Abstract
Patients with Mendelian susceptibility to mycobacterial disease have severe, recurrent life-threatening infections with otherwise poorly pathogenic mycobacteria and salmonellae. The extreme susceptibility is the result of genetic defects in the interleukin-12/interferon-γ (IL-12/IFN-γ) pathway. The infections are difficult to treat, and therapeutic options are limited. We explored the feasibility of antisense-mediated exon skipping as therapy for Mendelian susceptibility to mycobacterial disease with cells from a complete IL-12Rβ1−/− patient. Expression constructs were first studied to determine whether IL12RB1 lacking exon 2 encodes a functional protein. The IL-12Rβ1 expression construct lacking exon 2 was expressed on T cells. On IL-12 or IL-23 stimulation, this construct phosphorylated similar amounts of STAT1, STAT3, and STAT4 and induced similar amounts of IFN-γ compared with a normal IL-12Rβ1 construct. Antisense oligonucleotides (AONs) directed at exon 2 resulted in transcripts lacking exon 2 in both controls' and patients' T cells. In IL-12Rβ1−/− cells, skipping of exon 2 led to expression of IL-12Rβ1 on the cell surface and responsiveness to IL-12. We showed that IL12RB1 lacking exon 2 encodes a functional IL-12Rβ1. We demonstrated that T cells can be highly efficiently transduced with AONs and are amenable to antisense-mediated exon skipping. Furthermore, we showed that exon skipping (partly) corrects the IL-12Rβ1 deficiency in patients' cells.
Introduction
Human host immunity against pathogens is dependent on an effective cell-mediated immune response. Dendritic cells and macrophages recognize invading pathogens through innate pattern recognition receptors, such as Toll-like receptors and DC-SIGN, which are expressed on the membrane or intracellularly. This recognition results in production of interleukin-23 (IL-23), IL-18, and tumor necrosis factor. IL-23 initially drives the production of interferon-γ (IFN-γ) by natural killer (NK)–like T cells.1 IFN-γ in turn activates the macrophages to initiate various mechanisms to kill phagocytosed pathogens and to produce IL-12, which drives Th1 polarization. IL-23– and IL-12–activated NK- and T-cell subsets in addition provide an important link between innate and acquired immunity by diverse mechanisms including activating memory T cells.2
Patients with Mendelian susceptibility to mycobacterial disease (MSMD) suffer severe, life-threatening illnesses from infections with otherwise nonpathogenic mycobacteria and salmonellae resulting from defects in the IL-12/IFN-γ pathway. In immunodeficient MSMD patients, mutations have been found in the genes encoding the IL-12p40 subunit of IL-12 and IL-23 (IL12B), the IL12Rβ1 subunit of the IL-12 and IL-23 receptor (IL12RB1), the IFN-γR1 and R2 subunits of the IFN-γ receptor (IFNGR1 and IFNGR2), and in one of the signal transducers of this pathway; STAT1 (STAT1).2 The infections in MSMD patients are difficult to treat and are often recurrent. In patients with complete defects in these genes, the mortality before the age of 11 years is high: IL-12p40, 31%; IL-12Rβ1, 10%; IFN-γR1, 27%; and STAT1, 100%.2 Presently, there are no effective therapies for MSMD. Patients are treated with a long-term course of intense antibiotic therapy, which can be extended indefinitely to prevent new infections.3 Some patients (with IL-12p40 or IL12Rβ1 defects) benefit from additional administration of IFN-γ. Although bone marrow transplantations have been performed in several patients with complete IFN-γR1 defects, they were unsuccessful as half of the patients died and the other half had complications and a high incidence of graft failure.4
A new approach to therapy for MSMD may be through correction of certain defects in the patients' RNA, which can be achieved by a technique called exon skipping. Using antisense oligonucleotides (AONs), alternative splicing of the RNA can be forced to create an mRNA that encodes a shortened, yet functional, protein. Exon skipping has already been successfully applied for correction of various genetic diseases in patient cells ex vivo (eg, β-thalassemia, cystic fibrosis, ocular albinism type 15-7 ). In mouse models, application in vivo has been successful in the treatment of, for example, myotonic dystrophy8 and β-thalassemia.9 The results of the first clinical trial to treat patients with Duchenne muscular dystrophy in vivo with AONs are promising.10
In MSMD patients, the exon skip technique may be applicable in homozygous recessive defects (such as found in IL12B, IL12RB1, IFNGR1, IFNGR2, and STAT1) and in dominant-negative defects (such as found in IFNGR1 and STAT1) where the affected allele can be specifically targeted. The target cells (monocytes/macrophages or T cells, depending on the gene affected) are present in the circulation; therefore, the experiments to test these therapies can be carried out ex vivo.
We explored the feasibility of exon skipping as therapy for MSMD using cells from a patient with complete IL-12Rβ1 deficiency. After initial experiments with IL-12Rβ1 expression vectors to determine whether the shortened protein would be functional, activated T cells of the patient were treated with AONs. We assayed the effect on RNA splicing, protein expression, and IL-12 responses.
Methods
Patients and controls
Cells were obtained from 2 unrelated patients with the same homozygous Q32X null mutation in IL-12Rβ1 resulting from a single nucleotide substitution (r.94C >T) in exon 2. One of the patients has been described before as Patient 1 by de Jong et al.11 Cells from healthy donors were used as controls. All research involving patient material was approved by the Leiden University Medical Center-Medical Ethical Committee under number P07.48. This research was conducted in accordance with the Declaration of Helsinki.
IL-12Rβ1 and IL-23R expression constructs
Normal full-length IL12RB1 and IL23R coding sequences were cloned before into the expression vectors pLZRS-IRES-GFP and pLZRS-IRES-ΔNGFR, respectively.12,13 IL12RB1 lacking exon 2 was introduced in the construct by site-directed mutagenesis.14 As negative controls, vectors without insert were applied. Helper-free recombinant retrovirus was produced after introducing the constructs into a 293T-based amphotropic retroviral packaging cell line, Phoenix,15 using a calcium-phosphate transfection kit (Invitrogen, Carlsbad, CA). The virus-producing cells were subsequently cultured for 2 weeks under 2 μg/mL puromycin (Clontech, Mountain View, CA) selection after which a 20-hour supernatant was harvested.
Cells, culture conditions, and retroviral transduction of expression constructs
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by Ficoll-Amidotrizoate density gradient centrifugation. Cells were cultured in Iscove modified Dulbecco medium (Lonza Walkersville, Walkersville, MD) supplemented with 20 mM GlutaMAX (Invitrogen), 10% fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin (Invitrogen), and 30 U/mL IL-2 (Chiron, Emeryville, CA). To generate activated T cells, PBMCs were stimulated in the previous culture medium supplemented with 800 ng/mL PHA-16 (Murex Diagnostics, Dartford, United Kingdom). On day 2, after phytohemagglutinin (PHA) stimulation, 0.5 × 106 activated T cells were transduced with virus particles in CH-296-coated (RetroNectin, Takara, Kyoto, Japan) 48-well plate according to the protocol by Heemskerk et al16 with minor modifications. On day 14, cells were sorted with fluorescence-activated cell sorter (FACS) on green fluorescent protein (GFP) signal, after which 0.3 to 0.5 × 106 cells were restimulated at least twice with PHA in the presence of 106 irradiated allogeneic T cells (pool from 5 donors) and 0.1 × 106 irradiated B-lymphoblastoid cell line (B-LCL). After each restimulation, cells were allowed to proliferate for 14 days.
FACS and functional analyses of expression constructs
Cells were labeled directly with phycoerythrin (PE)-conjugated mouse anti–human IL-12Rβ1 mAb 2.4E6 (BD Biosciences PharMingen, San Diego, CA), F14R or DU-1 (Santa Cruz Biotechnology, Santa Cruz, CA), or labeled indirectly with rat anti–human IL-12Rβ1 mAb 2B10 in combination with the biotin-conjugated mouse anti–rat mAb G28-5 and streptavidin-PE (BD Biosciences, San Diego, CA) to determine IL-12Rβ1 expression by FACS analysis. Cells were tested for their ability to respond to human recombinant IL-12 (R&D Systems, Minneapolis, MN), IL-18 (MBL International, Woburn, MA), and IL-23 (R&D Systems). Mitogenic stimuli were anti-CD3 (OKT3) or anti-CD2 (CLB-T11.1/1 and CLB-T11.2/1 at 1:1; Sanquin) in combination with anti-CD28 (CLB-CD28/1; Sanquin Reagents, Amsterdam, The Netherlands). The concentration of IFN-γ and IL-10 in the supernatant was determined by cytokine-specific enzyme-linked immunosorbent assays (ELISAs; PeliKine/Sanquin). To study STAT phosphorylation, cells were fixed with paraformaldehyde and permeabilized with methanol. Then the cells were washed with phosphate-buffered saline, 0.2% bovine serum albumin, blocked with normal goat serum, and stained with the phospho-specific antibodies pY701-STAT1-Alexa 647, pY705-STAT3-PE, or pY693-STAT4-Alexa 647 (BD Biosciences PharMingen). Before analyzing by FACS, the cells were washed twice.
AON design and chemistry
AONs were designed to target exonic splice enhancers (ESEs), the sequences within an exon that are recognized by splicing factors to include an exon in a transcript. The locations of potential ESEs were predicted by ESEfinder (http://rulai.cshl.edu/tools/ESE/).17 The secondary structure of the pre-mRNA as predicted by M-FOLD (http://frontend.bioinfo.rpi.edu/applications/mfold/)18 was also taken into account.
AONs were high-performance liquid chromatography-purified, 21-mer 2′O-methyl RNA oligonucleotides with a full phosphorothioate backbone. Sequences of the AONs are from 5′ to 3′: AON63 ccugaaaacagcacucacuggu, AON64 gucugcauccggauauggcggg, AON128 ccuaaaaacagcacucacuggu (Prosensa, Leiden, The Netherlands), AON01 auauggcggguccugaaaaca, AON02 cugagucugcauccggauaugg, AON03 agcacucacugguucugcaggc (Eurogentec, Seraing, Belgium). AON63 was also available with a 5′ FAM label (Prosensa).
Cell culture and transduction of AONs
PBMCs were isolated from heparinized blood by Ficoll-Amidotrizoate density gradient centrifugation, and cells were stored frozen until use. Cells were cultured in hTC culture medium (Amaxa Biosystems, Gaithersburg, MD) supplemented with 20 mM GlutaMAX (Invitrogen), 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin (Invitrogen), and 30 U/mL IL-2 (Chiron Mimotypes). To generate activated T cells, PBMCs were stimulated in the previous culture medium supplemented with 1000 ng/mL PHA-16 (Murex Diagnostics). Typically, 6 days after PHA stimulation, 106 cells per well were transfected by electroporation with 20 μg/mL AON with the Nucleofector kit for human T cells (VPA-1002, Amaxa Biosystems) according to the manufacturer's protocols. Cells were immediately transferred to hTC culture medium supplemented with 10% FCS and incubated for 24 hours at 37°C, 5% CO2.
RNA isolation and reverse-transcription PCR
RNA was isolated from 106 cells 24 hours after transfection with Trizol reagent (Invitrogen). The RNA was reverse transcribed in a reaction containing 0.8 μg oligo-dT primer (Isogen Life Sciences, De Meern, The Netherlands), room temperature buffer (Invitrogen), 10 mM dithiothreitol, 500 μM deoxynucleoside triphosphates, 24 U RNAsin (Promega, Madison, WI), and 200 U SuperScript reverse transcriptase (Invitrogen). A total of 2 μL of cDNA was used in a polymerase chain reaction (PCR) in GoTaq Flexibuffer (Promega), 2.5 mM MgCl2, 200 μM deoxynucleoside triphosphates (Invitrogen), 10 pmol primers, and 0.8 U GoTaq DNA polymerase (Promega) in a total volume of 50 μL. Primer sequences are from 5′ to 3′ GAPDH-F: catcaccatcttccaggagc, GAPDH-R: gagtccttccacgatacc, IL12RB1-1F: tcgcaggtggcagagag, IL12RB1-4R: gggtcacctcaggagacttct, IL12RB1-1/3F: tgctgtccaggcagggcggctc, IL12RB1-17R: tagcctcgggcgagtca. PCR products were analyzed on agarose gels. For sequencing, PCR products were purified with a QIAquick PCR purification kit (QIAGEN, Valencia, CA). Products were sequenced using the BigDye Terminators, version 2.0 Cycle Sequencing kit (Applied Biosystems, Foster City, CA) and an ABI PRISM3700 DNA analyzer (Applied Biosystems).
Cytokine production
A total of 1.25 × 105 AON-transfected cells/well were stimulated in 200 μL hTC medium, supplemented with 20 mM GlutaMAX (Invitrogen), 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, with 2 μg/mL anti-CD2, 2 μg/mL anti-CD28, 1000 pg/mL IL-12 (R&D Systems), and/or 75 ng/mL IL-18 (BioSource International, Camarillo, CA). To measure cytokine production, supernatant of each well was removed after 2 to 6 days. The concentrations of IFN-γ were determined by cytokine-specific ELISAs (BioSource International).
Results
IL-12Rβ1 construct lacking exon 2 is expressed on the membrane
To determine feasibility of exon skipping for correction of MSMD, we chose to test the technique in cells from 2 unrelated patients with the same complete IL-12Rβ1 deficiency. Both patients harbor homozygous single nucleotide substitutions (r.94C >T) in exon 2 of the IL12RB1 gene, resulting in a premature stop of protein translation (Q32X). First, we determined whether IL-12Rβ1 lacking exon 2 would be a functional protein. Hereto, an IL12RB1 expression construct lacking exon 2 was generated by altering a wild-type IL12RB1 expression construct that we reported before.12 The constructs were made in the retroviral expression vector pLZRS, which ensures that the IL12RB1 and GFP genes are transcribed and expressed in tandem and allows for selection of transduced cells by FACS sorting on GFP signal. Constructs were transduced into activated T cells from the patients; as a negative control, the pLZRS vector without insert was used.
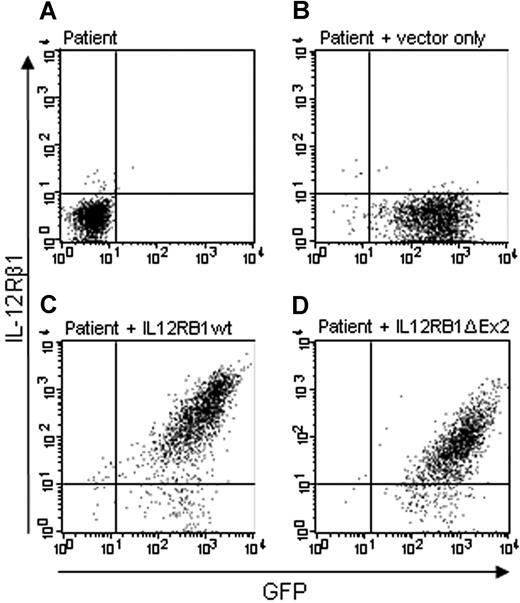
We analyzed expression of the IL-12Rβ1 on the cell surface with 4 IL-12Rβ1 antibodies. In untransduced cells from a patient, no GFP signal is detected and with the antibody DU-1 (raised against full-length IL-12Rβ1) and no expression of IL-12Rβ1 is detected on the cell surface (Figure 1A). Cells from the patient transduced with the empty pLZRS vector did not show IL-12Rβ1 expression on the cell surface but do show GFP expression (Figure 1B). After transduction of the wild-type IL12RB1 construct (IL12RB1wt), in cells from the patient, IL-12Rβ1 expression on the cell surface is restored (Figure 1C). After transduction of the IL12RB1 construct lacking exon 2 (IL12RB1Δex2), IL-12Rβ1 expression on the cell surface is present (Figure 1D), although detected at a 5- to 6-fold lower amount than the wild-type construct (compare with Figure 1C). Intracellular staining of IL-12Rβ1 with antibody DU-1 gave similar results. Extracellular staining with antibody 2B10 gave results similar to DU-1, except that IL-12Rβ1 expression on the cell surface of IL12RB1Δex2 was detected at an approximately 10-fold lower amount than the wild-type construct. No intracellular staining was performed because 2B10 is labeled indirectly. With 2 other IL-12Rβ1 antibodies (2.4E6 and F14R), we were unable to detect expression of the IL12RB1 construct lacking exon 2 (data not shown).
IL-12Rβ1 lacking exon 2 is expressed on the membrane. Expression of IL-12Rβ1 on the cell membrane of activated T cells from an IL-12Rβ1−/− patient was analyzed with the antibody DU-1. (A) Untransduced cells. (B) Cells transduced with GFP vector only. (C) Cells transduced with a wild-type IL12RB1 construct. (D) Cells transduced with the IL12RB1 construct lacking exon 2.
IL-12Rβ1 lacking exon 2 is expressed on the membrane. Expression of IL-12Rβ1 on the cell membrane of activated T cells from an IL-12Rβ1−/− patient was analyzed with the antibody DU-1. (A) Untransduced cells. (B) Cells transduced with GFP vector only. (C) Cells transduced with a wild-type IL12RB1 construct. (D) Cells transduced with the IL12RB1 construct lacking exon 2.
IL-12Rβ1 construct lacking exon 2 is functional
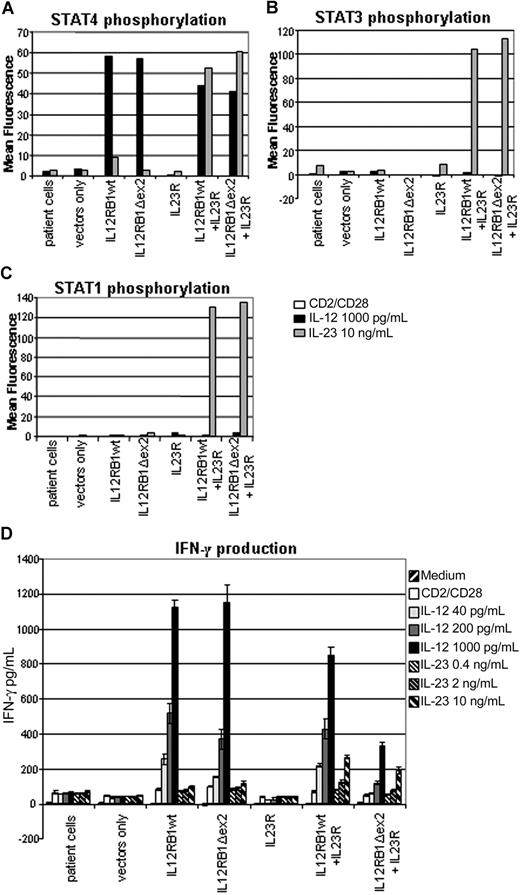
IL-12Rβ1 composes together with IL-12Rβ2 the IL-12 receptor and composes together with IL-23R the IL-23 receptor. IL-12 signaling is known to induce STAT4 phosphorylation, and IL-23 signaling is known to induce STAT1, STAT3, and STAT4 phosphorylation, although both result in subsequent IFN-γ and IL-10 production.12,13 To determine whether the IL12RB1Δex2 construct encodes a functional IL-12Rβ1 protein, activated IL-12Rβ1−/− T cells transduced with IL12RB1 constructs were stimulated with IL-12 and IL-23. We subsequently determined STAT phosphorylation by FACS and cytokine production by ELISA. IL-12 stimulation induced STAT4 phosphorylation and IFN-γ production in cells transduced with IL12RB1wt or IL12RB1Δex2 but not in untransduced cells or cells transduced with vector only (Figure 2A,D). Similar results were obtained for IL-10 production (data not shown).
IL-12Rβ1 lacking exon 2 is a functional protein. Response to IL-12 or IL-23 stimulation of IL-12Rβ1 constructs of activated T cells from an IL-12Rβ1−/− patient. Because IL-23R expression is normally low in PHA-activated T cells, an IL23R construct was transfected as well to allow detection of IL-23 responses. Cells were prestimulated for 18 hours with anti-CD2/CD28. (A) STAT4 phosphorylation 60 minutes after stimulation. (B) STAT3 phosphorylation 15 minutes after stimulation. (C) STAT1 phosphorylation 15 minutes after stimulation. (D) IFN-γ production measured after 72 hours of stimulation of 105 cells with various concentrations of IL-12 and IL-23 in the presence of anti-CD2/CD28.
IL-12Rβ1 lacking exon 2 is a functional protein. Response to IL-12 or IL-23 stimulation of IL-12Rβ1 constructs of activated T cells from an IL-12Rβ1−/− patient. Because IL-23R expression is normally low in PHA-activated T cells, an IL23R construct was transfected as well to allow detection of IL-23 responses. Cells were prestimulated for 18 hours with anti-CD2/CD28. (A) STAT4 phosphorylation 60 minutes after stimulation. (B) STAT3 phosphorylation 15 minutes after stimulation. (C) STAT1 phosphorylation 15 minutes after stimulation. (D) IFN-γ production measured after 72 hours of stimulation of 105 cells with various concentrations of IL-12 and IL-23 in the presence of anti-CD2/CD28.
Because PHA-activated T cells do not normally express the IL-23R, constructs expressing wild-type IL-23R13 were transduced into the cells as well to determine the function of IL-12Rβ1Δex2 in the IL-23 receptor complex. The IL23R construct transcribes and expresses the IL23R and the extracellular part of NGFR genes in tandem, thus allowing for selection of transduced cells by FACS sorting on NGFR signal.
In cells where both the IL12RB1wt or IL12RB1Δex2 and the IL23R were transduced, IL-23 stimulation induced STAT4, STAT3, and STAT1 phosphorylation (Figure 2A-C). This response was absent in untransduced cells, vector-transduced cells, and cells where only the IL23R or one of the IL12RB1 constructs was transduced (Figure 2A-C). A small amount of STAT4 phosphorylation is seen in response to IL-23 in the IL12RB1wt transduced cells, resulting from a minor IL-12-like effect of IL-23 that we have reported before.13 Significant IFN-γ production was induced by IL-23 in cells in which both an IL12RB1wt or IL12RB1Δex2 construct and an IL23R construct were present (Figure 2D). No IFN-γ was produced in untransduced cells, control vector-transduced cells, and cells where only the IL23R or one of the IL12RB1 constructs was present (Figure 2D). Similar results were obtained for IL-10 production (data not shown).
Highly efficient transfection of AONs into activated T cells
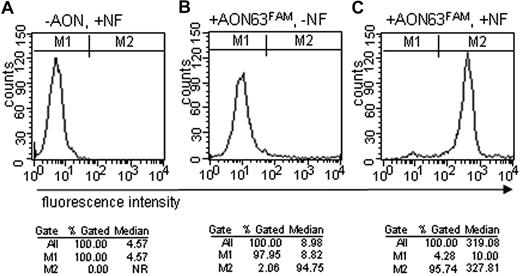
A FAM-labeled AON and activated T cells of a healthy control were used to optimize nucleofection protocols. Transfection efficiency was initially analyzed by microscopy, but whether AONs were located inside or outside the cells could not easily be distinguished (data not shown). Transfection efficiency of labeled AONs was assessed more accurately by FACS (Figure 3). In cells with nucleofection where only nucleofection reagent but no AON was added, fluorescence was detected in none of the cells (Figure 3A). In cells with the FAM-labeled AON where the actual nucleofection step was omitted, 2% of the cells show a moderate intensity of fluorescence (Figure 3B). In 96% of the cells where a FAM-labeled AON was added, a high intensity of fluorescence was detectable 24 hours after nucleofection (Figure 3C).
AONs enter the cells with high efficiency on nucleofection (NF). FACS analysis of 106 activated T cells from a normal control after NF with a FAM-labeled AON. Two gates, M1 and M2, were set to determine transfection efficiency (%) and median fluorescence of the cells. (A) No AON added, with nucleofection. (B) FAM-labeled AON added, without nucleofection. (C) FAM-labeled AON added with nucleofection. NR indicates not relevant.
AONs enter the cells with high efficiency on nucleofection (NF). FACS analysis of 106 activated T cells from a normal control after NF with a FAM-labeled AON. Two gates, M1 and M2, were set to determine transfection efficiency (%) and median fluorescence of the cells. (A) No AON added, with nucleofection. (B) FAM-labeled AON added, without nucleofection. (C) FAM-labeled AON added with nucleofection. NR indicates not relevant.
Nucleofection of AONs results in exon skipping
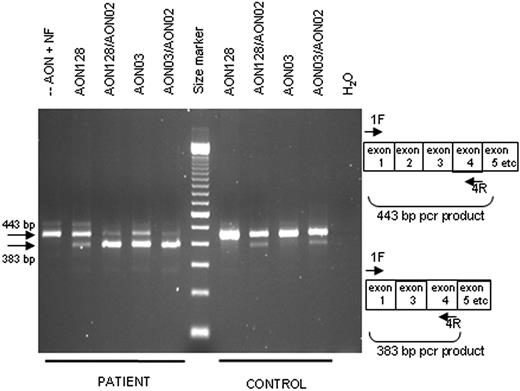
RNA was isolated at 24 hours after nucleofection of various AONs into activated T cells of a normal control and of patients to determine whether exon skipping had occurred in the pre-mRNA. Transcripts were analyzed with a primer set that can detect normally spliced transcripts (443 bp) as well as transcripts in which exon 2 is spliced out (383 bp). In control cells, the most abundant PCR product was 443 bp, whereas a 383-bp product was usually also detectable, indicating that exon skipping occurred but at a low efficiency (Figure 4). In patient cells with nucleofection but without AON always, only a 443-bp product was present (Figure 4). In addition, when using primers designed specifically to detect the exon-skipped transcript (ie, forward primer 1/3F directed at the junction of exon 1 and 3), no PCR product was detectable in these cells (data not shown). In patient cells nucleofected with various AONs, the most abundant PCR product was usually of 383 bp, although variation between effectiveness was clear (Figure 4). Several of the 383-bp products were isolated from gel and sequenced; these all revealed splicing directly from exon 1 to exon 3, lacking the 60 bp of exon 2. Transcripts of the household gene GAPDH were analyzed in all samples to allow comparison of total RNA present; GAPDH PCR products appeared not to differ between samples (data not shown). To determine whether any additional exons were unintentionally skipped, full-length IL12RB1 PCR products, generated with primers annealing to exon 1 (primer 1F) and exon 17 (primer 17R), were analyzed as well. No other transcripts were however detected (data not shown).
Nucleofection of AONs induces specific exon skipping. RT-PCR analysis of RNA isolated from T cells from an IL-12Rβ1−/− patient and a control 24 hours after nucleofection with various AONs. The primers 1F and 4R are indicated on the right: without exon skipping, a 443-bp product is expected; with exon skipping, a 383-bp product is expected. First lane represents no AON added, with nucleofection; and last lane, water control of the PCR. The size marker is a 100-bp ladder.
Nucleofection of AONs induces specific exon skipping. RT-PCR analysis of RNA isolated from T cells from an IL-12Rβ1−/− patient and a control 24 hours after nucleofection with various AONs. The primers 1F and 4R are indicated on the right: without exon skipping, a 443-bp product is expected; with exon skipping, a 383-bp product is expected. First lane represents no AON added, with nucleofection; and last lane, water control of the PCR. The size marker is a 100-bp ladder.
Three AONs (AON02, AON03, and AON64) were directed at sequences in exon 2 that were identical in patients and controls. Two AONs (AON01 and AON63) were specifically directed at wild-type sequences; AON128 was directed specifically at sequences in the patient cells. AON64 did not have an effect on splicing in any of the cells; all other AONs had comparable effects on splicing, except for AON03, which, in patient cells, always showed a stronger effect. Combinations of nonoverlapping AONs (AON02/03, AON128/02) worked better than single AONs in both control and patient cells (Figure 4).
Exon skipping results in correction of IL-12Rβ1 expression on the cell surface
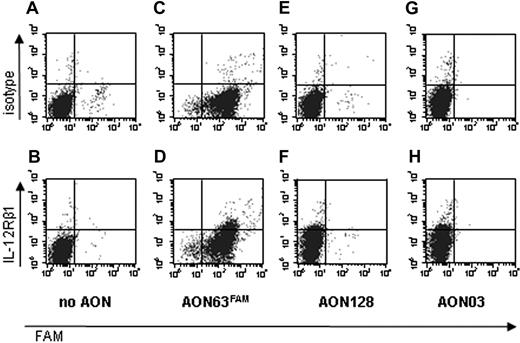
To determine whether exon skipping resulted in IL-12Rβ1 expression on the cell surface, cells were labeled with antibodies against IL-12Rβ1 or with an isotype control and analyzed by FACS. In cells nucleofected without AON, no IL-12Rβ1 expression was detected on the cell surface (Figure 5B). In cells nucleofected with AONs, IL-12Rβ1 expression could be detected on the cell surface as early as 24 hours after nucleofection (Figure 5D,F,H), whereas cells labeled with an isotype control (Figure 5A,C,E,G) showed that this was not the result of aspecific staining.
Nucleofection of AONs results in correction of IL-12Rβ1 expression. Activated T cells from an IL-12Rβ1−/− patient were nucleofected (A,B) without AON, with (C,D) AON63FAM, (E,F) AON128, or (G,H) AON03. At 24 hours after nucleofection, the cells were labeled with an isotype control (A,C,E,G) or with the antihuman IL-12Rβ1 antibody 2B10 (B,D,F,H) and analyzed by FACS.
Nucleofection of AONs results in correction of IL-12Rβ1 expression. Activated T cells from an IL-12Rβ1−/− patient were nucleofected (A,B) without AON, with (C,D) AON63FAM, (E,F) AON128, or (G,H) AON03. At 24 hours after nucleofection, the cells were labeled with an isotype control (A,C,E,G) or with the antihuman IL-12Rβ1 antibody 2B10 (B,D,F,H) and analyzed by FACS.
Exon skipping results in functional correction of the IL-12Rβ1 defect
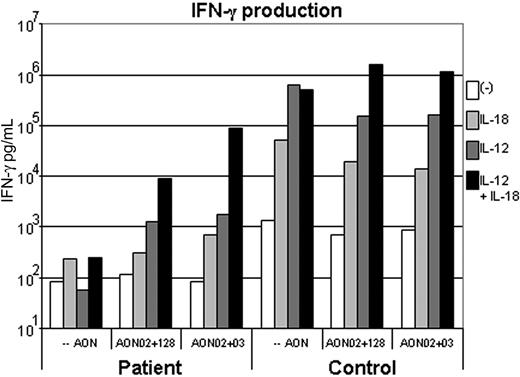
Efficient exon skipping is, based on the results in “IL-12Rβ1 construct lacking exon 2 is functional” with expression constructs, expected to (at least partly) restore the function of the IL-12Rβ1 in the targeted cells. Activated T cells from a healthy control and from a patient nucleofected with AONs or nucleofected without AONs were stimulated for 6 days with IL-12, IL-18, or IL-12 in combination with IL-18, after which the IFN-γ production was measured by ELISA. Control cells nucleofected without AONs produce abundant amounts of IFN-γ in response to IL-12, which appears slightly reduced on nucleofection with AONs. Patient cells nucleofected without AONs do not produce any IFN-γ in response to IL-12 (Figure 6). Patient cells nucleofected with AONs do produce IFN-γ in response to IL-12 (Figure 6). Because IL-12 is known to show a strong synergy with IL-18 in IFN-γ production, also IL-12 responses in combination with IL-18 were determined. Control cells nucleofected without AONs produce abundant amounts of IFN-γ in response to IL-12 in synergy with IL-18. Patient cells nucleofected without AONs do not produce more IFN-γ in response to IL-12 in synergy with IL-18 than to IL-18 alone, whereas nucleofection with AONs results in considerable IFN-γ production in response to IL-12 in synergy with IL-18 (Figure 6).
Nucleofection of AONs results in correction of IL-12 response. A total of 106 activated T cells per well from an IL-12Rβ1−/− patient and a control were nucleofected without AONs, with AON02 plus AON03, or with AON02 plus AON128, and subsequently stimulated for 6 days with 75 ng/mL IL-18 or 5000 ng/mL IL-12 or 5000 ng/mL IL-12 and 75 ng/mL IL-18. IFN-γ production was analyzed by ELISA. The bars represent averages of an experiment performed in duplicate.
Nucleofection of AONs results in correction of IL-12 response. A total of 106 activated T cells per well from an IL-12Rβ1−/− patient and a control were nucleofected without AONs, with AON02 plus AON03, or with AON02 plus AON128, and subsequently stimulated for 6 days with 75 ng/mL IL-18 or 5000 ng/mL IL-12 or 5000 ng/mL IL-12 and 75 ng/mL IL-18. IFN-γ production was analyzed by ELISA. The bars represent averages of an experiment performed in duplicate.
Discussion
Patients with complete defects of one of the genes involved in the type 1 cytokine pathway have severe, life-threatening infections with otherwise nonpathogenic mycobacteria and salmonellae. For these patients, no effective therapy is available. We have shown here, with a specific IL-12Rβ1 defect as an example, that antisense-mediated exon skipping may in the future provide an additional therapeutic option for a selection of these patients.
Antisense-mediated exon skipping is a technique that has been shown to achieve phenotypical correction of certain genetic defects in vitro5-7 and in vivo.10 The success of this technique is highly dependent on the cell type targeted, the transfection efficiency, the sequences targeted, and, very importantly, on whether the protein missing certain exon(s) is properly expressed and is (partly) functional. The cells we target here (T cells) are readily available from the blood from patients and controls. The transfection efficiency in these cells is, at 96%, very high. The sequence we target, in exon 2 of the IL12RB1, proved amenable to antisense-mediated exon skipping. Moreover, the exon skipping appears to be more efficient in patient cells than in controls cells. This is probably because of the effect the mutation has on one of the predicted ESEs. According to the program ESEfinder,17 2 of the 9 predicted ESE motifs in exon 2 (an Srp40 and a Sc35 motif) are obliterated because of the mutation. Normally, splicing factors bind to ESEs and recruit splicing machinery to actively include an exon in a transcript. The obliterated Sc35 motif has the highest score of the 9 ESE motifs in exon 2. Unfortunately, for these 2 patients, the disruption of the 2 ESEs by the mutation is not enough to induce exon skipping without nucleofection of AONs, as was shown by the absence of shorter transcripts and the total lack of IL-12 response in untransduced cells. In combination with nucleofection of AONs, however, exon skipping appears to be highly efficient in these cells.
The expression constructs reveal that IL-12Rβ1 lacking the domain encoded by exon 2 is expressed on the membrane of cells. The expression detected appears a factor 5 to 10 lower than that of the wild-type construct, depending on the antibody used. Four possible explanations for this observation follow: (1) The antibodies used (DU-1 and 2B10) do not bind as efficient to the IL-12Rβ1Δex2 as to the wild-type protein. This is very plausible because even single amino acid substitutions can, in some cases, completely abolish binding of an antibody to the protein, as was for instance found for the IL-12Rβ1 antibody 2.4E6 while detecting the L77P variation.12 In addition, 2 other antibodies (F14R and 2.4E6) do not detect the IL-12Rβ1Δex2 protein at all. (2) The bulk of the protein is, similar to IL-12Rβ1 proteins harboring various simple amino acid substitutions,12 retained inside the cell. This is observed when the protein quality control system in the endoplasmic reticulum prevents transport of mutant, misfolded, or incorrectly complexed proteins.19 IL-12Rβ1 variants sequestered within the cell have thus far always been detectable at expression levels similar to wild-type IL-12Rβ1.12 Intracellular FACS analysis of IL-12Rβ1Δex2, however, detected the same lower expression inside the cell as well, making this explanation therefore doubtful. (3) The IL12RB1Δex2 construct is transcribed or translated less efficiently. The expression constructs, however, transcribe and translate GFP in tandem with IL-12Rβ1 or IL-12Rβ1Δex2, allowing for selection or FACS gating of cells with similar GFP expression, which consequently also have similar IL-12Rβ1 or IL-12Rβ1Δex2 expression. (4) The half-life of the IL-12Rβ1Δex2 protein is reduced. The IL-12Rβ1 and IL-12Rβ1Δex2 expression constructs show, however, very similar responses to IL-12, suggesting that similar amounts of protein are present. The most probable explanation for the lower detection of IL-12Rβ1Δex2 on the cell surface therefore appears to be that binding of the antibodies is hampered.
The most important question was whether skipping of exon 2 would lead to a functional protein. When analyzing expression constructs, the response of the IL-12Rβ1Δex2 protein to IL-12 or to IL-23 is comparable with the response of the wild-type IL-12Rβ1 protein. When the IL-23R is (over)expressed in the same cell, the IL-12 response is reduced of both the IL-12Rβ1Δex2 and the wild-type IL-12Rβ1, suggesting that overexpression of the IL-23R successfully competes with IL-12Rβ2 for IL-12Rβ1 binding.
Treatment of patient cells with AONs results not only in cell surface expression of IL-12Rβ1 protein but also in a restored IL-12 response, both alone and in synergy with IL-18. This IL-12 response is, however, lower than that of control cells. Although the effect of the AONs on exon skipping as seen in the RT-PCRs is extensive, the translation of these transcripts into protein may take a long time. Little is known about normal IL-12Rβ1 transcription and translation dynamics or protein turnover. In addition, the translation efficiency of the IL-12Rβ1 lacking exon 2 may be altered. At the time points at which we measured the IL-12 response, only a small amount of IL-12Rβ1 protein was detectable on the cell surface. This is largely explained by the antibodies binding less well to IL-12Rβ1 lacking exon 2 but may also be partly the result of only a small amount of transcripts having been translated. Nonetheless, the small amount of protein was enough to generate a considerable IL-12 response. Limitations to the amount of patient material available have restricted us in the number of conditions we were able to test; there is, however, a great potential for improvement of the effect of the experiments in up-regulation of IL-12Rβ1 expression.
The great advantage of exon skipping to correct IL-12Rβ1 deficiency in patients is that the lymphocytes can be treated ex vivo and are in easily accessible tissue for treatment in vivo if desired. In addition, treatment may be only needed during infections with mycobacteria or salmonellae and may result in permanent protection if memory T cells can be generated effectively. A drawback of antisense-mediated exon skipping is that AONs, although they can be detected for 3 to 4 weeks after delivery,20 are diluted when cells divide, thus reducing the duration of the effect. A potential pitfall is that antibodies may be generated against the corrected IL-12Rβ1 protein, similar to the antibodies generated in 10% to 30% of the hemophilia patients who receive factor VIII.21 If antibodies should be generated, the expression of IL-12Rβ1 is entirely reversible because of the limited life span of the lymphocytes.
Apart from the 2 patients in this study, skipping of exon 2 would be possible in at least 4 other patients with defects in exon 2: patients 17.II.2, 20.II.1, and 21.II.2 reported by Fieschi et al22 and a patient reported by Haerynck et al.23 We are currently investigating whether other IL12RB1 exons are also amenable to exon skipping. For various IL12RB1 mutations, multiple exons will have to be skipped to maintain an open reading frame. Although we expect most internal exons to be amenable to exon skipping, this will not always result in correction of IL-12Rβ1 deficiency because, for example, the cytokine binding region, the transmembrane domain, and the signal transduction domain cannot be shortened or omitted. Exon skipping to correct recessive IFN-γR1 and IFN-γR2 deficiencies may not be possible because of the functional domains encoded by each exon. The most common, dominant-negative defect in IFN-γR1, however, which is present in approximately 25% of MSMD patients and is caused by a 4-bp deletion, can probably be corrected by exon skipping with AONs specific for the mutated allele.
We have shown here that antisense-mediated exon skipping can be applied in activated T cells, which may be relevant not only for the treatment of IL-12Rβ1 deficient patients but also for the treatment of other genetic disorders that affect the function of proteins expressed in lymphocytes. Exon skipping resulted in a considerable improvement of the function of the IL-12Rβ1 that, however, not yet matches the functional potential we observed with the IL-12Rβ1Δex2 construct. Future improvements may be achieved by increasing AON stability through changes in chemical structure and by influencing IL-12Rβ1 translation dynamics.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.v.d.V. designed the research, analyzed data, and wrote the paper; J.T.v.D. designed the research; and E.M.V. and R.A.d.P. performed the research; and G.J.P., J.C.T.v.D., and A.A.-R. designed AONs and advised on exon skip techniques.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Esther van de Vosse, Department of Infectious Diseases, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands; e-mail: E.van_de_Vosse@LUMC.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal