 2 NOS).
Upper limb (UL) cases include all of upper extremity and shoulder.
Lower limb (LL) cases include all of lower extremity and hip.
This group includes small lymphocytic lymphoma, lymphoplasmacytic lymphoma, mantle cell lymphoma, and Burkitt lymphoma.
Survival rates
Overall 5-year relative survival rates for patients with CTCL and CBCL were high (85% and 87%, respectively; Table 4). CTCL survival rates ranged from 91% for patients with MF to 40% for patients with Sézary syndrome. CBCL survival rates ranged from 93% to 96% for patients with cutaneous follicle center lymphoma or cutaneous marginal B-cell lymphoma. Survival rates were much lower among patients with pcDLBCL-leg (46%) than pcDLBCL-other (81%). Five-year relative survival rates were similar among males and females with CTCL (84% and 88%, respectively) and CBCL (both 86%). Overall CTCL and CBCL survival rates were highest among whites (both 87%) and lowest in blacks (82% and 83%, respectively).
Five-year relative survival rates among patients diagnosed with cutaneous lymphoma during 1992-2000 in the 16 SEER program registries by histologic type, sex, and race
| . | Total . | Males . | Females . | Whites . | Blacks . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | SR, % . | SE, % . | No. . | SR, % . | SE, % . | No. . | SR, % . | SE, % . | No. . | SR, % . | SE, % . | No. . | SR, % . | SE, % . | |
| Mature T-cell and NK-cell neoplasms (CTCLs) | 2270 | 85.4 | 1.0 | 1331 | 84.5 | 1.0 | 939 | 87.6 | 1.5 | 1636 | 87.3 | 1.2 | 306 | 81.8 | 2.8 |
| Mycosis fungoides (MF) | 1403 | 90.9 | 1.2 | 793 | 91.9 | 1.0 | 610 | 91.3 | 1.7 | 976 | 92.8 | 1.5 | 219 | 85.1 | 3.2 |
| Sézary syndrome (SS) | 45 | 39.5 | 8.6 | 28 | 31.6 | 10.6 | 17 | 50.8 | 14.1 | 35 | 45.0 | 10.3 | 5 | ∼† | ∼ |
| CD30+ lymphoproliferative disorders (CD30+ LPD) | 100‡ | 73.1 | 5.2 | 72 | 67.3 | 5.0 | 28 | 80.6 | 9.0 | 70 | 78.6 | 5.9 | 11 | 75.6 | 14.5 |
| Primary cutaneous peripheral T-cell lymphoma (pcPTL) | 722 | 79.1 | 2.0 | 438 | 75.7 | 2.0 | 284 | 81.8 | 2.9 | 555 | 80.8 | 2.2 | 71 | 75.2 | 6.3 |
| Mature B-cell neoplasms (CBCLs) | 610 | 86.5 | 2.1 | 348 | 86.2 | 2.7 | 262 | 85.7 | 3.4 | 498 | 87.1 | 2.4 | 25 | 83.3 | 9.9 |
| Cutaneous marginal zone B-cell lymphoma (pcMZL) | 98‡ | 93.1 | 4.4 | 56 | 91.5 | 5.9 | 42 | 93.9 | 6.5 | 70 | 93.9 | 5.4 | 3 | ∼ | ∼ |
| Cutaneous follicle center lymphoma (pcFCL) | 162 | 96.2 | 3.5 | 84 | 94.7 | 4.5 | 78 | 96.0 | 5.4 | 146 | 95.1 | 3.8 | 6 | ∼ | ∼ |
| Cutaneous diffuse large B-cell lymphoma (pcDLBCL) | 290 | 77.3 | 3.4 | 179 | 80.7 | 4.0 | 111 | 68.8 | 6.0 | 238 | 79.7 | 3.8 | 14 | 72.2 | 14.9 |
| pcDLBCL-leg | 48 | 46.3 | 9.4 | 31 | 48.9 | 11.7 | 17 | 38.4 | 15.7 | 41 | 49.7 | 10.7 | 3 | ∼ | ∼ |
| pcDLBCL-other | 242 | 81.2 | 3.4 | 148 | 85.9 | 4.0 | 94 | 72.8 | 6.3 | 197 | 83.8 | 3.8 | 11 | 85.7 | 13.9 |
| Extracutaneous lymphomas involving the skin* | 60 | 88.6 | 5.9 | 29 | 75.7 | 9.8 | 31 | 98.1 | 6.1 | 44 | 83.9 | 7.5 | 2 | ∼ | ∼ |
| . | Total . | Males . | Females . | Whites . | Blacks . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | SR, % . | SE, % . | No. . | SR, % . | SE, % . | No. . | SR, % . | SE, % . | No. . | SR, % . | SE, % . | No. . | SR, % . | SE, % . | |
| Mature T-cell and NK-cell neoplasms (CTCLs) | 2270 | 85.4 | 1.0 | 1331 | 84.5 | 1.0 | 939 | 87.6 | 1.5 | 1636 | 87.3 | 1.2 | 306 | 81.8 | 2.8 |
| Mycosis fungoides (MF) | 1403 | 90.9 | 1.2 | 793 | 91.9 | 1.0 | 610 | 91.3 | 1.7 | 976 | 92.8 | 1.5 | 219 | 85.1 | 3.2 |
| Sézary syndrome (SS) | 45 | 39.5 | 8.6 | 28 | 31.6 | 10.6 | 17 | 50.8 | 14.1 | 35 | 45.0 | 10.3 | 5 | ∼† | ∼ |
| CD30+ lymphoproliferative disorders (CD30+ LPD) | 100‡ | 73.1 | 5.2 | 72 | 67.3 | 5.0 | 28 | 80.6 | 9.0 | 70 | 78.6 | 5.9 | 11 | 75.6 | 14.5 |
| Primary cutaneous peripheral T-cell lymphoma (pcPTL) | 722 | 79.1 | 2.0 | 438 | 75.7 | 2.0 | 284 | 81.8 | 2.9 | 555 | 80.8 | 2.2 | 71 | 75.2 | 6.3 |
| Mature B-cell neoplasms (CBCLs) | 610 | 86.5 | 2.1 | 348 | 86.2 | 2.7 | 262 | 85.7 | 3.4 | 498 | 87.1 | 2.4 | 25 | 83.3 | 9.9 |
| Cutaneous marginal zone B-cell lymphoma (pcMZL) | 98‡ | 93.1 | 4.4 | 56 | 91.5 | 5.9 | 42 | 93.9 | 6.5 | 70 | 93.9 | 5.4 | 3 | ∼ | ∼ |
| Cutaneous follicle center lymphoma (pcFCL) | 162 | 96.2 | 3.5 | 84 | 94.7 | 4.5 | 78 | 96.0 | 5.4 | 146 | 95.1 | 3.8 | 6 | ∼ | ∼ |
| Cutaneous diffuse large B-cell lymphoma (pcDLBCL) | 290 | 77.3 | 3.4 | 179 | 80.7 | 4.0 | 111 | 68.8 | 6.0 | 238 | 79.7 | 3.8 | 14 | 72.2 | 14.9 |
| pcDLBCL-leg | 48 | 46.3 | 9.4 | 31 | 48.9 | 11.7 | 17 | 38.4 | 15.7 | 41 | 49.7 | 10.7 | 3 | ∼ | ∼ |
| pcDLBCL-other | 242 | 81.2 | 3.4 | 148 | 85.9 | 4.0 | 94 | 72.8 | 6.3 | 197 | 83.8 | 3.8 | 11 | 85.7 | 13.9 |
| Extracutaneous lymphomas involving the skin* | 60 | 88.6 | 5.9 | 29 | 75.7 | 9.8 | 31 | 98.1 | 6.1 | 44 | 83.9 | 7.5 | 2 | ∼ | ∼ |
The period of survival was from the date of diagnosis to the date of last contact, death, or December 31, 2005.
The registries include 2318 cases diagnosed during 1992-1999 in the SEER 12 and 562 cases diagnosed during 2000 in the SEER 16 registries.
Relative survival rates were available only for whites and blacks and were not yet available for Hispanics and Asian/Pacific Islanders, thus Hispanics and Asian/Pacific Islanders were excluded from race-specific survival analyses (n = 415).
SR indicates survival rate; and SE, standard error.
This group includes small lymphocytic lymphoma, lymphoplasmacytic lymphoma, mantle cell lymphoma, and Burkitt lymphoma.
Statistic not shown, based on fewer than 10 cases.
CD30+ LPD and cutaneous marginal zone B-cell lymphoma were first recognized in the third edition of the WHO's ICD-O. There were 100 cases of CD30+ LPD and 98 cases of pcMBL diagnosed in 2000.
We analyzed the CL overall survival rates in patients diagnosed before age 40 years. We found that the overall 5-year relative survival rates of patients with CTCL and CBCL were 91.8% and 85.6%, respectively, with males and females having similar rates. There were 18 patients with cutaneous lymphoma diagnosed before age 15 years (17 of which had CTCL), and their overall 5-year relative survival rate was 93.9%.
Discussion
In this study, we calculated population-based IR and survival rates of CLs diagnosed among residents of 16 SEER registries from 2001-2005, accounting for approximately 23% of the US population. This report includes 3884 cases of CLs. Unique and important findings include variation in incidence patterns of cutaneous T-cell and B-cell lymphomas by sex, race, and histologic types, suggesting that these lymphomas are etiologically distinct. In addition, we report new findings regarding CL incidence and survival patterns in Hispanic whites and Asian/Pacific Islanders.
In our study, CTCLs constituted the majority (71%) of CLs similar to previous studies (75%-85%).2,4,6,21,22 We found that the CTCL IR was 7.7/1 000 000 person-years, similar to that (6.4/1 000 000 person-years) reported by a recent SEER-based study using data from the original 9 registries analyzing 4783 cases of CTCL from 1973-2002.4 We found that CBCLs were much less common than CTCLs, accounting for 29% of CLs overall. This finding is consistent with 2 previous studies in which CBCL constituted 24% of CLs.2,6 For the first time to our knowledge, we report comprehensive IRs for CBCL overall (3.1/1 000 000 person-years) and subtypes in the United States. The CTCL IR was more than twice that of CBCL. A population-based study conducted in Florence, Italy reported a CBCL IR of 7/1 000 000 person-years.5 We found distinct differences in the anatomic distribution among CLs. Most CTCLs (39%) did not have a skin site specified, whereas CBCLs were classified mostly distributed on the head and neck (50%), similar to previous reports.23,24 We found cutaneous follicle center lymphoma had the highest frequency (70%) of head and neck tumors consistent with an earlier study (75%).25
Our study revealed major racial differences in IRs among CL subtypes. Blacks had statistically higher IR of CTCL and MF than other races, consistent with previous studies.4,26 To our knowledge, for the first time we reported that the CTCL IRs among Asian/Pacific Islanders and Hispanic whites were lower than among blacks and non-Hispanic whites. In contrast to MF, IRs for primary cutaneous peripheral T-cell lymphoma and cutaneous CD30+ T-cell lymphoproliferative disease were similar among blacks and non-Hispanic whites. In addition, we found that during 1992-2000, the IR of primary cutaneous peripheral T-cell lymphoma among non-Hispanic whites was higher than among blacks, and in recent years the IRs of blacks and non-Hispanic whites converged.
In contrast to CTCL, our study showed that CBCL was almost exclusively a disease of whites with higher IRs for CBCL overall, cutaneous marginal zone B-cell lymphoma, and follicle center lymphoma among non-Hispanic whites compared with other races. Cutaneous marginal zone B-cell lymphoma consisted of 7% of all CL similar to the Dutch and Austrian Cutaneous Lymphoma Group (7%).6 Borrelia burgdorferi infection has been reported to be associated with a subgroup of primary cutaneous marginal zone B-cell lymphoma in European27 but not in Asian28 or US29,30 cases.
Our study revealed some unique findings in Asian/Pacific Islanders. Asian/Pacific Islanders compared with non-Hispanic whites had significantly lower IRs for CTCL and CBCL subtypes, except for pcDLBCL in which IRs were similar and higher, respectively. CL IRs among Asian/Pacific Islanders were lower than other racial groups across US SEER registries and not restricted to just Hawaii (data not shown). pcDLBCL has been reported as the most frequent (89%) CBCL subtype in Japanese patients.31 Epidemiologic investigations have shown similar incidence patterns of lymphomas among foreign-born and US-born Asians, supporting the role of host susceptibility in etiology.32
We found a consistent male predominance for all CL subtypes. The predominance of males with CTCL overall is consistent with previous reports.4,7,33 We also showed a male predominance across 2 other CTCL subtypes: primary cutaneous peripheral T-cell lymphoma and cutaneous CD30+ T-cell lymphoproliferative disorders. The IRRs were statistically significantly higher for all CL subtypes with few exceptions (eg, Sézary syndrome).
We observed that age-specific IR of CBCL increased exponentially with age. This finding is consistent with a previous case report where the median age at diagnosis was 68 years.34 A similar age-specific trend has been reported for extracutaneous DLBCL.32 Aging and age-related effects such as immune senescence may be particularly important for CLs in which IRs increase steeply with age. In addition, chronic inflammation, DNA damage, and diminished immune surveillance that occur with older age35 may also contribute to lymphoma development. We also found that the age-specific IRs as well as the M/F IRRs of CTCL increased with age, in contract to CBCL in which the M/F IRRs decreased with age.
The 5-year relative survival rate for patients with CTCL was 85%. Patients with MF had the highest 5-year survival rate (91%) among patients with CTCL, similar to a study by the Dutch and Austrian Cutaneous Lymphoma Group (88%).6 A recent population-based study of 821 patients at the Thames Cancer Registry in Southeast England showed significant improved survival among MF patients over the past 20 years.36 Nevertheless, it is not clear whether this increase is primarily due to increased diagnosis at early stages of disease or to other factors, such as improved treatments.37 Our study showed that patients with Sézary syndrome had the lowest 5-year survival rate among patients with CTCL. This survival rate is higher than a previous report (24%).6 We found a higher 5-year relative survival rate (79%) for pcPTL patients compared with those reported (20%-50%) by the French Study Group on Cutaneous Lymphomas38 and the Dutch and Austrian Cancer Registries.6,39,40 The differences in survival among studies may be secondary to the populations studied and histologic subtypes constituting pcPTL. Many of the studies reported disease-specific and observed survival rates,6,39,40 which also may contribute to the differences in survival. We also observed a high 5-year relative survival rate (87%) for patients with CBCL. Patients with cutaneous marginal zone B-cell lymphoma and cutaneous follicular center lymphoma had the highest 5-year relative survival rates (93% and 96%, respectively), similar to previous studies (99% and 95%, respectively).6 Our study revealed that patients with pcDLBCL-leg (46%) had decreased survival consistent with previous reports6,38,41,42 and similar survival rates to patients with pcDLBCL-leg type in the Dutch Cutaneous Lymphoma Working Group (55%-58%)41 and the French Study Group on Cutaneous Lymphomas (41%).42
Examination of the temporal trends of CL showed that from 1980-1982 to 2001-2003 the IRs of CL overall and CTCL and CBCL have increased (Figure 3) similar to a previous study using the original 9 SEER registry areas that reported an increased in IR of CTCL.4 This pattern was also observed in NHL overall in which there was an estimated 50% increase in US age-adjusted IR from 1970-1990, then a stabilization of rates after 1990.32 Changes in diagnostic practice over time and the emergence of acquired immunodeficiency syndrome pandemic in the early 1980s contributed to the rise in NHL, but were not sufficient to explain entirely the dramatic increases.32 There are other possible explanations for the overall long-term increase in IRs. Population-based studies and cancer registries have used 3 different editions of ICD-O and 2 Field Trial editions to classify CL since 1978.43 Each new ICD-O edition had additional histologic classifications or revisions (1986,44 1988,45 1990,46 and 200047 ). New lymphoma classifications resulted in new additions and redistributions of broadly defined cases of CL among the more specific subclassifications.6 Hence, these changes have created inconsistencies over time in classification.
Most recently, during 2001-2003 through 2004-2005, the CL IRs (including CTCL and CBCL) apparently decreased. The CL IR during 2004-2005 is inconsistent with the previous observed trend. There are possible explanations for this finding. Delayed identification and reporting of CL cases may have contributed to the recent change. To correct for this, in recent years SEER has been computing “delay-adjusted” rates.48 This has been shown to be particularly important for cutaneous melanomas, with a recent approximate 5% increase in delay-adjusted rates compared with rates initially reported. We compared the rates for CL during 2001-2003 as initially reported and delayed-adjusted 2 years later and found a 5% increase in rates between submissions. Similarly, Dores et al recently reported a nonsignificant 6% increase between submissions for MF-SS during 1990 thorough 1994.49 Thus, if we adjust the most recent (2004-2005) CL rate by 5%, this would account for perhaps half the observed apparent change. Alternatively, our findings could indicate that recent IRs are leveling off. Future studies should examine recent IRs for CLs over time carefully when additional data are available before conclusions are made on the recent trends for CLs.
In addition, a potential limitation of the study is complete inclusion of all patients diagnosed at dermatologist's offices into SEER. However, this may not be a main limitation because most CL cases are referred to specialty clinics at university hospitals for further workup and treatment, and thus would be identified by SEER. In addition, any pathologic specimen sent from a dermatologist's office to a hospital for diagnosis would be identified by SEER staff and included if a resident of the SEER catchment area. Lastly, SEER has mounted considerable effort to identify those cases diagnosed at a dermatologist's office and not identified otherwise.
We found that pcDLBCL accounted for 44% of CBCLs similar to a recent report2 but in contrast to a previous study.50 Distinguishing between pcDLBCL and primary cutaneous follicular center lymphoma is challenging. Classification on the basis of morphology is difficult and associated with high intraobserver variation. Various studies have shown that some primary cutaneous follicular center lymphomas in which the majority of tumor cells are centroblasts previously have been categorized as DLBCL by most observers.1,34,51-53 Despite the predominance of centroblasts, clinical studies have suggested that these lymphomas have a benign clinical course. A recent study in The Netherlands by Senff et al found that of 167 patients with primary CL classified as DLBCL using the WHO classification, 109 (65%) were reclassified as primary cutaneous follicular center lymphoma after histologic examination.50 It is possible that some cases in our study coded as pcDLBCL would probably be classified today by an expert hematopathologist as primary cutaneous follicular center lymphoma. In conclusion, our result of primary cutaneous follicular center lymphoma and pcDLCL should be interpreted with caution until a future population-based study conducts a detailed histologic review of CBCL in the US and our findings are confirmed.
In comparing CLs with NHL overall, we found several differences and similarities. Whereas B-cell lymphomas comprised the majority (90.4%) of NHL overall,54 we found that CTCL accounted for the majority of primary CLs (71%). Interestingly, both cutaneous B-cell lymphomas and NHL overall shared similar distribution of histologic subtypes. The most common nodal B-cell lymphoma subtypes are DLBCL (30%-40%) and follicle center lymphoma (20%-30%).31,53 Similarly, we also found that pcDLBCL and primary cutaneous follicular center lymphoma accounted for 40% and 30% of cases, respectively. In contrast to T-cell lymphomas overall in which peripheral T-cell lymphoma is the most common type (33%), followed by MF/Sézary syndrome (28.5%),32 we found MF to be the most common CTCL subtype (54%), followed by primary cutaneous peripheral T-cell lymphoma (29%). In both cutaneous and overall B-cell lymphomas, IR were higher in whites, whereas blacks had higher rates of cutaneous and overall T-cell lymphomas.55
We conducted a population-based study, which avoids the biases associated with hospital and clinical series, and provided us with enough statistical power to calculate incidence and survival rates. The strengths of this study were the large sample size of rare lymphomas and unbiased ascertainment and assessment of cases. This study has shown variation in incidence patterns by race, sex, age, and histologic type, supporting the notion that CLs represent distinct disease entities. Our study showed previously unrecognized epidemiologic features that may ultimately be characteristic findings of the various CL subtypes. Whereas blacks had statistically higher IRs of CTCL, non-Hispanic whites had statistically higher rates of CBCL. A consistent male predominance was observed in the majority of CL subtypes. We also found that CTCL M/F IRRs increased with age, as opposed to CBCL in which M/F IRRs decreased with age. CBCL cases were mostly anatomically distributed on the head and neck, in contrast to CTCL. Overall, patients with CL had relatively high survival rates, with the exception of Sézary syndrome. Further investigations using large populations and molecular tools are warranted to elucidate the etiology of the diverse spectrum of CLs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the SEER program staff at the National Cancer Institute and the registries for their invaluable work and Mr John Lahey of IMS for figure preparation.
This research was supported in part by the Intramural Program of Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: J.R.T. conceived of and designed the study; P.T.B. and J.R.T provided administrative support; P.T.B., S.S.D., and J.R.T. provided study materials or patients, collected and assembled data, and wrote the paper; P.T.B., S.S.D., and W.F.A. performed statistical analyses; and P.T.B., S.S.D., W.F.A., and J.R.T. interpreted the data and approved the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jorge R. Toro, Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, 6120 Executive Blvd, Executive Plaza South, Rm 7012, Rockville, MD 20892-7231; e-mail: toroj@mail.nih.gov.