To the editor:
Porkka et al demonstrated that dasatinib crosses the blood-brain barrier and exerts antitumor activity in a mouse model of intracranial chronic myelogenous leukemia (CML).1 They reported that systemic dasatinib induced tumor cell clearance from cerebrospinal fluid (CSF) in patients with Philadelphia chromosome–positive (Ph+) leukemias and central nervous system (CNS) involvement.1 Mutational analysis of CSF leukemic cells suggested that progression was due to selection of drug-resistant clones within the CNS.1
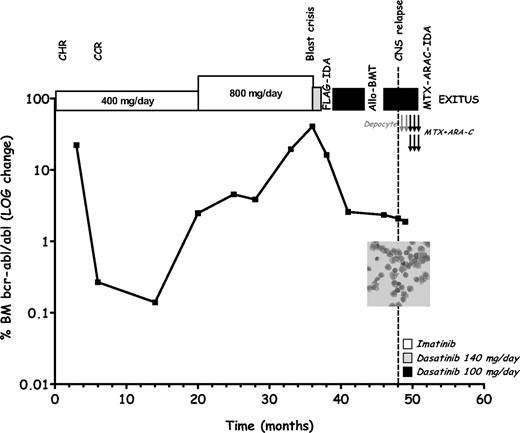
We report on a 46-year-old man with Ph+ chronic-phase (CP) CML without additional cytogenetic abnormalities. He received imatinib (400 mg/day) and achieved a complete hematologic response after 1 month, a complete cytogenetic response at 6 months, and a major molecular response at 12 months (Figure 1).2 Bone marrow (BM) examination after 20 months of imatinib disclosed a 1-log rise of BCR-ABL transcripts. Imatinib was escalated (800 mg/day) but BCR-ABL levels showed a progressive increase leading to myeloid blast crisis (BC) 33 months after diagnosis. Dasatinib (140 mg/day) was started but discontinued after 4 weeks due to hematologic toxicity. A fludarabine (30 mg/m2 per day on days 1-5), cytarabine (2 g/m2 per day on days 1-5), and idarubicin (10 mg/m2 per day on days 3-5) combination (FLAG-IDA)3 course induced a second CP, which was consolidated by allogeneic bone marrow transplantation (allo-BMT). Because of the high relapse risk, dasatinib (100 mg per day) was administered between chemotherapy and allo-BMT, resulting in approximately 1-log reduction in BCR-ABL level, and resumed after transplantation with the patient in CP and stable BCR-ABL levels. After 2 months an isolated leukemic CNS relapse occurred, despite further reduction of BCR-ABL levels and without BM blastosis. The patient succumbed to CNS disease 3 months afterward, despite CNS-directed chemotherapy. Sequencing of the BCR-ABL kinase domain from BM blasts, at the time of BC, and CSF tumor cells, at CNS relapse, did not show any mutation. The lack of BM blastosis, the reduced marrow BCR-ABL levels and the absence of a mutated clone, in BM and CSF, at the onset of meningeal leukemia, indicate that systemic dasatinib failed to prevent CSF disease while still controlling extra-CNS leukemia. Thus, relapse was most probably due to suboptimal penetrance/activity of the drug in CNS given the low dasatinib dose (100 mg per day) used being the patient in CP. In the 4 months preceding CNS progression, the patient was not given comedications known to decrease the plasma concentrations of dasatinib,4,5 including CYP4503A4 inducers, but rather received the CYP4503A4 inhibitor itraconazole. Therefore, even though we did not assess plasma levels, it is reasonable to exclude a negative influence of comedications on dasatinib bioavailability.
Clinical course of a patient with Philadelphia chromosome–positive chronic myelogenous leukemia developing isolated central nervous system relapse while under dasatinib therapy. At the time of blast crisis, morphologic and flow cytometry studies evidenced 55% of myeloid blasts in the bone marrow (BM) with a CD33+CD13+CD10−CD20−CD45+ immunophenotype (not shown). BCR-ABL levels in BM were quantified by real-time polymerase chain reaction according to a described standard protocol.2 Gray arrow indicates intrathecal therapy with liposomal cytarabine (Depocyte, 50 mg); black arrows indicate intrathecal therapy with methotrexate (MTX, 12 mg) and cytarabine (ARA-C, 50 mg). Inset displays a May-Grünvald-Giemsa–stained cytospin slide of cerebrospinal fluid (CSF) showing myeloid blasts with a CD33+CD13+CD10−CD20−CD45+ at flow cytometry (not shown). At the time of documented leptomeningeal disease, the bone marrow examination was consistent with chronic-phase disease with a blast differential count of 4%. CHR indicates complete hematologic response; CCR, complete cytogenetic response; FLAG-IDA, fludarabine (30 mg/m2 days 1-5), cytarabine (2 g/m2 days 1-5), and idarubicin (10 mg/m2 days 3-5) combination chemotherapy; Allo-BMT, allogeneic bone marrow transplantation; and MTX-ARA-C-IDA, systemic therapy with high-dose methotrexate (3.5 g/m2 day 1) and cytarabine (2 g/m2 days 2-3) plus idarubicin (8 mg/m2 days 2-3).
Clinical course of a patient with Philadelphia chromosome–positive chronic myelogenous leukemia developing isolated central nervous system relapse while under dasatinib therapy. At the time of blast crisis, morphologic and flow cytometry studies evidenced 55% of myeloid blasts in the bone marrow (BM) with a CD33+CD13+CD10−CD20−CD45+ immunophenotype (not shown). BCR-ABL levels in BM were quantified by real-time polymerase chain reaction according to a described standard protocol.2 Gray arrow indicates intrathecal therapy with liposomal cytarabine (Depocyte, 50 mg); black arrows indicate intrathecal therapy with methotrexate (MTX, 12 mg) and cytarabine (ARA-C, 50 mg). Inset displays a May-Grünvald-Giemsa–stained cytospin slide of cerebrospinal fluid (CSF) showing myeloid blasts with a CD33+CD13+CD10−CD20−CD45+ at flow cytometry (not shown). At the time of documented leptomeningeal disease, the bone marrow examination was consistent with chronic-phase disease with a blast differential count of 4%. CHR indicates complete hematologic response; CCR, complete cytogenetic response; FLAG-IDA, fludarabine (30 mg/m2 days 1-5), cytarabine (2 g/m2 days 1-5), and idarubicin (10 mg/m2 days 3-5) combination chemotherapy; Allo-BMT, allogeneic bone marrow transplantation; and MTX-ARA-C-IDA, systemic therapy with high-dose methotrexate (3.5 g/m2 day 1) and cytarabine (2 g/m2 days 2-3) plus idarubicin (8 mg/m2 days 2-3).
Dasatinib achieves CSF concentrations of 5.0%to28% of those found in the plasma and Authors speculated that the biologic potency of dasatinib and/or the lack of protein binding may explain antitumor activity at low CSF levels.1 However, approximately 50% of patients received steroids and/or other intrathecal agents before, together with, or soon after dasatinib, suggesting that this agent alone is unable exert a proper control of CNS disease in all cases.1,6 Factors other than tumor cell resistance might influence dasatinib activity in the CNS, including suboptimal systemic dosing. Further pharmacokinetic studies are needed to identify patients in whom dasatinib alone may effectively control CNS disease.
Authorship
Acknowledgments: This work was supported by contract grant sponsors Ministero della Salute, Ricerca Finalizzata, Servizio Sanitario Nazionale, and Istituto di Ricovero e Cura a Carattere Scientifico (Rome, Italy).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonio Pinto, National Cancer Institute, Fondazione Pascale, IRCCS, Via Mariano Semmola, Naples, Italy 80131; e-mail: apinto.int.napoli@tin.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal