In human blood, 1% to 5% of lymphocytes are γδ T cells; they mostly express the γδ T-cell receptor (TCR)Vγ9, recognize nonpeptide phosphoantigens (PAgs) produced by microbes and tumor cells, and mediate different modes of lytic activities directed against tumor target cells. Antibody-dependent cell-mediated cytotoxicity (ADCC) mediated by cytolytic lymphoid cells is essential for the clinical activity of anticancer monoclonal antibodies (mAbs), but whether PAgs affect ADCC by γδ T cells is unknown. Here we report that, in association with the CD20+-specific mAb rituximab (RTX), the synthetic PAg bromohydrin pyrophosphate (BrHPP) increased TCRVγ9+ cell binding to CD20+ lymphoma cells in vitro. This combination activated phospho-ZAP70 and phospho-ERK1/2 signaling in TCRVγ9+ cells and strongly enhanced their ADCC activity. We obtained similar results with BrHPP in the context of the mAbs alemtuzumab and trastuzumab. Furthermore, BrHPP enhanced RTX-mediated depletion of CD20+ cells in vitro from peripheral blood mononuclear cells of healthy subjects and enhanced ADCC by γδ T cells from patients with chronic lymphocytic leukemia. In cynomolgus macaques, a regimen combining RTX, BrHPP, and IL2 activated TCRVγ9+ lymphocytes and enhanced B-cell depletion from blood and lymph nodes. Thus, the combination with BrHPP PAg is able to improve the efficacy of cancer immunotherapy by therapeutic mAbs.
Introduction
The success of therapeutic monoclonal antibodies (mAbs) in the treatment of cancer can be attributed to their multiple bioactivities. Their mechanism of action combines antibody-dependent cellular cytotoxicity (ADCC), complement-mediated cytotoxicity, antibody-dependent phagocytosis, direct cytotoxic activity, and inhibition of receptor signaling.1 ADCC occurs when cytolytic effector cells expressing a receptor for the Fc region of the IgG class of antibodies (Fcγ receptors) bind to antibodies on the surface of target cells. In humans, Fcγ receptors comprise CD16 (FcγRIIIA-B), CD32 (FcγRIIA-C), and CD64 (FcγRI), which all bind the same region on IgG Fc but with low-to-medium (CD16, CD32) or high (CD64) affinities.2
Several lines of evidence suggest that enhancing ADCC induced by therapeutic mAbs may directly improve their clinical efficacy. First, in mice bearing xenografted tumors, the efficacy of the therapeutic mAbs rituximab (RTX) and trastuzumab (TTZ) relies upon cell-surface expression of FcγR.3 Second, ADCC is essential for the clinical efficacy of RTX in B-cell lymphoma patients and depends on the affinity of FcγRIIIA for the IgG.4,5 Third, optimizing the affinity of RTX, TTZ, and alemtuzumab (ALZ) for FcγRIIIA increases their ADCC and their efficacy in preclinical and clinical studies.6,–8 Finally, recruitment and activation of additional cell effectors for ADCC might also enhance the cytolytic activity of anticancer mAbs.9,10
The cytolytic effector cells involved in ADCC are CD16+ (ie, FcγRIIIA)–positive natural killer (NK) cells and other CD8+ cytolytic T lymphocytes, which release perforin through immunologic synapses to kill target cells. In addition, human CD4−CD8− γδ T cells from peripheral blood might provide an important reservoir of cytolytic effector cells for ADCC. In most humans and nonhuman primate species, the majority of circulating γδ T lymphocytes expresses the Vγ9 T-cell receptor, with CD4−CD8− TCRVγ9+ cells representing 1% to 3% of mononuclear cells. All these cells respond to stimulation with nonpeptide phosphoantigens (PAgs), which are small, phosphorylated metabolites produced by the cholesterol pathway in microbial pathogens and tumor cells. In addition to natural PAgs, the synthetic analog BrHPP11 selectively stimulates TCRVγ9+ γδ T lymphocytes. PAg-stimulated γδ T cells proliferate, secrete pro-inflammatory cytokines and chemokines, and, most importantly, kill leukemia, lymphoma, and carcinoma cells.12,13 Several studies involving macaque monkeys14,15 and clinical studies in cancer patients16,,,,,–22 have demonstrated in vivo the potential of PAg-activated TCRVγ9+ γδ T lymphocytes for cancer immunotherapy.
The mechanism by which PAgs stimulate γδ T cell–mediated cancer cell killing is unclear. The number of circulating γδ T lymphocytes increases 50- to 100-fold in humans treated with BrHPP and IL2 (our unpublished observations), thus expanding substantially the reservoir of these effector cells. Also, stimulation by PAg drives γδ T-cell maturation from naive to effector memory cells, some of which express CD16 at their cell surface,23,24 and CD16+ effector memory γδ T cells are better than other cells at spontaneously binding and killing CD20+ lymphoma cells.25,26 This involvement of CD16 suggests that PAg might stimulate ADCC by γδ T cells. Consistent with this, zoledronate, a drug that up-regulates the level of endogenous PAg metabolites in treated cells, also up-regulates ADCC by γδ T lymphocytes.27
We set out to examine whether activating γδ T cells with the synthetic PAg BrHPP would improve the efficacy of therapeutic mAbs by strengthening ADCC. Here we present in vitro and in vivo evidence showing that BrHPP increases ADCC by γδ T cells in association with RTX, ALZ, or TTZ, suggesting this new drug can improve the bioactivity of current anticancer mAbs.
Methods
Reagents
The synthetic PAg bromohydrin pyrophosphate (BrHPP; IPH1101; Innate Pharma, Marseilles, France) was used at 400 nM. Rituximab, trastuzumab (Roche, Les Ulis, France), and alemtuzumab (Schering, Lille, France) antibodies were used at 10 μg/mL unless stated otherwise.
Cell culture and flow cytometry–based assays
All γδ T-lymphocyte and cancer-cell cultures were established and maintained as previously described.28,29 Briefly, PAg-activated γδ T lymphocytes (TCRVγ9+ γδ T cells > 95%) were derived from the peripheral blood mononuclear cells (PBMCs) of healthy subjects by culturing for 2 weeks in medium supplemented with 400 nM BrHPP and 300 IU/mL rhIL2 at day 0 followed by IL2 renewal every 3 days. Cells were stained with FITC-conjugated antibodies to CD5, CD19, CD20, CD52, and TCRVγ9; PE-Cy7–conjugated anti-CD3; PE-Cy5–conjugated anti-CD27, or with PE-conjugated antibodies to markers TCRVγ9, CD16, CD32, and CD64 and isotype-matched control conjugates (Beckman-Coulter-Immunotech, Marseille, France), or with PE-conjugated antibodies to CD45RA, PE-Cy5–conjugated anti-CD107a and isotype-matched control conjugates (BD Pharmingen, Le Pont-de-Claix, France). Staining of intracellular phosphoproteins from gated TCRVγ9+ lymphocytes (50 000-150 000 cells/experiment) was carried out according to the supplier's instructions using both FITC-conjugated anti-TCRVγ9, Ax647-conjugated antiphospho-ZAP70 (Y319), and PE-conjugated phospho-ERK1/2 (T202/Y204) antibodies (BD Pharmingen). For IgG1 binding to CD16, 1.5 × 105 CD16+ TCRVγ9+ γδ T cells per well were incubated with the specified concentrations of trastuzumab and rituximab in PBS for 1 hour at 4°C, washed with ice cold PBS containing 5% FCS, cross-linked with polyclonal goat anti–human IgG (Sigma) in PBS containing 5% FCS for 10 minutes at 4°C, and washed again. Cells were then stained for free CD16 by PC7-conjugated anti-CD16 (clone 3G8, Beckman Coulter) at the cell surface of CD3+ TCRVγ9+ lymphocytes. Cells were analyzed with an LSR-II flow cytometer (Becton Dickinson) and Diva software (BD Biosciences, Mountain View, CA). Results are shown as 10% probability contour plots with outlier dots. Because γδ T lymphocytes capture membrane markers from the cells they bind,28 the number of PKH67 fluorochrome (MESF) molecules transferred from cells previously stained with this dye provided a measure of γδ T-cell binding to cancer cell lines in various conditions. The flow cytometry–based measure of T-cell binding (5 × 104 gated TCRVγ9+ γδ cells per point) was calibrated using FITC-coupled Quantum beads (Bang Laboratories, Fishers, IN) to convert the mean fluorescence intensities into the number of molecules of equivalent soluble MESF; the increase from 0 to 60 minutes gave the transferred MESF data.30
Cytotoxicity
Cytotoxicity assays based on cell surface expression of CD107a by lytic γδ T cells after 4-hour coincubation in the specified conditions have been described elsewhere.28,30 Specific lysis by PAg-activated γδ T lymphocytes involved standard 4-hour 51Cr release assays with the indicated effector:target cell ratio. Cells were coincubated for 4 hours in the presence of RTX alone (10 μg/mL), of RTX-derived Fab'2 alone (10 μg/mL, produced in our laboratory), or of RTX (10 μg/mL) plus 1 mM MgCl2 and 1.5 mM EGTA. Data shown are means and SD from 3 independent experiments with different donors.
Western blot analysis
Western blot analysis of γδ T cells (106 cells) for phospho-ERK1/2 (T202/Y204), ERK1/2, phosphoZAP70 (Y493), and ZAP70 (Cell Signaling Technologies, St Quentin en Yvelines, France) were performed using enhanced chemiluminescence detection as described.29
In vitro human B-cell depletion assays
A total of 2 × 107 PBMC from healthy donors (n = 8, collected by Etablissements Français du Sang, Toulouse, France) or 4.5 × 107 PBMC from untreated chronic lymphocytic leukemia (CLL) patients (n = 8; CD5+CD20+ cells > 95%, obtained with patients' written informed consent in accordance with the Declaration of Helsinki, by Service d'Hematologie, Hopital Purpan) were incubated in vitro in complete medium supplemented with either RTX, PAg, or both as indicated, plus IL2 (15 ng/mL, renewed every 3 days) for CLL cells. B-cell counts in normal PBMC were obtained from total cell counts and percentage of viable CD19+ lymphocytes in culture at 0 and 24 hours. The CD20+ B-cell depletion and TCRVγ9+-cell amplification rates from CLL PBMCs were based on total cell numbers of viable CD19+ and TCRVγ9+-cells, respectively, in culture at days 0, 7, and 13. Statistical difference was based on 1-tailed paired Student t test. IRB approval for these studies was obtained from the Comite Ethique of Montpellier University.
B-cell depletion in macaques
Three groups of age and sex-matched cynomolgus macaques weighing 2.5 to 4.4 kg were treated weekly with intravenous RTX (5 mg/kg) for 3 weeks (on days 0, 7, 14, and 21), a regimen that fully depletes B cells from the macaque blood and partially depletes B cells from the lymph nodes.31 Animals from group 1 (n = 4) received RTX alone; those from group 2 (n = 4) received RTX plus 106 IU rhIL2 by subcutaneous injection (Proleukin; Chiron; Novartis Vaccines and Diagnostics, Suresnes, France) daily for 5 days over 8 weeks (on days 7-11, 28-32, and 49-53); and those from group 3 (n = 6) received RTX and rhIL2, as group 2 plus PAg (50 mg/kg per dose, intravenous injection 30-minute infusions on days 7, 28, and 49). Animals were monitored daily for morbidity, body weight, hematology, clinical chemistry, and RTX toxicokinetics. Plasma concentrations of RTX were measured by sandwich ELISA as described.32 Blood samples were withdrawn, and leucocytes were isolated, counted, and analyzed by flow cytometry for CD3-PerCP/CD20-FITC/CD21-PE and CD3-PerCP/Vγ9-APC/CCR5-PE/CD69-FITC profiles. We found the human CD20 marker convenient for monitoring the macaque CD20+ B-cell rates. All monkeys were euthanized on day 62, and organs were collected and weighed. Bone marrow and lymph nodes were processed for FACS analysis at necropsy. Statistical differences were calculated from 1-tailed Mann-Whitney tests. Animal care and handling were carried out at MDS Pharma Services (St Germain sur l'Arbresle, France) in accordance with standard guidelines, following a protocol approved by MDS Pharma Services.
Statistics
The normality and variance of each sample series was evaluated before statistical analysis by the specified tests, using α = 5% for significant differences with Bonferroni correction whenever appropriate Briefly, the 1-tailed, paired Student t test was used for data shown in Figure 1D; 1-way ANOVA on ranks and multiple comparison to control groups was used for data shown in Figures 2, 3, and 5; and a 1-tailed Mann-Whitney rank sum test was used for data shown in Figures 4 and 5. All statistical analyses were performed using Sigma Stat 3.0 (SPSS, Chicago, IL) and XL Stat 2008 (AddinSoft, Paris, France) software.
Results
Blood TCRVγ9+ γδ T lymphocytes are reactive to both PAg and mAbs
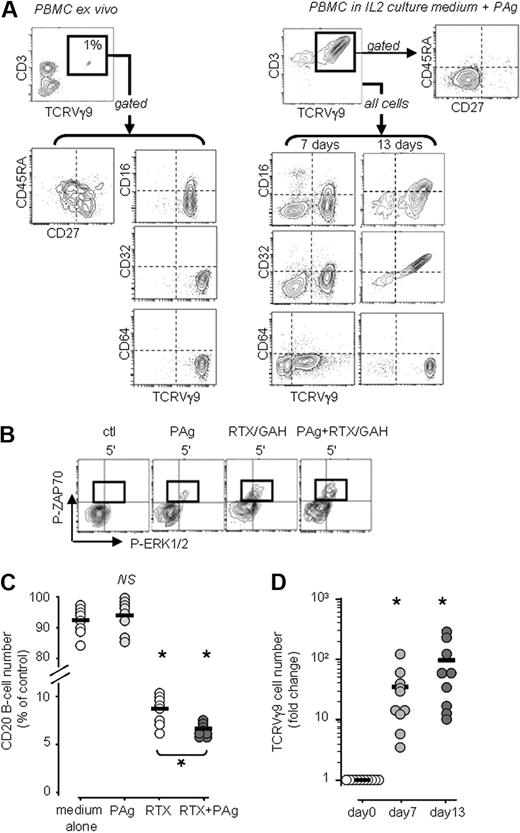
The blood of healthy human adults typically harbors around 1% of TCRVγ9+ γδ T lymphocytes, which comprise naive, central memory, and effector memory cells as identified by their CD27 and CD45RA phenotypes.24,25 In most donors, these lymphocytes express low levels of the Fcγ receptor CD16 and do not express the other IgG receptors CD32 and CD64 (a representative example is shown in Figure 1A left). This phenotype suggested that ligands for TCRVγ9 or CD16, such as PAg and RTX, respectively, might activate circulating TCRVγ9+ γδ T lymphocytes. We examined this possibility by purifying resting TCRVγ9+ T lymphocytes (≥ 98%) from PBMCs, stimulating these cells in vitro with PAg and cross-linked RTX, and looking for the appearance of phosphorylated signaling proteins by flow cytometry analysis after labeling for intracellular phosphoproteins (Figure 1B) and by Western blotting of the protein extracts. The isolation procedure caused no signaling activity, as shown by lack of phosphoZAP70 and phosphoERK1/2 in the control cells. By contrast, within 5 minutes, PAg alone triggered the appearance of both phosphoZAP70 (Y319) and phosphoERK1/2 (T202/Y204) in the cells. Although stimulation with RTX alone did not elicit signaling (data not shown), RTX cross-linked with goat anti–human IgG to mimic physiologic ligands induced both phosphoZAP70 and phosphoERK1/2 (T202/Y204) in the cells. When added together, PAg and cross-linked RTX enhanced the appearance of both phosphoZAP70 and phosphoERK1/2. Western blotting of the TCRVγ9+ T-cell protein extracts confirmed these findings and showed that a strong band of phosphoERK1/2 appeared 60 minutes after stimulation with RTX cross-linked with goat anti–human IgG (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Further experiments showed that PLCγ1, PKC, and PI3K were also involved (data not shown), indicating that PAg and cross-linked RTX induced intracellular signaling cascades that were previously found to drive activation in γδ T lymphocytes23 and cytotoxicity in NK cells.33
Freshly isolated TCRVγ9+ PBMCs are reactive to both PAg and RTX. (A) Representative phenotypes of unactivated and PAg-activated TCRVγ9+ T lymphocytes. (B) Flow cytometry of intracellular phosphoZAP70 and phosphoERK1/2 in gated TCRVγ9+ γδ T cells after stimulation with PAg and/or cross-linked RTX (a representative experiment of 6 performed, GAH: goat anti–human IgG). (C) Depletion of B cells from PBMCs of healthy subjects (n = 8) upon 24 hours of treatment with PAg (400nM BrHPP) and/or RTX (10 μg/mL) in vitro; *P < .05 versus control and RTX versus RTX+PAg by 1-way paired Student t test. (D) Culture with PAg (400 nM BrHPP) for 7 and 13 days in medium containing 100 U/mL IL-2 amplifies the number of TCRVγ9+ γδ T lymphocytes in the PBMCs of healthy subjects (n = 10; bars: group means); *P < .05 versus day 0 by 1-way paired t tests.
Freshly isolated TCRVγ9+ PBMCs are reactive to both PAg and RTX. (A) Representative phenotypes of unactivated and PAg-activated TCRVγ9+ T lymphocytes. (B) Flow cytometry of intracellular phosphoZAP70 and phosphoERK1/2 in gated TCRVγ9+ γδ T cells after stimulation with PAg and/or cross-linked RTX (a representative experiment of 6 performed, GAH: goat anti–human IgG). (C) Depletion of B cells from PBMCs of healthy subjects (n = 8) upon 24 hours of treatment with PAg (400nM BrHPP) and/or RTX (10 μg/mL) in vitro; *P < .05 versus control and RTX versus RTX+PAg by 1-way paired Student t test. (D) Culture with PAg (400 nM BrHPP) for 7 and 13 days in medium containing 100 U/mL IL-2 amplifies the number of TCRVγ9+ γδ T lymphocytes in the PBMCs of healthy subjects (n = 10; bars: group means); *P < .05 versus day 0 by 1-way paired t tests.
These data suggested that PAg might potentiate the depletive bioactivity of RTX. We tested this possibility by assaying for autologous CD20+ B-cell depletion from PBMCs in vitro. When PBMCs from healthy donors were cultured for 24 hours in complete medium with PAg alone (Figure 1C), no CD20+ B-cell depletion was observed, whereas culture with RTX alone (10 μg/mL) depleted, on average, 93% of CD20+ B cells. Culture with both PAg and RTX depleted significantly more than with RTX alone (P > .05, n = 8 donors): on average, 96% of CD20+ B cells were depleted. Together, these data confirm that circulating PBMCs from most healthy subjects comprise few TCRVγ9+ cells with a low level of cell surface CD16, which are, nevertheless, reactive to both PAg and human IgG.
When PBMCs from healthy donors were cultured for 7 to 13 days in IL2 culture medium with BrHPP, the TCRVγ9+ T cells proliferated strongly (mean expansion indices from n = 10 donors: 35-fold by 7 days and 99-fold by 13 days; P > .05; Figure 1D). These PAg-activated γδ cells acquired an effector memory phenotype (CD27−CD45RA−) with up-regulated expression of the Fcγ receptors CD1623,–25,34 and CD32, but not CD64 (Figure 1A right shows a representative result from the same donor as for the freshly isolated resting cells shown in Figure 1A left). RTX and TTZ IgG1 competed with anti-CD16 mAb (clone 3G8) for binding to the TCRVγ9+ γδ T-cell surface accordingly (Figure S2). Thus, resting TCRVγ9+ γδ T lymphocytes can bind human IgG and their activation with BrHPP PAg increases further this ability.
PAg promotes TCRVγ9+ γδ T-cell binding to mAb target cells
These findings suggested that mAb-induced γδ T-cell binding to cancer cells might be increased by PAg. We tested this using the PKH67 transfer assay, which measures a cell contact-dependent uptake of PKH67 tracker by conjugated lymphocytes.30,35,36 This assay crucially reflects binding to live cancer cells because the PKH67 transfer from live RAJI cells was abrogated when TCRVγ9+ γδ T lymphocytes were coincubated with dead 7-AAD+ PKH67+ cells (Figure S3). When incubated in vitro for 1 hour with live PKH67+ CD20+ RAJI cells alone, TCRVγ9+ cells captured 22 504 MESF (Figure 2A), showing that γδ cells bind spontaneously to this Burkitt lymphoma cell line, as already reported.26,36 This binding was increased in the presence of either the CD20-specific mAb RTX (10 μg/mL) or PAg (BrHPP; 400 nM) but not with the unrelated Her2/Neu-specific mAb TTZ (either alone or with PAg). Binding was enhanced even more, however, in the presence of both RTX and PAg. A representative experiment of more than 5 experiments with RAJI cells is shown in Figure 2A and composite data with γδ T cells from various healthy donors, and the CD20+ B-cell lymphoma cell lines RL and KARPAS-422 (follicular lymphomas), NCEB1, and ES-MOULT (mantle cell lymphomas) are shown in Figure 2B. Although the same γδ cells were less prone to bind spontaneously to the Her2/Neu+ mammary carcinoma cell line MCF7, this binding was increased with both TTZ and PAg but not with RTX and Ag (Figure S4). Consistent with these findings, γδ cell binding to the CD20+CD52+ cell line GRANTA was increased by either PAg plus RTX or by PAg plus the CD52-specific mAb ALZ (Figure 2C). Altogether, these data confirm that mAb-induced TCRVγ9+ γδ T-cell binding to cancer cells is enhanced by the PAg BrHPP.
BrHPP plus mAbs optimize binding of γδ T lymphocytes to cancer cells in vitro. (A) Representative experiment showing γδ T-cell binding to CD20+HER2/Neu− RAJI cells in various conditions. BrHPP (400 nM), TTZ (20 μg/mL), and RTX (10 μg/mL) were added to cells as indicated. Cell binding (acquired MESF) was obtained by subtracting the value at 0 minutes from that at 60 minutes. (B) Binding to CD20+ B-cell lymphoma cell lines by TCRVγ9+ γδ cells from different subjects in the specified conditions. The data are means and 1 SD from n = 3-8 different donors; nt, not tested; *P < .05 for significant difference of the BrHPP+RTX group to the other groups by 1-way ANOVA on ranks and multiple comparison. (C) Representative TCRVγ9+ γδ cell binding to the CD20+CD52+ mantle cell lymphoma cell line GRANTA in the various conditions described in panel B, including anti-CD52 ALZ (10 μg/mL).
BrHPP plus mAbs optimize binding of γδ T lymphocytes to cancer cells in vitro. (A) Representative experiment showing γδ T-cell binding to CD20+HER2/Neu− RAJI cells in various conditions. BrHPP (400 nM), TTZ (20 μg/mL), and RTX (10 μg/mL) were added to cells as indicated. Cell binding (acquired MESF) was obtained by subtracting the value at 0 minutes from that at 60 minutes. (B) Binding to CD20+ B-cell lymphoma cell lines by TCRVγ9+ γδ cells from different subjects in the specified conditions. The data are means and 1 SD from n = 3-8 different donors; nt, not tested; *P < .05 for significant difference of the BrHPP+RTX group to the other groups by 1-way ANOVA on ranks and multiple comparison. (C) Representative TCRVγ9+ γδ cell binding to the CD20+CD52+ mantle cell lymphoma cell line GRANTA in the various conditions described in panel B, including anti-CD52 ALZ (10 μg/mL).
PAg increases RTX-induced ADCC by TCRVγ9+ γδ T lymphocytes
We next examined the effect of PAg and RTX on ADCC mediated by γδ T cells. PAg-activated TCRVγ9+ γδ T cells were obtained from PBMCs cultured with PAg in IL2 culture medium for 13 days (Figure 1) then coincubated in vitro for 4 hours with a panel of CD20+ B-lymphoma cell targets. Exocytosis of cytolytic granules, indicating cytolytic T-cell activity, was assessed by immunofluorescent staining of the cell surface for TCRVγ9 and a marker of cytolytic granules, CD107a, and flow cytometry to determine the proportion of cells expressing the 2 antigens. Upon coincubation with the lymphoma cells alone, few TCRVγ9+ γδ T cells had cell surface CD107a (Figure 3A). Upon coincubation in the presence of RTX or PAg alone, somewhat more cells had the CD107a marker on their surface, but the proportion of CD107a+ TCRVγ9+ γδ T lymphocytes was highest in the presence of both RTX and PAg together with any of the lymphoma cell targets in the panel.
BrHPP plus mAbs increase TCRVγ9+ γδ T-cell–mediated ADCC. (A) The percentage of CD107a+ cells in total TCRVγ9 lymphocytes u pon 4-hour incubation with various CD20+ B-cell lymphomas in the specified conditions. (B) ADCC of various CD20+ lymphoma cells by RTX and BrHPP-activated γδ T cells. Tumor cell lysis by RTX alone (100, 50, and 10 μg/mL):  ; PAg-activated γδ T cells alone (E/T ratio 30:1, 10:1, and 1:1): ▨; RTX (10 μg/mL) plus PAg-activated γδ T cells (E/T ratio 30:1, 10:1, and 1:1):
; PAg-activated γδ T cells alone (E/T ratio 30:1, 10:1, and 1:1): ▨; RTX (10 μg/mL) plus PAg-activated γδ T cells (E/T ratio 30:1, 10:1, and 1:1):  ; data show means ± SD from 3 to 8 experiments, each with different donor. (C) Specific lysis of CD20+ RL cells by RTX and PAg-activated γδ T lymphocytes results from ADCC (means ± 1 SD from 3 experiments each with a different donor. (D) ADCC of CD52+ RL cells with ALZ plus PAg-activated γδ T cells, as in panel C. (E) ADCC of HER2+ SKBR3 mammary carcinoma cells with TTZ plus PAg-activated γδ T cells, as in panel C. P < .05 by Mann-Whitney rank sum tests.
; data show means ± SD from 3 to 8 experiments, each with different donor. (C) Specific lysis of CD20+ RL cells by RTX and PAg-activated γδ T lymphocytes results from ADCC (means ± 1 SD from 3 experiments each with a different donor. (D) ADCC of CD52+ RL cells with ALZ plus PAg-activated γδ T cells, as in panel C. (E) ADCC of HER2+ SKBR3 mammary carcinoma cells with TTZ plus PAg-activated γδ T cells, as in panel C. P < .05 by Mann-Whitney rank sum tests.
BrHPP plus mAbs increase TCRVγ9+ γδ T-cell–mediated ADCC. (A) The percentage of CD107a+ cells in total TCRVγ9 lymphocytes u pon 4-hour incubation with various CD20+ B-cell lymphomas in the specified conditions. (B) ADCC of various CD20+ lymphoma cells by RTX and BrHPP-activated γδ T cells. Tumor cell lysis by RTX alone (100, 50, and 10 μg/mL):  ; PAg-activated γδ T cells alone (E/T ratio 30:1, 10:1, and 1:1): ▨; RTX (10 μg/mL) plus PAg-activated γδ T cells (E/T ratio 30:1, 10:1, and 1:1):
; PAg-activated γδ T cells alone (E/T ratio 30:1, 10:1, and 1:1): ▨; RTX (10 μg/mL) plus PAg-activated γδ T cells (E/T ratio 30:1, 10:1, and 1:1):  ; data show means ± SD from 3 to 8 experiments, each with different donor. (C) Specific lysis of CD20+ RL cells by RTX and PAg-activated γδ T lymphocytes results from ADCC (means ± 1 SD from 3 experiments each with a different donor. (D) ADCC of CD52+ RL cells with ALZ plus PAg-activated γδ T cells, as in panel C. (E) ADCC of HER2+ SKBR3 mammary carcinoma cells with TTZ plus PAg-activated γδ T cells, as in panel C. P < .05 by Mann-Whitney rank sum tests.
; data show means ± SD from 3 to 8 experiments, each with different donor. (C) Specific lysis of CD20+ RL cells by RTX and PAg-activated γδ T lymphocytes results from ADCC (means ± 1 SD from 3 experiments each with a different donor. (D) ADCC of CD52+ RL cells with ALZ plus PAg-activated γδ T cells, as in panel C. (E) ADCC of HER2+ SKBR3 mammary carcinoma cells with TTZ plus PAg-activated γδ T cells, as in panel C. P < .05 by Mann-Whitney rank sum tests.
In these conditions, 51Cr-release assays showed that RTX alone (10, 50, or 100 μg/mL) killed none of the 6 CD20+ lymphoma cell lines (Figure 3B). By contrast, the PAg-activated TCRVγ9+ γδ T cells alone efficiently killed 2 of them (GRANTA and DAUDI), in line with DAUDI cell sensitivity to γδ T cell–mediated lysis.37 These lytic activities were not enhanced by addition of RTX. In the cases of the 4 other CD20+ lymphoma cell lines, however, the combination of mAb with PAg-activated γδ T-cell effectors significantly enhanced cell killing compared with mAb or PAg alone (P < .05, n = 3-8 experiments each with different PBMC donors). As illustrated with RL cells, the specific lysis mediated by the combination of PAg-activated γδ T cells with RTX resulted from perforin-mediated ADCC (Figure 3C): replacing RTX with its Fab′2 fragment or adding inhibitors of vesicular transport (MgCl2/EGTA)38 to the medium abrogated cell lysis, indicating that the cells were killed by ADCC. Similarly, in the presence of ALZ, PAg-activated γδ T cells mediated ADCC of the CD52+ lymphoma cell line RL, and, in the presence of TTZ, PAg-activated γδ T cells mediated ADCC of the HER2/Neu+ breast carcinoma cell line SKBR3 (Figure 3D,E). Collectively, these data show that the ADCC of allogeneic cancer cell lines can be optimized by treatment with a combination of the appropriate mAb and PAg-activated TCRVγ9+ lymphocytes from healthy subjects.
PAg increases the ADCC of CLL cells by autologous γδ T lymphocytes and RTX
PBMCs from patients with B-cell CLL comprise both TCR Vγ9 γδ T-cell effectors and malignant CD20+ B-cell targets, so they provide a useful model with which to assess depletion of autologous, malignant B cells by RTX and PAg. When autologous CD20+ B-cell depletion was tested as previously (Figure 1C) with whole PBMCs from CLL patients (n = 8; CD19+ CD20+ cells > 98%); however, no significant depletion was produced by either of RTX, PAg, or both (Figure 4A). In addition, spontaneous apoptosis of the CLL cells was very rare for up to 13 days of in vitro culture, consistent with the known resistance of CLL cells to RTX-induced apoptosis39 and their ability to escape T-cell binding.40 Because such culture conditions did not activate any TCRVγ9+ γδ T-cell proliferation (Figure 4A), we reasoned that insufficient γδ T cell–mediated ADCC might be involved. We thus attempted to expand TCRVγ9+ γδ T lymphocytes from CLL patient PBMCs to further assess their ADCC of autologous CLL B-cell targets induced by RTX.
Depletion of autologous B cells in vitro from PBMCs of CLL patients. (A) The percentage of B cells (□) and TCRVγ9+ γδ T cells ( ) remaining in the PBMCs of CLL patients (mean ± SD, n = 8) after 1, 7, and 13 days culture in medium supplemented with PAg and therapeutic mAbs, as indicated. Data are means plus or minus SD; NS: no significant change as compared with day 0, Mann-Whitney tests with α = 5%. (B) Amplification of TCRVγ9+ γδ T cells from PBMCs of the same CLL patients as in panel A after 13 days culture in medium supplemented with IL2 and the specified reagent; *P < .05 versus “control IL2 medium” by 1-way paired t tests. (C) Cytotoxic degranulation by TCRVγ9+ γδ T cells (amplified from patient CLL017 as specified) in response to 4-hour incubation with autologous PBMCs and PAg, as indicated. (D) 51Cr-release assays for specific lysis of target cells (autologous PBMCs or allogeneic MEC2 CLL, as specified) by CLL patients' PBMCs that were either untreated or activated by 13 days' culture with BrHPP and IL2 as specified. Data are means of triplicates from 1 of 3 experiments. (E,F) Same experiments as in panels C and D with RTX-treated CLL target cells.
) remaining in the PBMCs of CLL patients (mean ± SD, n = 8) after 1, 7, and 13 days culture in medium supplemented with PAg and therapeutic mAbs, as indicated. Data are means plus or minus SD; NS: no significant change as compared with day 0, Mann-Whitney tests with α = 5%. (B) Amplification of TCRVγ9+ γδ T cells from PBMCs of the same CLL patients as in panel A after 13 days culture in medium supplemented with IL2 and the specified reagent; *P < .05 versus “control IL2 medium” by 1-way paired t tests. (C) Cytotoxic degranulation by TCRVγ9+ γδ T cells (amplified from patient CLL017 as specified) in response to 4-hour incubation with autologous PBMCs and PAg, as indicated. (D) 51Cr-release assays for specific lysis of target cells (autologous PBMCs or allogeneic MEC2 CLL, as specified) by CLL patients' PBMCs that were either untreated or activated by 13 days' culture with BrHPP and IL2 as specified. Data are means of triplicates from 1 of 3 experiments. (E,F) Same experiments as in panels C and D with RTX-treated CLL target cells.
Depletion of autologous B cells in vitro from PBMCs of CLL patients. (A) The percentage of B cells (□) and TCRVγ9+ γδ T cells ( ) remaining in the PBMCs of CLL patients (mean ± SD, n = 8) after 1, 7, and 13 days culture in medium supplemented with PAg and therapeutic mAbs, as indicated. Data are means plus or minus SD; NS: no significant change as compared with day 0, Mann-Whitney tests with α = 5%. (B) Amplification of TCRVγ9+ γδ T cells from PBMCs of the same CLL patients as in panel A after 13 days culture in medium supplemented with IL2 and the specified reagent; *P < .05 versus “control IL2 medium” by 1-way paired t tests. (C) Cytotoxic degranulation by TCRVγ9+ γδ T cells (amplified from patient CLL017 as specified) in response to 4-hour incubation with autologous PBMCs and PAg, as indicated. (D) 51Cr-release assays for specific lysis of target cells (autologous PBMCs or allogeneic MEC2 CLL, as specified) by CLL patients' PBMCs that were either untreated or activated by 13 days' culture with BrHPP and IL2 as specified. Data are means of triplicates from 1 of 3 experiments. (E,F) Same experiments as in panels C and D with RTX-treated CLL target cells.
) remaining in the PBMCs of CLL patients (mean ± SD, n = 8) after 1, 7, and 13 days culture in medium supplemented with PAg and therapeutic mAbs, as indicated. Data are means plus or minus SD; NS: no significant change as compared with day 0, Mann-Whitney tests with α = 5%. (B) Amplification of TCRVγ9+ γδ T cells from PBMCs of the same CLL patients as in panel A after 13 days culture in medium supplemented with IL2 and the specified reagent; *P < .05 versus “control IL2 medium” by 1-way paired t tests. (C) Cytotoxic degranulation by TCRVγ9+ γδ T cells (amplified from patient CLL017 as specified) in response to 4-hour incubation with autologous PBMCs and PAg, as indicated. (D) 51Cr-release assays for specific lysis of target cells (autologous PBMCs or allogeneic MEC2 CLL, as specified) by CLL patients' PBMCs that were either untreated or activated by 13 days' culture with BrHPP and IL2 as specified. Data are means of triplicates from 1 of 3 experiments. (E,F) Same experiments as in panels C and D with RTX-treated CLL target cells.
In vitro culture of PBMCs from normal donors with BrHPP and IL2 increases both numbers and ADCC function of TCRVγ9+ γδ T lymphocytes (Figures 1,Figure 2–3). Likewise, after activation with PAg and 13 days in IL2 culture medium, the PBMCs from CLL patients showed significant TCRVγ9+ γδ T-cell proliferations (P < .05, n = 8; Figure 4B) that matched those of PBMCs from healthy subjects under the same conditions (Figure 1D).
The ADCC of autologous CLL cells mediated by these PAg-activated TCRVγ9+ γδ T lymphocytes was then tested by assaying exocytosis of cytolytic granules from ADCC effector cells and 51Cr-release from dying, autologous target cells after 4 hours of coincubation. The PAg-activated TCRVγ9+ cells from CLL patients had potential for cytolytic activity, as demonstrated for example by secondary restimulation with BrHPP, but in absence of RTX, they neither secreted lytic granules (Figure 4C) nor killed autologous CLL cells (Figure 4D). By contrast, when coincubated with RTX-treated autologous CLL cells, the PAg-activated TCRVγ9+ cells from CLL patients secreted lytic granules (Figure 4E) and efficiently killed autologous CLL cells (Figure 4F), although these CLL cell lines were resistant to RTX in such conditions (Figure S5). In control experiments, whole untreated CLL PBMC were able to kill the allogeneic MEC-2 CLL cell line but marginally lysed the autologous CLL targets with or without RTX. Together, these results indicated that although CLL is almost refractory to RTX-induced depletion of autologous malignant B cells, adequate combinations with PAg-driven cytolytic γδ T lymphocytes are able to reconstitute the ADCC bioactivity of RTX in unfavorable malignant conditions.
A regimen of BrHPP and IL2 enhances RTX bioactivity in cynomolgus macaques
The cynomolgus macaque monkey (Macacca fascicularis) is a useful model with which to assess autologous depletion of normal B cells by RTX together with γδ T-cell activation by PAg and IL2, as this macaque species responds as humans do to these compounds.15,41 We treated 3 groups of age- and sex-matched cynomolgus macaques with RTX alone (group 1, n = 4), with RTX and IL2 (group 2, n = 4), or with RTX, PAg and IL2 (group 3, n = 6) following the schedule indicated in Figure 5A. Blood samples were collected at intervals throughout the schedule for analysis of B cells (using the marker for human CD20) and TCRVγ9+ T cells by flow cytometry. Before the experiment, TCRVγ9+ γδ T cells comprised 1% to 5% of the peripheral blood leukocytes. In the group 3 animals, the circulating TCRVγ9+ T-cell population expanded significantly for approximately 10 days after each PAg injection, whereas no change in these cells was detected in the other groups (Figure 5A). By day 60, as expected,15 the level of blood TCRVγ9+ cells had returned to baseline in all 3 groups, and very similar levels of TCRVγ9+ γδ T-cell infiltrates were found in the bone marrow and lymph nodes of animals in the 3 groups at necropsy (on day 62; Figure 5A).
B-cell depletion in macaques is enhanced by treatment with RTX, PAg, and IL2. Age- and sex-matched cynomolgus macaques received intravenous RTX alone (group 1; n = 4; empty circles and bars), RTX and IL2 (group 2; n = 4; gray circles and bars) or RTX, PAg, and IL2 (group 3; n = 6; black circles and bars) at the intervals specified on the graphs. Data shown are group means for blood (graphs, left) and group means ± SEM for bone marrow and lymph nodes (histograms, right) showing the percentage of cells that were TCRVγ9+ cells (A) and CD20+ B cells (B). (C) Group means for serum concentrations of RTX; *P < .05 by 1-tailed Mann-Whitney tests.
B-cell depletion in macaques is enhanced by treatment with RTX, PAg, and IL2. Age- and sex-matched cynomolgus macaques received intravenous RTX alone (group 1; n = 4; empty circles and bars), RTX and IL2 (group 2; n = 4; gray circles and bars) or RTX, PAg, and IL2 (group 3; n = 6; black circles and bars) at the intervals specified on the graphs. Data shown are group means for blood (graphs, left) and group means ± SEM for bone marrow and lymph nodes (histograms, right) showing the percentage of cells that were TCRVγ9+ cells (A) and CD20+ B cells (B). (C) Group means for serum concentrations of RTX; *P < .05 by 1-tailed Mann-Whitney tests.
Before therapy, the circulating CD20+ B cells in cynomolgus macaques comprised, on average, 17% of the peripheral blood leukocytes (range 8%-23%), as described.41 Within 24 hours after the first RTX treatment, all the CD20+ B cells were cleared from the peripheral blood of the animals in all 3 groups (Figure 5B). No circulating CD20+ B cells were detected for 8 days in all animals from groups 1 and 2 and for 15 days in group 3 animals. Groups 1, 2, and 3 took 18, 15, and 34 days, respectively, to reconstitute 10% of their initial CD20+ B-cell blood count; by day 40, the circulating CD20+ B-cell range was 0.4% to 14% of PBMCs in groups 1 and 2 and 0.2% to 6% in group 3. Upon necropsy, the mean levels of CD20+ B cells of the animals in groups 1, 2, and 3 were, respectively, 13%, 9%, and 7% of leukocytes in bone marrow and 11%, 9%, and 6% of leukocytes in lymph nodes (Figure 5B). Thus, PAg increased the bioactivity of RTX in macaques. Consistent with these findings, after 20 days, a higher concentration of free RTX was detected in the sera of macaques from group 3 than from the animals in groups 1 and 2 (Figure 5C), suggesting that the PAg regimen also improved the pharmacokinetics of RTX, possibly by reducing the circulating target cells and any antibodies against the RTX that they might produce.
Discussion
We show here that the bioactivity of the therapeutic mAb RTX is improved by association with the PAg BrHPP. Our data indicate that this improvement is due to the optimization of 2 steps in the mechanism leading to target cell cytolysis. The first step is binding of effector T cells to the target tumor cells to form an immunologic synapse—a process that is fundamental for antitumor immunity. CLL cells are able to inhibit the formation of immunologic synapses with normal T lymphocytes,40 and several hematopoietic and nonhematopoietic tumor cell lines show impaired binding by normal allogeneic T lymphocytes,26 thus providing a route for tumor cells to escape immune cell recognition.42,43 The broadly effective anticancer drug lenalidomide appears to target this binding dysfunction in CLL.40 Hence, promoting the formation of immunologic synapses between effector cells and tumor cells has great potential for cancer immunotherapy. We demonstrate that BrHPP enhances γδ T-cell binding to various tumor cells coated with any one of the 3 therapeutic mAbs we tested: RTX, ALZ, and TTZ. The second step is activation of intracellular signaling cascades leading to exocytosis of lytic granules and tumor cell ADCC. We show that BrHPP and RTX activate multiple signaling cascades in γδ T lymphocytes, leading to increased secretion of lytic granules and ADCC of target cells. Thus, increasing the rates of circulating γδ T lymphocytes able to mediate ADCC in cancer patients might prove useful for therapeutic purposes. As a proof of concept, in healthy macaques, this therapeutic regimen with BrHPP triggered proliferation of peripheral γδ T lymphocytes, providing waves of FcγR+ effector cells able to mediate ADCC in the presence of the depleting antibody.
The outcome of activating cytolytic TCRVγ9+ γδ T lymphocytes with PAg and RTX is enhanced depletion of target cells, demonstrated here for both normal and malignant B cells in vitro and in vivo. The lack of B-cell depletion from CLL patients' PBMCs in culture was not surprising, however, as the treatment of this disease usually requires combined chemo-immunotherapy regimens.44,45 In addition, the onset of CLL disease is associated with impaired CLL cell binding40 and a deficit of CLL-specific immunity involving inhibitory cytokines and regulatory T cells.46,47 Our data indicate that BrHPP expands the pool of cytolytic γδ T cells in PBMCs from CLL patients as well as from healthy subjects. In association with RTX, BrHPP triggers CLL cell lysis. Thus, despite the defective immunity of CLL patients, these in vitro data suggest that the combined PAg and RTX regimen might give better clinical responses in patients with CD20+ B-cell malignancies. This view is supported by the strong B-cell depletion observed in healthy macaques, in which γδ T-cell activation was combined with the RTX treatment, with no evidence of toxicity. In addition, this strategy is not restricted to RTX treatment of B-cell tumors, because PAg and the other therapeutic mAbs we tested, ALZ and TTZ, also improved depletion of CD52+ and HER2/Neu+ tumor cells, respectively.
These conclusions are supported by recent in vitro data with combinations of RTX or TTZ and zoledronate,27 a bisphosphonate drug developed for preventing skeletal fractures. Zoledronate is a potent and highly selective inhibitor of farnesyl diphosphate synthase from the mevalonate pathway in mammalian peroxisomes. Although not a phosphoantigen itself, zoledronate up-regulates the level of endogenous PAg metabolites in treated cells, which further activate γδ T cells.48,49 The immunostimulation patterns of BrHPP and zoledronate are different yet ultimately involve the same TCRVγ9+ γδ T lymphocytes. The bisphophonate targets most human cells, it accumulates in bone, and its serum half-life is around a week. Its bioactivity requires cell internalization, it is statin-sensitive, and it produces long-lasting PAg accumulation in treated cells. BrHPP, by contrast, only targets TCRVγ9+ γδ T lymphocytes, does not accumulate in human tissues, and its serum half-life is around 5 minutes. Its bioactivity is statin-independent, it does not require processing or cell internalization, and it triggers immediate immunologic responses.11 Future therapeutic protocols might take advantage of these contrasting features in γδ T cell–based approaches.
The treatment of B-cell lymphoma and Her2/Neu+ breast tumors has benefited considerably from the use of therapeutic mAbs, either alone or in combination with standard chemotherapy. Frequent relapses, however, demonstrate that the bioactivity of these mAbs is still suboptimal. Potentiating the cytotoxicity induced by anticancer mAbs can be achieved not only by direct engineering of these drugs but also by strategies that target the downstream cytolytic effector cells. The recruitment of Fcγ receptor-dependent functions appears well suited in this regard, because FcγR-expressing γδ T cells can be amplified and fine-tuned for stronger cytolytic activity. Given the ease of γδ T-cell amplification and cytolytic activation in patients, our results are a major milestone toward improving immunotherapies based on monoclonal antibodies. Combining PAg and a depleting mAb might prove useful for a variety of malignant and autoimmune diseases requiring targeted cell depletion. In patients with non-Hodgkin follicular lymphoma, the response rates correlate strongly with the plasma levels of RTX and with ADCC.9,50 Thus, enhancing the efficacy of RTX treatment by its combination with BrHPP has considerable therapeutic potential, most especially for patients that FcγR alleles impair ADCC. On this premise, we recently initiated a clinical trial for relapsing follicular lymphoma patients with RTX, BrHPP, and IL2 as a combination regimen. Further investigation will determine the efficacy of this new therapeutic strategy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Carol Featherstone and Kelly Thornber for proofreading and editing the manuscript, Catherine Trichard and Gilles Paintaud (Université de Tours) for technical support, and Françoise Horand (MDS Pharma Services) for animal care.
J.J.F. is supported by institutional grants from the Inserm and by contracts from the Association pour la Recherche sur le Cancer (3757), Ligue Contre le Cancer, Agence Nationale de la Recherche (PICOSTIM), and Institut National du Cancer (Projets Libre V9V2TER and PAIR Lymphome RITUXOP).
Authorship
Contribution: J.G.-D., C. Bezombes, A.-H.C., D.C., E.G., and A.Q.-M. designed and performed the experiments; C. Bonnafous, V.S., S.I., and J.-F.L. designed and performed experiments and collected data; L.Y. and G.L. collected data; H.S. designed experiments and interpreted data; and J.-J.F. designed the research program, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: J.G.-D., C. Bezombes, G.L., H.S., and J.-J.F. hold a patent on therapeutic combinations of phosphoantigen and antibodies. C. Bonnafous, V.S., S.I., J.-F.L., and H.S. are employees of Innate Pharma SA. G.L. and J.-J.F. are consultants for and employees of Innate Pharma SA. The remaining authors declare no competing financial interests.
Correspondence: Jean-Jacques Fournié, Inserm U563, BP3028 CHU Purpan, 31024 Toulouse, France; e-mail: jean-jacques.fournie@inserm.fr.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal