Abstract
The transmembrane isoform of mucin 1 (MUC1/TM) is a well-recognized tumor antigen, contributing to tumorigenesis and immune evasion. Although MUC1/TM has been correlated with malignancy, we have previously reported on antitumor properties and prevention of tumor development by a secreted splice variant of MUC1 (MUC1/sec). Because myeloid-derived suppressor cells (MDSCs) play a critical role in tumor-induced immunosuppression, we investigated their recruitment by tumor cells expressing either MUC1/TM or MUC1/sec. DA-3 tumor cells expressing MUC1/sec recruit dramatically lower levels of MDSCs, relative to MUC1/TM-expressing DA-3 cells. Because MUC1/sec was previously shown to down-regulate tumor expression of urokinase plasminogen activator (uPA), a protease linked to tumor aggressiveness and metastasis, the potential role of uPA in MDSC recruitment was investigated. Tumor-derived uPA is capable of recruiting MDSCs, and correlates with tumor development. In addition to diminishing recruitment of MDSCs, the effect of MUC1/sec on MDSC-suppressive mechanisms was investigated. MUC1/sec, or its unique immunoenhancing peptide, is capable of blocking expression of arginase 1 and production of reactive oxygen species in MDSCs, implicated in the suppression of T cells. These findings demonstrate a new mechanism of MDSC recruitment, and provide evidence that MUC1/sec has antitumor properties affecting MDSCs.
Introduction
Mucin 1 (MUC1) is a tumor antigen that is overexpressed on the surface of various epithelial tumor cells. The transmembrane isoform of MUC1 can promote tumor progression by creating a barrier around tumor cells against immune cells, sequestering compounds that suppress immune responses, interacting with growth-promoting signaling molecules, and aiding in the metastatic process.1 Furthermore, MUC1 has been shown to accelerate premalignant inflammatory lesion transformation to malignancy.2 We have previously reported on antitumor properties of a splice variant of MUC1, which is secreted and known as MUC1/sec.3-5 MUC1/sec is found in normal6,7 and tumor tissue,8 but, interestingly, malignancy in ovarian lesions has been correlated with loss of its expression.9 DA-3 murine mammary tumor cells expressing MUC1/sec (DA-3/sec) are rejected from BALB/c mice, whereas expression of the transmembrane isoform of MUC1 (MUC1/TM; DA-3/TM) has little effect.3-5 DA-3/sec cells, however, lead to tumor development in immunocompromised BALB/c nude mice, or BALB/c mice depleted of T lymphocytes. Furthermore, depletion of innate immune cells allows initial tumor growth, followed by tumor regression once the adaptive immune response develops.3 We have determined that MUC1/sec modulates various factors involved in tumorigenesis, such as up-regulating signal transducer and activator of transcription 1 (Stat1), leading to the down-regulation of tumor-derived urokinase plasminogen activator (uPA), a serine protease correlated with tumor progression in human cancers.5
Other investigators have reported that tumor cell expression of MUC1/TM recruits immature dendritic cells (DCs).10 In our previous studies, we analyzed whether there was a difference in recruitment of immune cells to DA-3/TM or DA-3/sec cells, and we reported that DA-3/TM cells recruit more GR-1+ cells than DA-3/sec tumor cells.3 We and others have also previously described that a myeloid population that accumulates in many tumor models, and are known as CD11b+GR-1+ myeloid-derived suppressor cells (MDSCs),11-16 orchestrate immunosuppression by suppressing T cells.13 In this study, we show that GR-1+ cells that are recruited to high levels by DA-3/TM cells, but not DA-3/sec, are in fact MDSCs. Furthermore, we report for the first time that uPA, which is down-regulated by MUC1/sec, is capable of recruiting MDSCs. This is a new mechanism by which uPA may be augmenting and contributing to tumor development.
MUC1 has also been shown to impair differentiation and function of DCs,17 along with inducing a regulatory phenotype with immune-suppressive cytokine secretion.10,17,18 We therefore investigated whether in addition to modulating recruitment of MDSCs, MUC1/sec can alter MDSC-suppressive mechanisms. We found that whereas DA-3/TM cells induce high levels of arginase in MDSCs, associated with suppression,19 DA-3/sec cells block that induction. Furthermore, a unique peptide found in MUC1/sec and called immunoenhancing peptide (IEP), which we have reported to have antitumor properties,4 can also block induction of arginase in MDSCs. The production of reactive oxygen species (ROS) in MDSCs, which has been correlated with arginase levels and also contributes to immunosuppression,20 is likewise blocked by MUC1/sec. Thus, the antitumor properties of MUC1/sec include down-regulating tumor-induced recruitment of MDSCs via uPA, and also blocking MDSC-suppressive mechanisms.
Methods
Animals and cell lines
BALB/c mice (H-2d) were bred in our animal facility at the University of Miami according to guidelines of the National Institutes of Health (NIH; Bethesda, MD). BALB/cnu/nu mice were purchased from Charles River Laboratories (Wilmington, MA). DO11.10 transgenic mice were provided by Dr Becky Adkins (University of Miami, Miami, FL). FVB and matrix metalloprotease (MMP)–9−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). DA-3, DA-3/sec, and DA-3/TM cells were maintained and injected in mice, and tumors were measured, as previously described.3,5,12 For uPA studies, mice were injected intraperitoneally with 1 μg recombinant murine uPA (Molecular Innovations, Southfield, MI), 12 μg recombinant mouse plasminogen activator inhibitor 1 (PAI-1; EMD/Calbiochem, Gibbstown, NJ), or 0.9% saline. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Miami.
Cell harvesting/purification
Spleens were mashed through 70-μM cell strainers (BD Biosciences, San Jose, CA) to obtain single-cell suspensions. Bone marrow (BM) was harvested by cutting femur ends, and flushing BM with RPMI media using a 25-gauge needle. Peritoneal cavity was lavaged by injecting 5 mL cold phosphate-buffered saline (PBS), and shaking cavity using hemostats before collecting cells. Blood was collected into PBS/0.008% heparin (Sigma-Aldrich, St Louis, MO) by submandibular bleeding using animal lancets (Medipoint, Mineola, NY). Plasma was collected by bleeding into heparinized microvettes (Braintree Scientific, Braintree, MA), then spinning tubes at 3000g for 15 minutes, 4°C. uPA in plasma was analyzed by loading 100 μL plasma per well in a total uPA enzyme-linked immunosorbent assay (Molecular Innovations). Tumor-infiltrating cells were harvested using a digestion protocol by Dr Alan B. Frey (New York University, New York, NY).21 Purification of MDSCs was performed by staining cells using anti–GR-1 (RB6-8C5) antibody (BD Biosciences), followed by magnetic antibody cell separation (MACS) using goat anti–rat microbeads (Miltenyi Biotec, Auburn, CA). To analyze MDSCs histologically, 106 cells in RPMI were allowed to dry onto slides, then fixed in methanol, and stained with Harleco Hemcolor (EMD).
Flow cytometry
Cells were stained, washed, and resuspended in fluorescence-activated cell sorter buffer (PBS, 0.5% bovine serum albumin, 0.1% sodium azide) for analysis on a LSR II flow cytometer (BD Biosciences). Antibodies used were anti–GR-1 (RB6-8C5), anti-CD11b (M1/70), anti-CD11c, anti-CD86, anti-CD80, anti-interleukin (IL)–4Ra, and anti-PDL1 from BD Biosciences; pan anti–major histocompatibility complex (MHC) class II and anti–MCSF-R from eBioscience (San Diego, CA); and anti-F4/80 from Invitrogen (Carlsbad, CA). For ROS detection, cells were resuspended in 2 mL prewarmed 37°C Dulbecco modified Eagle medium (DMEM), and 5- (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA [DCFDA]; Invitrogen) was added for a final concentration of 1 μM. Cells were incubated at 37°C for 20 minutes, then washed twice in cold PBS, and labeled for surface markers.
Suppression assay
After red cell lysis, DO11.10 splenocytes were plated (2 × 105 cells/96 flat-bottom well), in HL-1 media (BioWhittaker, Walkersville, MD) containing penicillin/streptomycin, glutamax, 2-mercaptoethanol (2-ME), ovalbumin (OVA; 15 μg/mL; EMD/Calbiochem), 500 μM Nω-hydroxy-nor-l-arginine (NOHA), and NG-monomethyl-l-arginine (NMMA; EMD), and MDSCs were added at indicated concentrations. After 48 hours, 1 μCi [3H]thymidine was added per well. Eighteen hours later, cells were harvested onto filters and analyzed using a beta counter. For proliferation by CFSE (5- (and 6-)carboxyfluorescein diacetate succinimidyl ester) analysis, DO11.10 splenocytes were labeled with 2.5 μM CFSE at room temperature for 10 minutes, and then cocultured with MDSCs for 5 days before analysis of CFSE dilution by flow cytometry.
uPA gene knockdown/analysis
Retroviral vector encoding shRNA to murine uPA (clone V2MM_57 826; Open Biosystems, Huntsville, AL) was transfected into AMPHO packaging cells using Lipofectamine2000 (Invitrogen) for 6 hours, 37°C. Media were then replaced, and the next day media was again replaced, and packaging cells were incubated at 32°C. The next day, media was collected, filtered using 0.45-μM syringe filters, aliquoted, and frozen at −80°C. DA-3/TM cells plated the previous day (2 × 105 cells/6 wells) were transduced by replacing media with 1 mL retrovirus-containing media, and 1 μL 1000× polybrene (Millipore, Billerica, MA) for 24 hours, 37°C. Cells were washed 2 times with PBS and cultured for 2 days before selection using 1 ug/mL puromycin (Sigma-Aldrich). After selection, cloning was performed, and then clones were plated (4 × 105/6 wells) for RNA isolation. RNA was isolated using TRI reagent (Molecular Research Center, Cincinnati, OH). cDNA was made from 1 μg RNA using cDNA Synthesis Kit (Fermentas, Glen Burnie, MD). Semiquantitative polymerase chain reaction (PCR) was performed on 2 μL cDNA using a 2× PCR Master Mix (Fermentas), with the following primers: uPA forward (5′-GCTCCTATAATCCTGGAGAGATGAA), uPA reverse (5′-ACCTGTCTTTTCAGCTTCTTCCCTCC), β-actin forward (5′-GGGAATGGGTCAGAAGGACT), and β-actin reverse (5′-TTTGATGTCACGCACGATTT). PCR conditions were as follows: 95°C for 2 minutes, 30 cycles for uPA or 25 cycles for β-actin (cycle = 95°C, 30 seconds; 58°C, 30 seconds, 72°C, 30 seconds), and 72°C for 10 minutes. Densitometric analysis was performed using Scion Image Software (NIH). RNA levels were normalized to the signal of β-actin, and reported as relative density. uPA zymography gels were performed, as previously described.5
Arginase analysis
MDSCs were plated (5 × 106/24 wells) in MDSC media (DMEM/F12, 10% FCS, penicillin/streptomycin, 1× nonessential amino acids, 2 mM Na pyruvate, Glutamax, and 2-ME). After 24 hours, cell lysates were obtained, and 50 μg protein was loaded on gels for Western blot analysis, as previously described.5 Antibodies used included anti–arginase 1 (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-actin (Sigma-Aldrich). For functional arginase assay, MDSCs were plated (3 × 105/96-well flat-bottom) in MDSC media, with 20% conditioned media from tumor cells, 100 ng/mL recombinant IL-4 (BD Biosciences), and/or 10 μg/mL multiple antigenic peptide (MAP)–IEP/SCR.4 IgY control antibody, or a chicken antibody against IEP, was described previously.4 Size-separated DA-3/sec conditioned media was produced using Amicon Ultra 100 K centrifugal filters (Millipore). After 48 hours, cells were washed using PBS, and 50 μL lysis buffer (10 mL H2O, 1 tablet Complete Mini EDTA [ethylenediaminetetraacetic acid]–free protease inhibitor [Roche, Mannheim, Germany], and 0.1% Triton X-100 [Sigma-Aldrich]) was added per well. Cells were incubated at 37°C for 30 minutes, and lysates were transferred to microfuge tubes. A total of 50 μL of 25 mM Tris-HCL (Sigma-Aldrich) and 10 μL 2 mM MnCl2 (Alfa Aesar, Ward Hill, MA) was added to each sample. Samples were heated at 56°C for 10 minutes, and 100 μL of 500 mM l-arginine (Alfa Aesar), pH 9.7, was added. Arginine hydrolysis was stopped after 30-minute incubation at 37°C, with 800 μL acid solution (1:3:7, 96% H2SO4:85% H3PO4:H2O). A total of 40 μL 4% α-isonitrosopropiophenone (Sigma-Aldrich) in 100% ethanol was added to precipitate the urea by-product. Samples were heated at 100°C for 30 minutes. Optical density 540 nm was measured. A standard curve was performed by running samlples of urea (Alfa Aesar) in lysis buffer parallel to cell lysates.
Statistical analysis
Error bars represent SEM, and all P values were 2 sided (t test).
Results
MUC1/sec expression by tumor cells decreases accumulation of MDSCs
We have previously shown that tumor cell expression of MUC1/sec leads to tumor rejection by an immune-mediated antitumor response.3-5 Furthermore, in our attempt to dissect which immune cells play a role in the rejection, we performed recruitment studies in vivo and reported that DA-3/sec tumor cells recruit significantly lower levels of GR-1+ cells relative to DA-3/TM cells.3 To further analyze the GR-1+ cell population recruited, we analyzed whether they coexpress CD11b, characteristic of MDSCs that mediate immunosuppression.11,16 Furthermore, MDSCs have been correlated with tumor burden, and because DA-3/sec cells do not develop tumors in immune intact animals, MDSC levels were analyzed in immunocompromised BALB/c nude mice harboring DA-3/TM or DA-3/sec tumors. Spleens and tumors were harvested when tumors reached similar volumes (900-1000 mm3). Whereas spleens of DA-3/sec tumor-bearing animals had 11% MDSCs, spleens of DA-3/TM tumor-bearing animals had 30% (Figure 1A). Within DA-3/TM tumors we detected 13% MDSCs relative to 3% in DA-3/sec tumors (Figure 1A). The absolute numbers of MDSCs were also significantly different, with 16.3 × 106 splenic and 1.6 × 106 tumor MDSCs in DA-3/TM tumor-bearing animals, and 4.2 × 106 splenic and 0.5 × 106 tumor MDSCs in DA-3/sec tumor-bearing animals (P < .002 and .05; data not shown). The levels of blood MDSCs as a function of tumor size were analyzed. MDSCs accumulate to greater levels in the blood of DA-3/TM relative to DA-3/sec tumor-bearing BALB/cnu/nu mice in parallel to tumor volume (Figure 1B). Whereas the levels of MDSCs do increase in the blood of DA-3/sec tumor-bearing mice from 25% to 50%, the increase is far more dramatic in DA-3/TM tumor-bearing mice in which 80% of the circulating white blood cells are CD11b+GR-1+ (Figure 1C). Thus, lack of immunosuppressive MDSC accumulation and recruitment to DA-3/sec tumor cells may contribute to effective antitumor immune responses, leading to tumor rejection.
MUC1/sec-expressing tumor cells (DA-3/sec) accumulate less MDSCs, relative to MUC1/TM-expressing cells (DA-3/TM). BALB/cnu/nu mice were implanted with 106 DA-3/sec or DA-3/TM cells s.c. (A) Single-cell suspensions were produced from tumors and spleens of mice harboring tumors with volumes of 900 to 1000 mm3, then stained for myeloid-derived suppressor cell (MDSC) markers (CD11b+ and GR-1+) and analyzed by flow cytometry. (B) Blood from mice at different stages in tumor development was collected, and cells were stained and analyzed by flow cytometry for MDSC markers. (C) Blood from animals harboring similar sized DA-3/sec or DA-3/TM tumors was collected, and cells were stained and analyzed by flow cytometry for MDSC markers. (D) Plasma from normal animals and tumor bearers was assayed for urokinase plasminogen activator (uPA). Error bars representing SEM and P values are provided.
MUC1/sec-expressing tumor cells (DA-3/sec) accumulate less MDSCs, relative to MUC1/TM-expressing cells (DA-3/TM). BALB/cnu/nu mice were implanted with 106 DA-3/sec or DA-3/TM cells s.c. (A) Single-cell suspensions were produced from tumors and spleens of mice harboring tumors with volumes of 900 to 1000 mm3, then stained for myeloid-derived suppressor cell (MDSC) markers (CD11b+ and GR-1+) and analyzed by flow cytometry. (B) Blood from mice at different stages in tumor development was collected, and cells were stained and analyzed by flow cytometry for MDSC markers. (C) Blood from animals harboring similar sized DA-3/sec or DA-3/TM tumors was collected, and cells were stained and analyzed by flow cytometry for MDSC markers. (D) Plasma from normal animals and tumor bearers was assayed for urokinase plasminogen activator (uPA). Error bars representing SEM and P values are provided.
uPA recruits and leads to accumulation of MDSCs
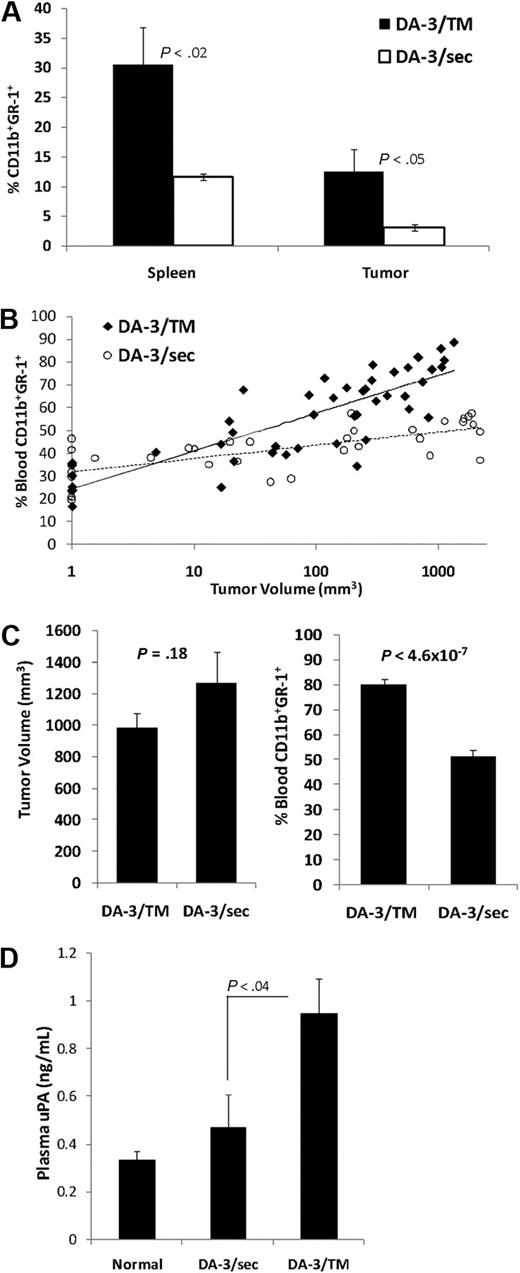
Because MDSC levels have been correlated with tumor-derived factors, we began to analyze what factors produced by the tumor cells may be responsible for the lack in accumulation of MDSCs by DA-3/sec cells. We have recently reported that MUC1/sec is capable of up-regulating the levels of Stat1 in murine and human tumors, and in doing so down-regulates a protumorigenic serine protease, urokinase plasminogen activator (uPA).5 We found that DA-3/TM tumors that produce uPA show elevated levels of uPA in plasma, whereas DA-3/sec tumors show near normal levels of plasma uPA (Figure 1D). Because uPA is known to be involved in migration of immune cells and tumor cells,22 we investigated whether uPA is capable of recruiting MDSCs in tumor-bearing mice that have high levels of MDSCs. Tumor-bearing mice were injected intraperitoneally with 1 μg uPA, or saline as control. Whereas splenic MDSC levels were similar in both control saline-injected mice and uPA-injected tumor-bearing mice, after 2 hours there was a prominent recruitment of CD11b+GR-1+ cells into the peritoneal cavity in mice that received uPA (Figure 2A). To ascertain whether we could recruit and accumulate MDSCs in normal mice, we injected BALB/c mice with 8 injections of uPA or saline every other day, and then cells were harvested 2 hours after the last injection. uPA recruited dramatic levels of MDSCs intraperitoneally, 36% relative to 2% in controls, and levels of circulating MDSCs in the blood doubled from 20% to 44% (Figure 2B). We also saw a modest, but significant increase of MDSCs, from 3.8% to 6.4%, in the spleens. However, there was no significant difference in the levels of MDSCs in the BM. To ascertain whether these recruited CD11b+GR-1+ cells functioned as MDSCs, we performed a T-cell suppression assay. Splenocytes from DO11.10 transgenic mice, which harbor CD4+ T cells specific for OVA, were stimulated in vitro using whole OVA protein. MDSCs, which were recruited intraperitoneally in normal BALB/c mice using uPA, or splenic MDSCs from DA-3/TM tumor bearers as a control, were added at various ratios. The recruited MDSCs were functionally suppressive of T-cell proliferation (Figure 2C).
uPA can recruit MDSCs. (A) DA-3/TM tumor-bearing mice were injected intraperitoneally with saline as control or 1 μg recombinant uPA. Two hours later, peritoneal lavages (IP) were performed and spleens were harvested for staining MDSCs and analyzing by flow cytometry. (B) Normal BALB/c mice were injected intraperitoneally 8 times every other day with either saline as control, or 1 μg recombinant uPA. Two hours after the last injection, the BM, spleen, blood, and IP cells were harvested and stained for MDSC analysis by flow cytometry. (C) Normal BALB/c mice were injected 2 times every other day with uPA intraperitoneally, and then 2 hours after the last injection IP cells were purified for MDSCs using an antibody to GR-1. Splenic MDSCs from DA-3/TM tumor-bearing mice were also purified. DO11.10 splenocytes were then cocultured with MDSCs at various ratios and activated with OVA. T-cell proliferation (counts per million [CPM]) was measured after providing cultures [3H]thymidine. Error bars representing SEM and P values are provided. Results are representative of at least 3 experiments.
uPA can recruit MDSCs. (A) DA-3/TM tumor-bearing mice were injected intraperitoneally with saline as control or 1 μg recombinant uPA. Two hours later, peritoneal lavages (IP) were performed and spleens were harvested for staining MDSCs and analyzing by flow cytometry. (B) Normal BALB/c mice were injected intraperitoneally 8 times every other day with either saline as control, or 1 μg recombinant uPA. Two hours after the last injection, the BM, spleen, blood, and IP cells were harvested and stained for MDSC analysis by flow cytometry. (C) Normal BALB/c mice were injected 2 times every other day with uPA intraperitoneally, and then 2 hours after the last injection IP cells were purified for MDSCs using an antibody to GR-1. Splenic MDSCs from DA-3/TM tumor-bearing mice were also purified. DO11.10 splenocytes were then cocultured with MDSCs at various ratios and activated with OVA. T-cell proliferation (counts per million [CPM]) was measured after providing cultures [3H]thymidine. Error bars representing SEM and P values are provided. Results are representative of at least 3 experiments.
Because we did not observe an increase of MDSC levels in the BM of mice given uPA, we investigated the source of the high levels of circulating MDSCs. One hypothesis was that uPA treatment prevented differentiation of MDSCs into other cell types, thus accumulating cells with the CD11b+GR-1+ markers in the periphery. If that were the case, one could expect to see a decrease in DCs or macrophages in the periphery. However, analyses of cells from the different compartments of BALB/c mice given uPA or saline as control did not result in a decrease in CD11c+ DCs or F4/80+ macrophages (Figure 3A,B). Another possibility is that uPA recruits cells out of the BM into circulation rapidly, and that analyzing the different compartments 2 hours after the last injection of uPA would prevent the detection of an increase in MDSC levels in the BM. Therefore, BALB/c mice were injected 2 times every other day with either uPA or saline, and then cells were harvested from the different compartments 2 days rather than 2 hours later. Whereas an increase in MDSC levels in the peritoneal cavity or spleen was no longer observed, we still saw high levels of MDSCs in the blood, 39% relative to 22%, and additionally, high levels of MDSCs in the BM, 60% relative to 37% in controls (Figure 3C). These results led us to question whether uPA causes the proliferation of MDSCs directly. Lineage-negative cells from normal BM were purified, and cultured in vitro for 5 days with or without uPA. There was no difference in the levels of MDSCs in the presence of uPA (data not shown), but these findings do not rule out the possibility that in vivo uPA can cooperate with other signals to induce proliferation of MDSCs.
Source and inhibition of uPA-mediated MDSC recruitment. Normal BALB/c mice were injected intraperitoneally 8 times every other day with either saline as control, or 1 μg recombinant uPA. Two hours after the last injection, the BM, spleen, blood, and IP cells were harvested and stained for (A) dendritic cells (CD11c) and (B) macrophages (F4/80), and analyzed by flow cytometry. (C) Normal BALB/c mice were injected intraperitoneally 2 times every other day with either saline as control, or 1 μg recombinant uPA. Two days after the last injection, the BM, spleen, blood, and IP cells were harvested and stained for MDSC analysis by flow cytometry. (D) FVB and FVB MMP-9−/− mice were injected intraperitoneally with 1 μg recombinant uPA, or saline as control, 2 times every other day. The IP cells were harvested 2 hours after the last injection and stained for MDSC analysis by flow cytometry. (E) Normal BALB/c mice were injected intraperitoneally with 1 μg recombinant uPA alone, or in conjunction with 12 μg recombinant PAI-1. Two hours later, IP cells were harvested and stained for MDSC analysis by flow cytometry. Error bars representing SEM and P values are provided.
Source and inhibition of uPA-mediated MDSC recruitment. Normal BALB/c mice were injected intraperitoneally 8 times every other day with either saline as control, or 1 μg recombinant uPA. Two hours after the last injection, the BM, spleen, blood, and IP cells were harvested and stained for (A) dendritic cells (CD11c) and (B) macrophages (F4/80), and analyzed by flow cytometry. (C) Normal BALB/c mice were injected intraperitoneally 2 times every other day with either saline as control, or 1 μg recombinant uPA. Two days after the last injection, the BM, spleen, blood, and IP cells were harvested and stained for MDSC analysis by flow cytometry. (D) FVB and FVB MMP-9−/− mice were injected intraperitoneally with 1 μg recombinant uPA, or saline as control, 2 times every other day. The IP cells were harvested 2 hours after the last injection and stained for MDSC analysis by flow cytometry. (E) Normal BALB/c mice were injected intraperitoneally with 1 μg recombinant uPA alone, or in conjunction with 12 μg recombinant PAI-1. Two hours later, IP cells were harvested and stained for MDSC analysis by flow cytometry. Error bars representing SEM and P values are provided.
uPA has been shown to activate MMPs,23 and it has previously been reported that MDSCs express high levels of MMP-9.24 In addition, MMP-9 made by MDSCs and BM progenitor cells is partly responsible for the expansion of MDSCs in tumor-bearing hosts.25 We therefore sought to analyze whether MMP-9 plays a role in the migration of MDSCs by uPA in vivo. FVB MMP-9−/− mice, and FVB mice as controls, injected with uPA intraperitoneally, led to the same level of MDSC recruitment (Figure 3D). However, this shows evidence that uPA can recruit MDSCs in different strains of mice (BALB/c, FVB, and C57BL/6 [data not shown]). A natural inhibitor of uPA, PAI-1 inhibits uPA activity by binding uPA and its receptor uPAR, leading to rapid endocystosis and degradation of uPA. MDSCs do express uPAR (data not shown), and so we tested whether PAI-1 could inhibit the recruitment of MDSCs by uPA. BALB/c mice were injected with 1 μg uPA intraperitoneally alone or in conjunction with 12 μg recombinant PAI-1, and a significant reduction of uPA-mediated MDSC recruitment was observed with the coinjection of PAI-1 (Figure 3E).
Tumor-derived uPA correlates with MDSC recruitment and tumor growth in vivo
To assess whether tumor-derived uPA affects MDSC recruitment and tumor growth, we decided to knock down expression of uPA by DA-3/TM cells. A replication-defective retrovirus was produced using a vector containing shRNA for murine uPA. DA-3/TM cells were infected with the retrovirus, selected, and cloned out. Sixty-five clones were screened by semiquantitative PCR for knockdown of uPA expression. Three clones were chosen for further study, and relative to DA-3/TM, semiquantitative PCR for uPA RNA expression in clones DA-3/TM-shuPA8, DA-3/TM-shuPA52, and DA-3/TM-shuPA56 showed 85, 73, and 49% knockdown in uPA expression at the RNA level, respectively (Figure 4A). A zymography assay to test functional uPA enzymatic activity was performed on 3-day culture supernatants from DA-3/TM cells and the 3 clones. Relative to DA-3/TM, conditioned media from the tumor cells showed that clones DA-3/TM-shuPA8, DA-3/TM-shuPA52, and DA-3/TM-shuPA56 were 74%, 68%, and 0% knocked down in the levels of secreted uPA, respectively (Figure 4B). Thus, DA-3/TM-shuPA8 and DA-3/TM-shuPA52 serve as 2 clones in which uPA activity was knocked down considerably, whereas DA-3/TM-shuPA56 serves as a control cell line that underwent all the same conditions of infection, selection, and cloning, but is not knocked down for uPA expression at the protein level. The various tumor cells were then tested in an in vivo assay for recruitment of MDSCs intraperitoneally, as we had done initially for DA-3/TM and DA-3/sec cells.3 Whereas the 2 cell lines secreting high levels of uPA, DA-3/TM and DA-3/TM-shuPA56, recruited similar levels of MDSCs intraperitoneally, the 2 cell lines making considerably less uPA, DA-3/TM-shuPA8 and DA-3/TM-shuPA52, recruited substantially less MDSCs (Figure 4C). These results are similar to our previous ones in which DA-3/sec tumor cells, which do not secrete uPA, recruit far less GR-1+ cells than DA-3/TM cells.3 To evaluate the effects of uPA down-regulation on tumor development, we implanted BALB/c mice with 106 tumor cells. DA-3/TM and DA-3/TM-shuPA56 developed tumors at similar growth rates, whereas DA-3/TM-shuPA8 and DA-3/TM-shuPA52, which secrete less uPA, developed tumors significantly slower (Figure 4D). To confirm that systemic uPA levels can augment tumor growth, BALB/c mice were implanted with DA-3/TM tumor cells, and administered 1 μg of recombinant uPA or saline intraperitoneally every other day while monitoring tumor development. Administration of uPA systemically leads to faster tumor progression (Figure 4E).
Tumor-derived uPA recruits MDSCs and augments tumor growth. DA-3/TM tumor cells were infected with a retrovirus encoding murine uPA shRNA, then selected, and cloned. Three clones were analyzed (DA-3/TM-shuPA8, -shuPA52, and -shuPA56). (A) Semiquantitative PCR for uPA and β-actin message was performed on RNA from DA-3/TM cells and the uPA knockdown clones. Relative density of uPA to β-actin is presented. (B) Conditioned media from tumor cells that were plated in serum-free media for 3 days were analyzed on an uPA-specific zymography gel. As a positive control, 50 ng recombinant uPA (Rt.uPA) was loaded in parallel. The high-molecular-weight (HMW) and low-molecular-weight (LMW) degradation product of uPA can be detected on the gel. The density of the HMW uPA bands is presented. (C) To analyze recruitment of MDSCs to the various tumor cells, 2 × 106 tumor cells were injected intraperitoneally, and 18 hours later IP cells were harvested and stained for MDSC analysis by flow cytometry. (D) Tumor development was monitored by implanting BALB/c mice with 106 cells subcutaneously. and measuring tumors every 3 to 4 days. (E) To determine whether systemic uPA can augment tumor development, BALB/c mice were injected with 106 DA-3/TM cells subcutaneously, and either 1 μg recombinant uPA or saline intraperitoneally every other day for 3 weeks while tracking tumor development every 3 to 4 days. Error bars representing SEM and P values are provided. *P < .001 between DA-3/TM-shuPA8 and DA-3/TM-shuPA56. **P < .05 between DA-3/TM-shuPA52 and DA-3/TM-shuPA56.
Tumor-derived uPA recruits MDSCs and augments tumor growth. DA-3/TM tumor cells were infected with a retrovirus encoding murine uPA shRNA, then selected, and cloned. Three clones were analyzed (DA-3/TM-shuPA8, -shuPA52, and -shuPA56). (A) Semiquantitative PCR for uPA and β-actin message was performed on RNA from DA-3/TM cells and the uPA knockdown clones. Relative density of uPA to β-actin is presented. (B) Conditioned media from tumor cells that were plated in serum-free media for 3 days were analyzed on an uPA-specific zymography gel. As a positive control, 50 ng recombinant uPA (Rt.uPA) was loaded in parallel. The high-molecular-weight (HMW) and low-molecular-weight (LMW) degradation product of uPA can be detected on the gel. The density of the HMW uPA bands is presented. (C) To analyze recruitment of MDSCs to the various tumor cells, 2 × 106 tumor cells were injected intraperitoneally, and 18 hours later IP cells were harvested and stained for MDSC analysis by flow cytometry. (D) Tumor development was monitored by implanting BALB/c mice with 106 cells subcutaneously. and measuring tumors every 3 to 4 days. (E) To determine whether systemic uPA can augment tumor development, BALB/c mice were injected with 106 DA-3/TM cells subcutaneously, and either 1 μg recombinant uPA or saline intraperitoneally every other day for 3 weeks while tracking tumor development every 3 to 4 days. Error bars representing SEM and P values are provided. *P < .001 between DA-3/TM-shuPA8 and DA-3/TM-shuPA56. **P < .05 between DA-3/TM-shuPA52 and DA-3/TM-shuPA56.
DA-3/sec MDSCs do not suppress T cells
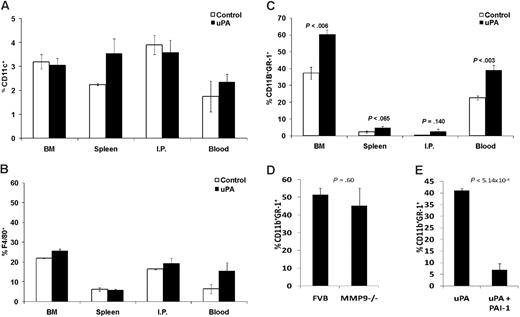
MDSCs have been shown to suppress T-cell proliferation by various mechanisms, so we analyzed whether MDSCs from BALB/cnu/nu mice with either DA-3/sec or DA-3/TM tumors suppressed in a similar manner. Splenic MDSCs were purified from tumor-bearing animals as well as from normal BALB/cnu/nu, and a T-cell suppression assay was performed using CFSE-labeled DO11.10 splenocytes stimulated with OVA protein in vitro. MDSCs from DA-3/TM tumor-bearing, but not normal or DA-3/sec tumor-bearing mice suppressed T-cell proliferation (Figure 5A). Because MDSCs have been reported to suppress via up-regulating the enzyme arginase 1,26 or production of nitric oxide (NO),27 we performed a suppression assay with splenic MDSCs from DA-3/TM tumor-bearing mice, adding the arginase inhibitor NOHA and/or the NO synthase inhibitor NMMA. Whereas NOHA reversed the suppression of T-cell proliferation by MDSCs, NMMA had no effect in our model (Figure 5B). Thus, MDSCs from DA-3/sec tumor bearers are not suppressive, whereas the enzyme arginase is responsible for DA-3/TM MDSC-associated T-cell suppression.
DA-3/sec MDSCs are not suppressive, and MDSCs suppress via the enzyme arginase 1. (A) BALB/cnu/nu mice were implanted with 106 DA-3/TM or DA-3/sec tumor cells. When tumors reached similar volumes, spleens of normal mice and tumor mice were harvested, and MDSCs were purified using a GR-1 antibody, then cocultured with CFSE-labeled DO11.10 splenocytes, and stimulated with OVA protein. Proliferation was measured by analyzing CFSE dilution by flow cytometry. (B) Splenic MDSCs were isolated from DA-3/TM tumor bearers and cultured in a suppression assay as in panel A, with the addition of the arginase inhibitor (NOHA) and/or the NO synthase inhibitor (NMMA). Proliferation (CPM) was measured after providing cultures [3H]thymidine. Error bars representing SEM are provided, and data are representative of at least 3 experiments.
DA-3/sec MDSCs are not suppressive, and MDSCs suppress via the enzyme arginase 1. (A) BALB/cnu/nu mice were implanted with 106 DA-3/TM or DA-3/sec tumor cells. When tumors reached similar volumes, spleens of normal mice and tumor mice were harvested, and MDSCs were purified using a GR-1 antibody, then cocultured with CFSE-labeled DO11.10 splenocytes, and stimulated with OVA protein. Proliferation was measured by analyzing CFSE dilution by flow cytometry. (B) Splenic MDSCs were isolated from DA-3/TM tumor bearers and cultured in a suppression assay as in panel A, with the addition of the arginase inhibitor (NOHA) and/or the NO synthase inhibitor (NMMA). Proliferation (CPM) was measured after providing cultures [3H]thymidine. Error bars representing SEM are provided, and data are representative of at least 3 experiments.
MUC1/sec blocks induction of arginase and ROS in MDSCs
Because MUC1/sec expression alters expression of tumorigenic factors in tumor cells, such as Stat1 and uPA,5 we investigated whether MUC1/sec, which is secreted by the tumor cells, can alter MDSCs. We isolated splenic MDSCs from DA-3/TM tumor bearers and cultured them in vitro for 24 hours in the presence of 20% conditioned media (CM) from DA-3/TM and/or DA-3/sec tumor cells. Western blot of MDSC lysates demonstrates that whereas DA-3/TM CM induces expression of arginase to high levels in MDSCs, DA-3/sec CM does not (Figure 6A). Furthermore, the induction of arginase by DA-3/TM CM can be blocked by the addition of DA-3/sec CM. These results were confirmed using an arginase assay, in which arginase in lysates of MDSCs is activated and allowed to hydrolyze exogenous arginine, releasing the measurable by-product urea. Using this assay, DA-3/TM CM also induces high levels of arginase in MDSCs, whereas DA-3/sec CM blocks its induction (Figure 6B). IL-4 was used as a positive control in this assay for induction of arginase, which when added to DA-3/TM CM was additive in its induction of arginase. Again, adding DA-3/sec CM with DA-3/TM CM and IL-4 considerably lowered the levels of arginase (Figure 6B). We have previously reported that MUC1/sec contains a unique 11 amino acid peptide, IEP, shown to have antitumor properties.4 To investigate whether IEP by itself could block the induction of arginase in MDSCs, splenic MDSCs from DA-3/TM tumor bearers were cultured with 20% DA-3/TM CM, with the addition of 10 μg/mL of either MAP-IEP format, or a peptide with the same amino acids in a scrambled order (MAP-SCR) as control. The MAP format contains 4 peptides conjugated to a branched lysine core, which in our previous studies offered protection to mice against tumor development.4 Whereas DA-3/TM CM induces high levels of arginase in MDSCs, the addition of MAP-IEP blocks this induction (Figure 6C). Furthermore, we filtered DA-3/sec CM using a 100-kDa filter separating the CM into the approximately 115-kDa MUC1/sec-containing fraction (sec[> 100 K]) and a fraction lacking MUC1/sec (sec[< 100 K]). The addition of IL-4 and the various DA-3/sec CM fractions to MDSC cultures indicates that only MUC1/sec-containing fractions can inhibit arginase (Figure 6D). In addition, the use of a chicken antibody specific for IEP4 can block arginase inhibition by MUC1/sec-containing fractions.
MUC1/sec and its unique IEP block induction of arginase in MDSCs. (A) Splenic MDSCs from DA-3/TM tumor-bearing mice were purified and cultured in vitro in the presence of 20% CM from DA-3/TM and/or DA-3/sec tumor cell cultures for 24 hours. MDSC lysates were then analyzed by Western blot for the enzyme arginase I and β-actin. Relative density of arginase to β-actin is presented. (B) Splenic MDSCs from DA-3/TM tumor-bearing mice were cultured as in panel A, and after 48 hours MDSC lysates were obtained and an arginase assay was performed to detect levels of arginase. Urea is measured as a by-product of exogenous arginine hydrolysis. IL-4 was used (100 ng/mL) as a positive control for induction of arginase in MDSCs. (C) MDSCs were cultured and assayed for the presence of arginase as in panel B, with the addition to the cultures of 10 μg/mL of the unique MUC1/sec peptide (MAP-IEP), or as a control a scrambled peptide sequence (MAP-SCR). (D) Splenic MDSCs were cultured as in panel B with IL-4 and DA-3/sec CM, or DA-3/sec CM separated using a 100-kDa filter into a MUC1/sec-containing fraction (sec > 100 K), and a fraction lacking MUC1/sec (sec < 100 K). Antibody specific for IEP (aIEP) or a control IgY was also added to MDSC cultures, and an arginase assay was performed. Error bars representing SEM are provided, and data are representative of at least 3 experiments.
MUC1/sec and its unique IEP block induction of arginase in MDSCs. (A) Splenic MDSCs from DA-3/TM tumor-bearing mice were purified and cultured in vitro in the presence of 20% CM from DA-3/TM and/or DA-3/sec tumor cell cultures for 24 hours. MDSC lysates were then analyzed by Western blot for the enzyme arginase I and β-actin. Relative density of arginase to β-actin is presented. (B) Splenic MDSCs from DA-3/TM tumor-bearing mice were cultured as in panel A, and after 48 hours MDSC lysates were obtained and an arginase assay was performed to detect levels of arginase. Urea is measured as a by-product of exogenous arginine hydrolysis. IL-4 was used (100 ng/mL) as a positive control for induction of arginase in MDSCs. (C) MDSCs were cultured and assayed for the presence of arginase as in panel B, with the addition to the cultures of 10 μg/mL of the unique MUC1/sec peptide (MAP-IEP), or as a control a scrambled peptide sequence (MAP-SCR). (D) Splenic MDSCs were cultured as in panel B with IL-4 and DA-3/sec CM, or DA-3/sec CM separated using a 100-kDa filter into a MUC1/sec-containing fraction (sec > 100 K), and a fraction lacking MUC1/sec (sec < 100 K). Antibody specific for IEP (aIEP) or a control IgY was also added to MDSC cultures, and an arginase assay was performed. Error bars representing SEM are provided, and data are representative of at least 3 experiments.
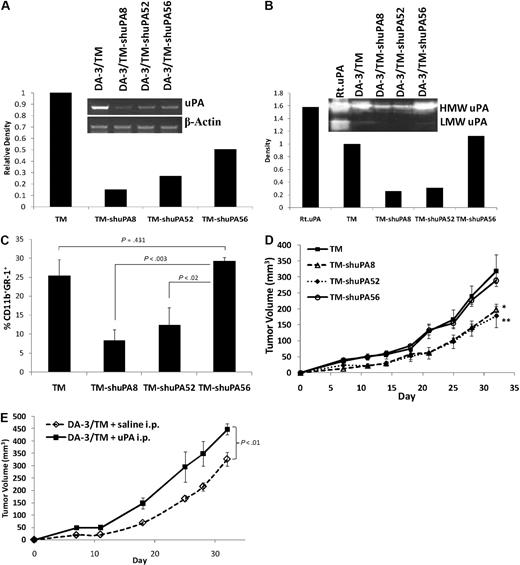
Several investigators have shown that arginase levels in MDSCs correlate with the production of ROS in these cells, and that ROS contributes to immunosuppression by MDSCs.14,20,28 We assessed whether MUC1/sec-containing media can block ROS production by MDSCs. Splenic MDSCs from DA-3/TM tumor-bearing mice were cultured in vitro in the presence of IL-4, 20% DA-3/TM CM, and/or DA-3/sec CM, sec(> 100 K), sec(< 100 K), for 18 hours. Cells were treated with DCFDA, a cell-permeable compound that is nonfluorescent until oxidized by ROS inside the cell. Treatment with DA-3/TM CM shows an up-regulation of the levels of ROS in MDSCs, whereas DA-3/sec CM causes the levels of ROS to decrease (Figure 7A). DA-3/sec CM and sec (> 100 K) CM block induction of ROS in MDSCs by DA-3/TM CM, whereas sec (< 100 K) CM does not. We confirmed that ROS levels correlate with arginase levels in MDSCs, as treating the cells with NOHA also blocks ROS in MDSCs, whereas IL-4 up-regulates the levels (Figure 7A). Furthermore, whereas sec(> 100 K) down-regulates ROS production induced by IL-4, anti-IEP reverses this effect (Figure 7B). To determine whether this occurs in vivo, ROS levels in blood or spleen MDSCs from BALB/cnu/nu animals harboring DA-3/TM or DA-3/sec tumors were compared. There is a dramatic difference in ROS production, with high levels in blood and spleen MDSCs from DA-3/TM relative to DA-3/sec tumor-bearing mice (Figure 7C). Blood MDSCs from DA-3/sec or DA-3/TM tumor-bearing mice were histologically similar, representing a heterogeneous population of immature cells, as described previously (Figure 7D). Splenic MDSCs were further characterized, and were negative for most markers tested, but had a modest increase in MHC class II and CD86 levels on MDSCs from DA-3/sec animals relative to MDSCs from DA-3/TM animals (Figure 7E).
MUC1/sec blocks ROS production in MDSCs. (A) Splenic MDSCs from DA-3/TM tumor-bearing mice were cultured in vitro for 18 hours in the presence of IL-4, 20% DA-3/TM CM, DA-3/sec CM, DA-3/sec CM separated into MUC1/sec-containing fraction (sec[> 100 K]), and/or DA-3/sec CM lacking MUC1/sec (sec[< 100 K]). NOHA was used in cultures as a control for inhibition of ROS production by MDSCs. ROS was detected by treating cells with 1 μM DCFDA, then staining cells for MDSCs and analyzing MDSCs for ROS fluorescence by flow cytometry. Gating was set based on NOHA-treated MDSCs. (B) Splenic MDSCs were cultured as in panel A, with the addition of an aIEP or a control IgY, with mean fluorescence intensities (MFIs) indicated. (C) Blood and spleens were harvested from BALB/cnu/nu mice harboring either DA-3/TM or DA-3/sec tumors, and cells were directly treated with DCFDA without in vitro culture, and stained and gated for MDSC analysis of ROS production. Histograms were overlayed and MFIs are indicated. (D) Blood MDSCs from DA-3/sec or DA-3/TM tumor-bearing BALB/cnu/nu mice were fixed on slides and stained for histology. (E) Splenocytes from DA-3/sec or DA-3/TM tumor-bearing BALB/cnu/nu mice were stained and gated for MDSCs, and the indicated markers were analyzed, with MFIs listed. Results are representative of at least 3 experiments.
MUC1/sec blocks ROS production in MDSCs. (A) Splenic MDSCs from DA-3/TM tumor-bearing mice were cultured in vitro for 18 hours in the presence of IL-4, 20% DA-3/TM CM, DA-3/sec CM, DA-3/sec CM separated into MUC1/sec-containing fraction (sec[> 100 K]), and/or DA-3/sec CM lacking MUC1/sec (sec[< 100 K]). NOHA was used in cultures as a control for inhibition of ROS production by MDSCs. ROS was detected by treating cells with 1 μM DCFDA, then staining cells for MDSCs and analyzing MDSCs for ROS fluorescence by flow cytometry. Gating was set based on NOHA-treated MDSCs. (B) Splenic MDSCs were cultured as in panel A, with the addition of an aIEP or a control IgY, with mean fluorescence intensities (MFIs) indicated. (C) Blood and spleens were harvested from BALB/cnu/nu mice harboring either DA-3/TM or DA-3/sec tumors, and cells were directly treated with DCFDA without in vitro culture, and stained and gated for MDSC analysis of ROS production. Histograms were overlayed and MFIs are indicated. (D) Blood MDSCs from DA-3/sec or DA-3/TM tumor-bearing BALB/cnu/nu mice were fixed on slides and stained for histology. (E) Splenocytes from DA-3/sec or DA-3/TM tumor-bearing BALB/cnu/nu mice were stained and gated for MDSCs, and the indicated markers were analyzed, with MFIs listed. Results are representative of at least 3 experiments.
Discussion
Immunosuppression plays a key role in regulating antitumor responses, and a key cell population that is an important effector is MDSCs. Close to 20 years ago, we reported that these myeloid cells accumulate in tumor-bearing mice due to tumor-derived factors,12 and that they down-regulate polyclonal and antigen-specific T- and B-cell responses via different mechanisms involving either prostaglandin E2 (PGE2), or a cell contact–dependent interaction.13 These cells are now recognized as CD11b+GR-1+ immature myeloid cells, and found to mediate immunosuppression in cancer,14,29,30 traumatic stress,31 burns,32 and infection.33 Furthermore, they have been found to block natural killer (NK)–cell cytotoxicity,34 cross-talk with macrophages,35 induce development of T regulatory cells,36,37 and most importantly block T-cell function.14 Although the glycoprotein MUC1 (MUC1/TM) has been linked to tumor aggressiveness and contributes to various stages of tumorigenesis, we have previously reported on antitumor properties and protection against tumor development by a secreted splice variant MUC1/sec.3-5 Furthermore, in ovarian cancer patients, MUC1/sec was found in benign lesions, and its loss was correlated with malignant lesions.9 In our attempt to determine the mechanisms of MUC1/sec antitumor properties, we investigated the recruitment of immune cells to tumor cells expressing either MUC1/TM or MUC1/sec. We showed previously that DA-3/sec tumor cells recruited far less GR-1+ cells than DA-3/TM cells.3 We now present evidence that these cells are MDSCs, and that MUC1/sec expression by tumor cells reduces MDSC accumulation in the tumor, the spleen, and the blood of tumor bearers (Figure 1).
MDSC accumulation has been correlated to tumor burden (Figure 1B), due to greater production of tumor-derived factors. We report for the first time that uPA, produced by various mouse and human tumors, is capable of mediating MDSC recruitment (Figure 2). We previously demonstrated that MUC1/sec can up-regulate expression of Stat1 in tumor cells, leading to the down-regulation of uPA,5 and whereas DA-3/sec tumor bearers have normal circulating plasma uPA levels, DA-3/TM tumor bearers have 2-fold higher circulating uPA levels (Figure 1D). Thus, DA-3/sec cells most likely recruit less MDSCs due to their down-regulation of uPA. It has previously been shown that uPA is critical for macrophage chemotaxis,38 basophil chemotaxis,39 migration, and invasion of immature DCs,40 and uPA−/− mice fail to recruit inflammatory cells to sites of infections.41 Furthermore, it has been shown in human neutrophils that uPA can form a trimolecular complex with its receptor, uPAR, and the CD11b integrin, resulting in enhanced adhesion and migration.42 We now show that uPA can lead to systemic accumulation of MDSCs in normal mice (Figure 2B), can recruit MDSCs that are capable of suppressing T cells (Figure 2C), and can augment tumor development (Figure 4E). The role of uPA in immunosuppression has been implicated previously with NKT cell–derived uPA, leading to the indirect activation of transforming growth factor-β and peripheral tolerance in the eye.43 With our studies, we provide additional evidence that uPA is involved in immunosuppression by recruiting MDSCs. Furthermore, we showed that different levels of tumor-derived uPA correlate with MDSC recruitment to tumor cells, and tumor development (Figure 4). From our in vitro data (data not shown), we do not have evidence that would suggest that uPA directly causes MDSC proliferation; however, rapid recruitment of MDSCs out of the BM most likely leads to a reactive homeostatic proliferation (Figure 3C). It has also been shown that a soluble fragment of human uPAR, which can be cleaved by uPA, can mobilize CD34+ mouse and human hematopoietic stem and progenitor cells,44 but the chemotactic peptide sequence of uPAR (88-92, SRSRY) used is found in human uPAR, but not mouse uPAR. Furthermore, we showed that the natural inhibitor of uPA, PAI-1, can block the migration of MDSCs by uPA (Figure 3E).
MUC1 has been shown by several groups to induce regulatory DCs with high IL-10 and decreased IL-12 secretion, associated with immunosuppression.10,17,18 We investigated whether MUC1/sec, besides altering recruitment of MDSCs, can affect their suppressive mechanisms because MDSCs developing in the presence of DA-3/sec tumors are not suppressive (Figure 5A). MDSCs have been shown to suppress T cells via either production of NO or up-regulating arginase.45 In our tumor model, MDSCs suppress T cells via the enzyme arginase (Figure 5B), so we investigated whether MUC1/sec can alter arginase expression. By culturing splenic MDSCs with conditioned media from DA-3/TM or DA-3/sec, containing secreted MUC1/sec, we determined that DA-3/TM CM induces high levels of arginase expression, and DA-3/sec CM does not (Figure 6). In addition to not inducing arginase, DA-3/sec CM blocked induction of this enzyme by DA-3/TM CM or IL-4. We have previously described that MUC1/sec contains the unique peptide IEP, not found in MUC1/TM or any other protein, and has protective properties against tumor development.4 MAP-IEP was also capable of blocking induction of arginase in MDSCs by DA-3/TM CM. Induction of arginase by DA-3/TM cells is in part due to PGE2 (data not shown), which is made by both DA-3/TM and DA-3/sec cells, and others have shown it to induce arginase expression and suppression.26,46 In addition, we investigated whether uPA, produced by DA-3/TM cells, can also induce MDSC-suppressive mechanisms such as arginase. Although MDSCs recruited intraperitoneally by uPA were suppressive (Figure 2C), uPA did not induce arginase in MDSCs in vitro (data not shown). It is possible that uPA along with other signals in the peritoneal cavity induce a suppressive program in intraperitoneally recruited MDSCs in vivo. In addition to limiting arginine availability and increasing levels of urea, arginase is correlated with the levels of ROS production.20 Furthermore, it has been shown that all-trans retinoic acid eliminates MDSCs by differentiating them into mature myeloid cells, in a mechanism that involves neutralizing ROS.28 In this study, we confirm that arginase levels correlate with ROS production in MDSCs, and that MUC1/sec is capable of down-regulating ROS, like arginase, in MDSCs in vitro and in vivo (Figure 7). Whereas uPA down-regulation in tumor cells expressing MUC1/sec plays a role in their rejection, this is not the sole contributing factor. For example, we have shown previously that DA-3/sec cells and other mouse and human tumor cells exposed to MUC1/sec up-regulate Stat1 to high levels, which makes them susceptible to IFNs.5 Thus, expression of uPA alone would not allow for DA-3/sec cells to develop tumors in immune intact animals, as they would remain susceptible to IFNs, and to MUC1/sec and IEP immune-modulating effects such as blocking MDSC-suppressive mechanisms.
Evidence is accumulating that MDSCs play a role in human malignancy-induced immunosuppression,47 and in immunotherapeutic strategies.48 Our results demonstrate that a naturally occurring protein, MUC1/sec, has antitumor properties, which include down-regulating tumor production of uPA, leading to decreased MDSC recruitment, and down-regulation of MDSC-suppressive mechanisms, namely arginase and ROS. Furthermore, IEP, which has antitumor properties, contributes to this process. Discovering that uPA leads to MDSC recruitment and enhancement of immunosuppression also contributes to our understanding as to why it has been correlated with poor prognosis and cancer aggressiveness among its other protumorigenic functions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are particularly grateful for the advice and assistance provided by Mary Ellen Handel-Fernandez, Lynn M. Herbert, and Mantley Dorsey Jr. We also thank Dr Carolyn Cray and Dr Norman H. Altman for their advice.
This work was supported in part by Grants R01 CA25583 and F31 GM079805 from NIH, a National Cancer Center (Plainview, NY) predoctoral fellowship, and a BD Biosciences (San Jose, CA) Immunology Research Grant.
National Institutes of Health
Authorship
Contribution: D.I. performed and designed experiments and wrote the manuscript; and D.M.L. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Diana M. Lopez, Department of Microbiology and Immunology, Miller School of Medicine, University of Miami, PO Box 016960 (R-138), Miami, FL 33101; e-mail: dlopez@med.miami.edu.

![Figure 2. uPA can recruit MDSCs. (A) DA-3/TM tumor-bearing mice were injected intraperitoneally with saline as control or 1 μg recombinant uPA. Two hours later, peritoneal lavages (IP) were performed and spleens were harvested for staining MDSCs and analyzing by flow cytometry. (B) Normal BALB/c mice were injected intraperitoneally 8 times every other day with either saline as control, or 1 μg recombinant uPA. Two hours after the last injection, the BM, spleen, blood, and IP cells were harvested and stained for MDSC analysis by flow cytometry. (C) Normal BALB/c mice were injected 2 times every other day with uPA intraperitoneally, and then 2 hours after the last injection IP cells were purified for MDSCs using an antibody to GR-1. Splenic MDSCs from DA-3/TM tumor-bearing mice were also purified. DO11.10 splenocytes were then cocultured with MDSCs at various ratios and activated with OVA. T-cell proliferation (counts per million [CPM]) was measured after providing cultures [3H]thymidine. Error bars representing SEM and P values are provided. Results are representative of at least 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/19/10.1182_blood-2008-08-176438/7/m_zh80170934150002.jpeg?Expires=1769128289&Signature=twX-f3E4Eo4zMBMyloQGEGbfVuN61jD8-0pqMxPt7NbetjrgTjrPg04SS8n6nrGuBvWUyyj1OtWXNVfxtBspr2elOg7lUjj~Qjj4Xm7tkA-tKTpsaLsEzxp~psdsIjExV6dcjrCLgViJDoW5gWS58kvTD9RYE-LXeH5LwdMVoSvizDGMhPvvm~GphUgXS1p5Ohquj~Rj-WSFMscK8BJMWymiO-M~5Y9EE2xopIpW5EGthlMKrSA9zU~SXkVaZfD7tjEN0SQNAO05iusQAVxDWtCZJdSPM97qGzbEtxmLiCynewQbKT~hMK~ODZ3PpdiCEbB9J0QGIgBUwMt5z3dFcQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. DA-3/sec MDSCs are not suppressive, and MDSCs suppress via the enzyme arginase 1. (A) BALB/cnu/nu mice were implanted with 106 DA-3/TM or DA-3/sec tumor cells. When tumors reached similar volumes, spleens of normal mice and tumor mice were harvested, and MDSCs were purified using a GR-1 antibody, then cocultured with CFSE-labeled DO11.10 splenocytes, and stimulated with OVA protein. Proliferation was measured by analyzing CFSE dilution by flow cytometry. (B) Splenic MDSCs were isolated from DA-3/TM tumor bearers and cultured in a suppression assay as in panel A, with the addition of the arginase inhibitor (NOHA) and/or the NO synthase inhibitor (NMMA). Proliferation (CPM) was measured after providing cultures [3H]thymidine. Error bars representing SEM are provided, and data are representative of at least 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/19/10.1182_blood-2008-08-176438/7/m_zh80170934150005.jpeg?Expires=1769128289&Signature=yMhO5m6LSgmN16VLovHBvDP0REpHcw7THVmjyEHlf8CXC-tzy2LuVoWriacLi2FH6gSJi1BUnL7hPttyWgm2N6GWltTlftr4FKE9R-etndpOlWHTId~X7MPaL2P2r4lRh5VIpK6gSgmBHBBCBv3~~gFfv29fe1IanrK7bLr7x2tQJ5xpDCFPxWrOPs2nrekZxVB7Dy0tOK1QW8kyEN0idSM-h-8YLEzdnHDP7OIjbra6t1neW8f4On5eCdW0avmUqliqGq5U170ZQYO23M~uvoAxZ9XFo7ycdZ9ut3SN4dt2mOGmxSvhFE7A-VgFR5EUPfrRnxWPA9WSENK6d-DDTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. MUC1/sec blocks ROS production in MDSCs. (A) Splenic MDSCs from DA-3/TM tumor-bearing mice were cultured in vitro for 18 hours in the presence of IL-4, 20% DA-3/TM CM, DA-3/sec CM, DA-3/sec CM separated into MUC1/sec-containing fraction (sec[> 100 K]), and/or DA-3/sec CM lacking MUC1/sec (sec[< 100 K]). NOHA was used in cultures as a control for inhibition of ROS production by MDSCs. ROS was detected by treating cells with 1 μM DCFDA, then staining cells for MDSCs and analyzing MDSCs for ROS fluorescence by flow cytometry. Gating was set based on NOHA-treated MDSCs. (B) Splenic MDSCs were cultured as in panel A, with the addition of an aIEP or a control IgY, with mean fluorescence intensities (MFIs) indicated. (C) Blood and spleens were harvested from BALB/cnu/nu mice harboring either DA-3/TM or DA-3/sec tumors, and cells were directly treated with DCFDA without in vitro culture, and stained and gated for MDSC analysis of ROS production. Histograms were overlayed and MFIs are indicated. (D) Blood MDSCs from DA-3/sec or DA-3/TM tumor-bearing BALB/cnu/nu mice were fixed on slides and stained for histology. (E) Splenocytes from DA-3/sec or DA-3/TM tumor-bearing BALB/cnu/nu mice were stained and gated for MDSCs, and the indicated markers were analyzed, with MFIs listed. Results are representative of at least 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/19/10.1182_blood-2008-08-176438/7/m_zh80170934150007.jpeg?Expires=1769128289&Signature=Ey93PtCP6MgPAXJgZhRjh9lmGJJqrEaiHOCCvim6P92c1S2MWlxMUNQEME4aeSo9WwmXCrDZUuI4nRCkFA2szm-U3RD8d3gig3YNUHmgHEt6G9KRg9wHT7A0g3oYOX3FgWOUw5X2AH-H-XDuYXkNXz8ts7MKTWcovmZwHDP9Rh9cskGk4FP07zCuu9yCBHEp26mJ0EgU9swCZQw~w-7QCfyn1ivO4M-eexf991HE~XbrhqmplIuLDVk1GKJKo4N9p8s1N6kRuTutL56Sfk5w8v-xkF3ic2IBsI~nJXlm4R8edk3rTu0aPKkkV1wb6GOvs6aAQBtYjq-j7qrptI7RYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal