Abstract
High-risk neuroblastoma remains a clinically challenging disease. Here, we report that a multifaceted immunotherapeutic approach including syngeneic hematopoietic stem cell transplantation (HSCT), adoptive transfer of sensitized T cells (from syngeneic donors vaccinated to tumor antigens), and early posttransplantation tumor vaccination can effectively treat mice with established neuroblastoma. Vaccination was an important component of this immunotherapy, as it resulted in enhanced and prolonged tumor-specific CD8 T-cell activity and improved antitumor efficacy. Surprisingly, CD4 cell depletion of mice given sensitized T cells resulted in better tumor-free survival, which was associated with an early increased expansion of CD8 T cells with an effector phenotype, increased numbers of tumor-reactive CD8 T cells, and increased tumor infiltration by CD8 T cells. However, in the absence of CD4 T cells, development of long-term tumor immunity (memory) was severely compromised as reflected by diminished CD8 T-cell recall responses and an inability to resist tumor rechallenge in vivo. Based on these results, a major challenge with this immunotherapeutic approach is how to obtain the ideal initial antitumor response but still preserve antitumor immune memory. These data suggest that identification and selective depletion of immune inhibitory CD4 T cells may be a strategy to enhance early antitumor immunity and induce a long-lasting tumor response after HSCT.
Introduction
Neuroblastoma is the second most common solid tumor in children and it is responsible for approximately 15% of childhood cancer deaths. Whereas patients with stage I or II disease respond to conventional treatment regimens, those older than 1 year of age diagnosed with high risk disease (stage III and IV) continue to have a poor prognosis. Intensive chemotherapy treatment followed by autologous hematopoietic stem cell transplantation (HSCT) has resulted in improved event-free survival for patients with high-risk neuroblastoma, but despite this aggressive treatment, neuroblastoma reoccurs in more than 50% of these patients.1 Given this high incidence of relapse, more effective treatments targeting minimal residual disease and metastasis are required.
We previously determined that vaccination of mice with tumor cells that express the costimulatory molecules CD54, CD80, CD86, and CD137L induces a strong cell-mediated immune response that can protect mice against tumor challenge.2 Despite these encouraging results, this cell-based vaccine failed to eliminate established tumors in resting mice. In the current study, we explored whether a treatment consisting of myeloablation, syngeneic HSCT, adoptive transfer of tumor-reactive T cells, and posttransplantation cell-based tumor vaccination could treat established tumors.
Lymphodepletion followed by adoptive transfer of tumor-reactive lymphocytes, derived from autologous tumor, represents a promising approach for the treatment of metastatic cancer in humans.3 In a clinical trial, infusion of tumor-reactive T cells induced regression of metastatic melanoma in approximately 50% of patients.4 Several animal studies demonstrated that the persistence, proliferation, and antitumor efficacy of adoptively transferred effector T cells were substantially enhanced in lymphopenic hosts.5 Lymphodepletion can also eradicate suppressive immune cells in the host, including regulatory T cells and myeloid-derived suppressor cells.6-8 Furthermore, induction of lymphopenia can enhance cytokine production or increase homeostatic cytokine levels (IL-7, IL-15) by removing cells that compete for these endogenous cytokines.9,10 Finally, experimental data indicate that complete (lethal) myeloablation followed by syngeneic hematopoietic stem cell rescue can enhance adoptive immunotherapy efficacy, and it was found that hematopoietic stem cells in the graft directly promoted the expansion and function of adoptively transferred tumor-reactive CD8 T cells.11
Vaccination to tumor antigens during post-HSCT immune reconstitution has demonstrated skewing of the T-cell repertoire toward the specific antigen(s).12 In addition, vaccination during homeostatic proliferation may facilitate an immune response to weak self-antigens and enhance T cell–mediated antitumor immunity.13-16 Given these data, the combination of HSCT-related lymphopenia, adoptive T-cell transfer, and vaccination may offer a promising strategy to elicit effective antitumor immunity.
The role of CD4 T cells in antitumor immunity after autologous HSCT and adoptive immunotherapy has not been well defined. CD4 T cells play a fundamental role in the activation, expansion, and survival of primary CD8 T cells, and they provide signals that support the establishment and maintenance of memory CD8 T cells.17 In addition, CD4 T cells can directly or indirectly mediate the destruction of tumor cells.18-21 Animal studies demonstrated the importance of including antigen-specific CD4 T-helper cells together with CD8 T cells for long-term persistence of effector cells.22-25 However, CD4 T cells also normally include a subset of immune suppressive CD25+Foxp3+ regulatory cells. Regulatory T cells also prevent lymphopenia-induced proliferation of CD4 and CD8 T cells.26,27 The transfer of CD4 T cells containing CD4+CD25+ cells prevented effective adoptive immunotherapy with tumor specific CD8 T cells.6 Endogenous CD4 T cells that survived myeloablative conditioning were also able to inhibit CD8-mediated antitumor immunity.11 Our previous data demonstrated that the ratio of regulatory CD25+Foxp3+CD4 to CD25−Foxp3−CD4 helper T cells was significantly increased in HSCT recipients given adoptively transferred T cells,28 suggesting that the antitumor efficacy offered by post-HSCT adoptive T-cell transfer may be attenuated by the regulatory cells. In the current study, we evaluated the influence of CD4 T cells on treatment of established tumors using our multifaceted immunotherapy. The results demonstrate that depletion of CD4 T cells increases immunotherapeutic efficacy; however, the CD4 cell depletion results in loss of T-cell memory to tumor antigens. Therefore, approaches that restore T-cell memory will need to be identified to avoid the risk for “late” tumor relapse.
Methods
Mice
A/J mice (6-8 weeks of age) were purchased from The Jackson Laboratory (Bar Harbor, ME). The animals were housed in the Medical College of Wisconsin (MCW) Biomedical Resource Center (Milwaukee, WI), which has been accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC). All studies were approved by the MCW Institutional Animal Care and Use Committee.
Tumor cells
Neuro-2a, a mouse neuroblastoma of strain A origin, was obtained from ATCC (Manassas, Virginia). An aggressive subclone, designated AGN2a, was derived through sequential in vivo passage in mice, as previously described. The AGN2a variant is MHC class I–positive and MHC class II–negative.2
AGN2a cells engineered to overexpress Renilla luciferase (Rluc) were generated by transfection with a plasmid expression vector (pcDNA3.1[-]; Invitrogen, Carlsbad, CA) encoding Rluc. A permanent AGN2a cell line expressing Rluc was obtained by cloning. To generate “established” tumors, AGN2a-Rluc cells were injected subcutaneously into the flanks of A/J mice. AGN2a-Rluc+ tumor growth was monitored weekly by whole-body bioluminescent imaging. Briefly, mice were injected intravenously with colenterazine (0.7 μ/g; Xenogen, Alameda, CA), and imaged using an IVIS Imaging System (Xenogen) to assess bioluminescence. Regions of interest (ROI) surrounding the tumor bioluminescent signal were manually drawn using the LIVING IMAGE software (Xenogen), and results were reported as total photon flux within a ROI. Tumor growth was also monitored by routine caliper measurements, and mice were considered moribund and euthanized when tumors exceeded 250 mm2.
Antibodies
The following monoclonal antibodies (mAbs), with or without a fluorescent conjugate, were obtained from BD Biosciences Pharmingen (San Diego, CA): anti-CD4 (GK1.5 and RM4-5), anti-CD8 (53-6.7), anti-CD16/CD32 (2.4G2), anti-CD54 (3E2), anti–4-1BBL (TKS-1), anti-CD80 (16-10A1), anti-CD86 (37.51), anti-CD44 (1M7), and anti–rat IgG2a (RG7/1.30). Control antibodies included purified mouse IgG2b and rat IgG2b. Anti-CD62L (MEL-14) mAb was obtained from eBioscience (San Diego, CA).
Hybridomas producing anti-CD4 mAb (GK1.5) and anti-CD8 mAb (2.43) were obtained from ATCC. These mAbs were produced in our laboratory using Integra CL 1000 bioreactors (Chur, Switzerland).
T-cell enrichment
A/J spleens were collected and processed into single-cell suspensions. For CD8 T-cell enrichment, splenocytes were incubated with anti–CD8-conjugated microbeads (Miltenyi Biotec, Auburn, CA), and the CD8 T cells positively selected by automated immunomagnetic sorting (AutoMACS). CD8 T-cell purity after positive selection was more than 98%. Total T cells were negatively enriched from splenocytes using a pan T-cell isolation kit (Miltenyi Biotec). T-cell purity after negative selection was greater than 85% (not shown).
Tumor vaccine
To generate the cell-based tumor vaccine, AGN2a cells were transfected with separate plasmids containing gene inserts for CD54 (pcDNA3.1/Hygro vector; Invitrogen, Carlsbad, CA), CD80, CD86, and CD137L (each in pCI-neo vectors; Promega, Madison, WI) using nucleofection (Amaxa Biosystems, Koeln, Germany) as described previously.2 The AGN2a-CD54/80/86/137L+ cells, also referred to as AGN2a-4P, were harvested 24 hours after transfection, irradiated, and administered subcutaneously as a tumor vaccine.
Syngeneic HSCT, adoptive T-cell transfer, and tumor vaccination
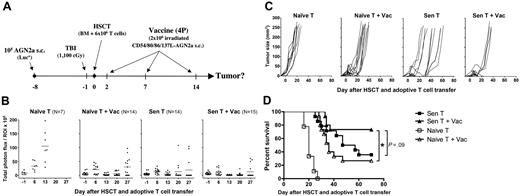
For treatment of mice with established tumors (Figure 1A), A/J mice were inoculated subcutaneously with 105 AGN2a-Rluc cells. Seven days after tumor inoculation, the mice were lethally irradiated with 1100 cGy, and 24 hours later they received transplants with a single intravenous injection of 107 syngeneic bone marrow (BM) cells supplemented with 6 × 106 T cells. Donor cells (both BM and T cells) were harvested from either naive mice or mice that had been sensitized to tumor antigens by 2 weekly vaccinations with AGN2a-4P cells. The sensitized T cells were harvested from the donor mice 5 days after the last vaccination. In some experiments, T cells were labeled for 10 minutes at room temperature with carboxyfluorescein succinimidyl ester (CFSE, 5 μM) in phosphate-buffered saline (PBS), washed once with 10% fetal calf serum (FCS), twice with PBS, and resuspended in sterile PBS for injection. Some of the tumor-bearing mice were vaccinated subcutaneously with 2 × 106 irradiated AGN2a-4P cells on days 2, 7, and 14 after HSCT. In some experiments, mice were depleted of T-cell subsets in vivo by intraperitoneal injection of 250 μg anti-CD4 (GK1.5) or anti-CD8 (2.43) mAbs on days −1, 2, 5, and 8 after HSCT.
A combination of myeloablative therapy, HSCT, adoptive immunotherapy, and tumor vaccination was able to eliminate established mouse neuroblastoma. (A) Overall experimental design for these studies: tumors were established in A/J mice by subcutaneous injection of 105 AGN2A-Luc cells on day −8. On day −1, bioluminescent imaging was done to ensure that the mice had comparable tumor burden before treatment, and the mice were then treated with lethal TBI. On day 0, the mice received transplants of 107 syngeneic BM cells supplemented with 6 × 106 T cells. Adoptively transferred T cells were either derived from naive donor mice (Naive T) or derived from mice prevaccinated with the AGN2a-4P vaccine (Sen T). On days 2, 7, and 14 after HSCT, some mice were treated with the irradiated AGN2a-4P vaccine (Vac). For analysis, mice were grouped according to the therapy received as Naive T, Naive T + Vac, Sen T, Sen T + Vac. Tumor burdens were monitored by (B) bioluminescent imaging, depicted as total photon flux, and (C) caliper measurements. (D) Kaplan-Meier survival curves. The data represent the combined results of 3 independent experiments (10-15 mice per group). Survival of mice that received naive T cells without vaccine was significantly different from all other treatment groups at P < .001. (*P < .05.) The Sen T and Sen T + Vac survival curves were not significantly different (P = .09). Statistics were computed using the log-rank test.
A combination of myeloablative therapy, HSCT, adoptive immunotherapy, and tumor vaccination was able to eliminate established mouse neuroblastoma. (A) Overall experimental design for these studies: tumors were established in A/J mice by subcutaneous injection of 105 AGN2A-Luc cells on day −8. On day −1, bioluminescent imaging was done to ensure that the mice had comparable tumor burden before treatment, and the mice were then treated with lethal TBI. On day 0, the mice received transplants of 107 syngeneic BM cells supplemented with 6 × 106 T cells. Adoptively transferred T cells were either derived from naive donor mice (Naive T) or derived from mice prevaccinated with the AGN2a-4P vaccine (Sen T). On days 2, 7, and 14 after HSCT, some mice were treated with the irradiated AGN2a-4P vaccine (Vac). For analysis, mice were grouped according to the therapy received as Naive T, Naive T + Vac, Sen T, Sen T + Vac. Tumor burdens were monitored by (B) bioluminescent imaging, depicted as total photon flux, and (C) caliper measurements. (D) Kaplan-Meier survival curves. The data represent the combined results of 3 independent experiments (10-15 mice per group). Survival of mice that received naive T cells without vaccine was significantly different from all other treatment groups at P < .001. (*P < .05.) The Sen T and Sen T + Vac survival curves were not significantly different (P = .09). Statistics were computed using the log-rank test.
Interferon-γ enzyme-linked immunosorbent spot assays
To assess for presence of tumor-reactive interferon-γ (IFNγ)–secreting CD8 T cells, T cells harvested from spleens and draining lymph nodes were purified by immunomagnetic sorting as previously detailed. Enzyme-linked immunosorbent spot (ELISPOT) assays were done using the mouse IFN-γ ELISPOT Kit from BD Biosciences, as described previously.28 The numbers of spots were quantitated using a Cellular Technology Limited (CTL) ImmunoSpot Analyzer (CTL Analyzers, Cleveland, OH).
Immunohistochemistry
Tumors were harvested, embedded in optimal cutting temperature (OCT) compound, and frozen at −80°C. Frozen sections (5 μm thick) were fixed in zinc-formalin for 5 seconds and then washed with distilled water. For immunohistochemical staining, endogenous peroxidase was inhibited with peroxidase block (Dako, Carpinteria, CA). Nonspecific antibody binding was blocked by incubating with serum-free protein block (Dako) for 15 minutes. Staining for CD8 T cells was done by first incubating with anti–mouse CD8 (BD PharMingen, San Diego, CA) at a 1/50 dilution for 1 hour. Biotin-conjugated secondary antibody was used for development using the DAB chromogen system (Dako). Tissue sections were analyzed on an AxioImager Z1 microscope (Carl Zeiss Microimaging, Thronwood, NY) using a 20× Plan Apochromat objective. Images were recorded with an Axiocam HRc camera and AxioVision Rel 4.6 digital imaging software (both from Carl Zeiss Microimaging).
Annexin V staining
Apoptosis was assessed by flow cytometry using fluorescent-conjugated annexin V antibody (BD Pharmingen). Cells were labeled with anti-CD8 (53-6.7) then washed and incubated with annexin V at a 1/20 dilution in annexin V binding buffer for 15 minutes at room temperature. The cells were then washed, resuspended in annexin V binding buffer, and immediately analyzed by flow cytometry. Annexin V labeled cells were colabeled with propidium iodide (PI) to differentiate early apoptotic cells (annexin V+ PI−) from late apoptotic/necrotic cells (annexin V+ PI+).
Statistics
Survival curves were compared by log-rank analysis. The Student t test was used to compare ELISPOT data. Statistical analyses were performed using Prism 5.0a software (GraphPad, San Diego, CA). P values less than .05 were considered significant.
Results
The combination of myeloablative therapy, HSCT, adoptive immunotherapy, and cell-based tumor vaccination was able to eliminate established neuroblastoma
We have previously demonstrated that “protective” immunity to neuroblastoma could be induced after syngeneic HSCT using a combination of adoptive T-cell transfer and cell-based tumor vaccination.28 We hypothesized that a similar immunotherapeutic approach would effectively treat mice with established tumors. To test this hypothesis, mice were inoculated with luciferase-expressing AGN2a cells 7 days before treatment with lethal total body irradiation (TBI; day −1), followed by HSCT (day 0) consisting of bone marrow supplemented with 6 × 106 T cells either from naive or sensitized (ie, previously vaccinated with AGN2a-4P cells) donor mice (see Figure 1A for experimental design). On days 2, 7, and 14 after HSCT, tumor-bearing mice were vaccinated with the AGN2a-4P tumor vaccine (Figure 1A).
When mice were given adoptive transfer with T cells from syngeneic naive mice, tumors progressed in all mice (Figure 1B,C, Naive T group) resulting in 100% mortality by day 30 after HSCT (Figure 1D). In contrast, when mice given naive T cells were also administered the AGN2a-4P cell-based vaccine, tumor regressions were observed in all recipients (Figure 1B,C, Naive T + Vac group); however, only 27% of these mice survived long-term, because tumor regressions in the majority of recipients were transient (Figure 1B-D). Adoptive transfer of T cells from only sensitized animals also induced tumor regression in nearly all recipients, but most regressions were again transient, resulting in a 36% survival rate (Figure 1B-D, Sen T group). AGN2a-4P vaccination of mice given sensitized T cells resulted in the best overall survival rate (73%; Figure 1D, Sen T + Vac group). Therefore, administration of both T cells and the AGN2a-4P vaccine contributed to the antitumor effects, and both were required to achieve optimal antitumor immunity.
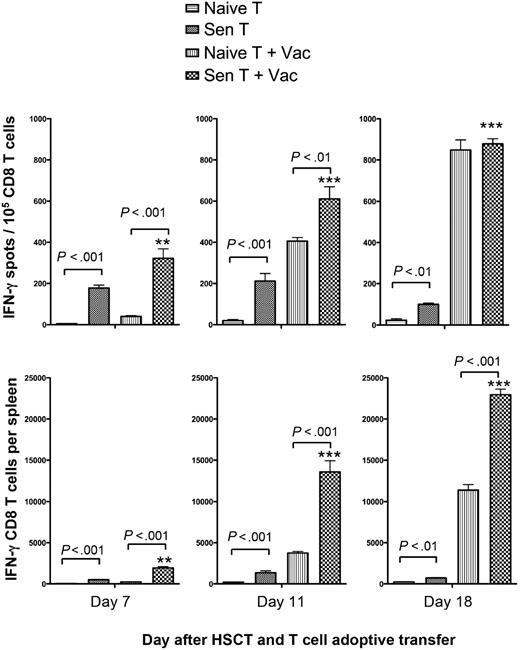
Administration of the AGN2a-4P vaccine was associated with a significant increase in splenic tumor-reactive CD8 T cells
Next, we examined the kinetics of tumor-reactive CD8 T cells present in the spleens of mice from each of the treatment groups in Figure 1. The frequencies and absolute numbers of splenic CD8 T cells that secreted IFN-γ in response to AGN2a were measured at 7, 11, and 18 days after HSCT. Tumor-reactive CD8 T cells could not be detected at any time-point in nonvaccinated mice given naive T cells (Figure 2). There was a significant increase in IFN-γ–producing tumor-reactive CD8 T cells in nonvaccinated mice given sensitized T-cell transfer (P < .01) compared with nonvaccinated mice given naive T cells. The frequencies and numbers of tumor-reactive CD8 T cells in these mice remained relatively constant at each time point. Although relatively few tumor-reactive CD8 T cells were observed after the first vaccine (day 7) when naive T-cell transfer was combined with AGN2a-4P vaccination, a dramatic increase in tumor-reactive CD8 cells was seen after the second vaccine (day 11; Figure 2). The kinetics of this response was different from that elicited in mice given sensitized T cells plus the AGN2a-4P vaccine, where significantly higher frequencies and absolute numbers of IFN-γ-producing tumor-reactive CD8 T cells were present both 7 and 11 days after HSCT (P < .01). For mice given tumor-sensitized T cells, vaccination led to significantly increased numbers of splenic tumor-reactive CD8 T cells at all time points (P < .01). CD8 reactivity was never observed against SaI, another tumor of strain A origin (data not shown), indicating that the T-cell reactivity observed in these assays was specific to the AGN2a neuroblastoma. Overall, the frequencies and absolute numbers of IFN-γ-producing CD8 T cells on days 11 and 18 after HSCT correlated with the survival data shown in Figure 1D.
Posttransplantation vaccination significantly increased frequencies and absolute numbers of tumor-reactive CD8 T cells in the spleens of treated mice. Tumor-bearing A/J mice received transplants of syngeneic BM cells plus 6 × 106 T cells from naive (Naive T) or pre-vaccinated (Sen T) donor mice. Some mice were treated on days 2, 7, and 14 after HSCT with the AGN2a-4P vaccine (Vac). On days 7, 11, and 18 after HSCT (5 days after first, 4 days after second, and 4 days after third vaccine), spleens were harvested and CD8 T cells isolated by immunomagnetic sorting. The CD8 cells were assayed in IFN-γ ELISPOT assays with tumor cell stimulators to determine tumor-reactive IFN-γ–secreting cell frequencies and absolute numbers. The data are from 1 of 3 replicate experiments, and the CD8 T cells were isolated from the pooled splenocytes of 3 mice. **P < .01; ***P < .001 when Sen T results were compared with Sen T + Vac results at the indicated times.
Posttransplantation vaccination significantly increased frequencies and absolute numbers of tumor-reactive CD8 T cells in the spleens of treated mice. Tumor-bearing A/J mice received transplants of syngeneic BM cells plus 6 × 106 T cells from naive (Naive T) or pre-vaccinated (Sen T) donor mice. Some mice were treated on days 2, 7, and 14 after HSCT with the AGN2a-4P vaccine (Vac). On days 7, 11, and 18 after HSCT (5 days after first, 4 days after second, and 4 days after third vaccine), spleens were harvested and CD8 T cells isolated by immunomagnetic sorting. The CD8 cells were assayed in IFN-γ ELISPOT assays with tumor cell stimulators to determine tumor-reactive IFN-γ–secreting cell frequencies and absolute numbers. The data are from 1 of 3 replicate experiments, and the CD8 T cells were isolated from the pooled splenocytes of 3 mice. **P < .01; ***P < .001 when Sen T results were compared with Sen T + Vac results at the indicated times.
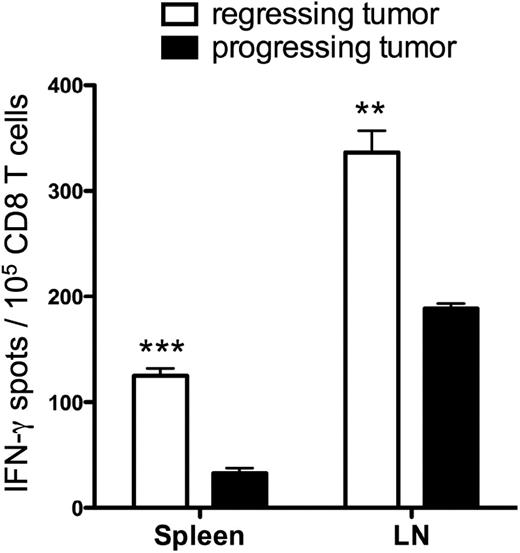
Successful antitumor immunity correlated with frequencies of tumor-reactive CD8 T cells in peripheral lymphoid tissues
When mice were treated with HSCT, naive T-cell transfer and AGN2a-4P vaccination, many of the tumors progressed after an initial regression, but some tumors were completely eliminated (Figure 1C, Naive T + Vac). To determine whether the magnitude of the immune response was associated with treatment outcome, we compared tumor-reactive CD8 T-cell frequencies in the spleens and tumor draining lymph nodes (LNs) of mice that had “progressing” tumors with those that had “regressing” tumors. Thirty days after HSCT, tissues were harvested and tumor-reactive CD8 T-cell frequencies were determined in IFN-γ ELISPOT assays. There were significantly increased frequencies of tumor-reactive CD8 T cells in both the spleens (P < .001) and lymph nodes (P < .01) of mice with regressing tumors compared with those with progressing tumors (Figure 3). These data suggest that tumor-reactive T cells must be generated and/or persist to ensure the complete elimination of established tumors.
The magnitude of CD8 antitumor reactivity correlated with tumor regression. CD8 T cells were isolated from spleens and tumor draining lymph nodes 30 days after HSCT, from mice treated with naive T cell adoptive transfer and AGN2a-4P vaccination. The CD8 cells were assayed in IFN-γ ELISPOT assays with tumor cell stimulators to determine tumor-reactive IFN-γ–secreting cell frequencies. The experiment is representative of 2 independent experiments in which the CD8 T cells were pooled from 2 or 3 individual mice. ***P < .001; **P < .01 when mice with regressing tumors were compared with mice with progressing tumors.
The magnitude of CD8 antitumor reactivity correlated with tumor regression. CD8 T cells were isolated from spleens and tumor draining lymph nodes 30 days after HSCT, from mice treated with naive T cell adoptive transfer and AGN2a-4P vaccination. The CD8 cells were assayed in IFN-γ ELISPOT assays with tumor cell stimulators to determine tumor-reactive IFN-γ–secreting cell frequencies. The experiment is representative of 2 independent experiments in which the CD8 T cells were pooled from 2 or 3 individual mice. ***P < .001; **P < .01 when mice with regressing tumors were compared with mice with progressing tumors.
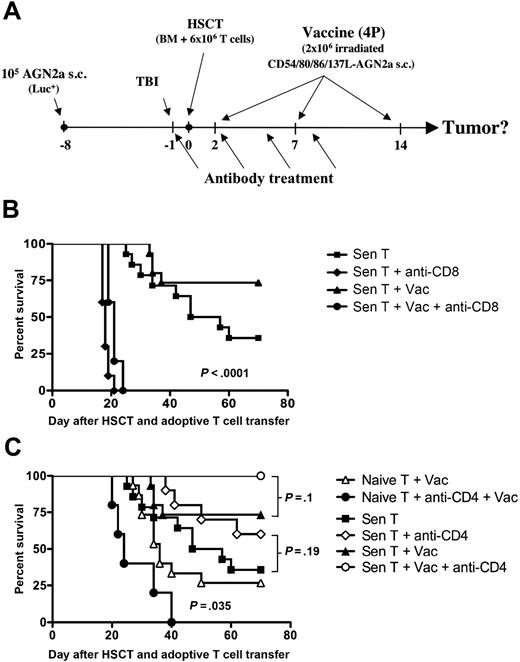
While CD8 T cells were required for immunotherapy-induced tumor rejection, depletion of CD4 T cells had a differential impact on the antitumor response based on the source of transferred T cells
The roles of CD8 and CD4 T cells in elimination of established tumors were examined by depleting the respective T-cell subset in vivo using monoclonal antibodies (see Figure 4A for design). In hosts given tumor-sensitized T cells with or without the AGN2a-4P vaccine, depletion of CD8 cells completely eliminated antitumor reactivity (Figure 4B). These results confirmed the crucial role of CD8 T cells in the immune response to AGN2a, as we have previously demonstrated.28,29
CD8 T cells were required for antitumor efficacy, while the role of CD4 T cells depended upon the source of adoptively transferred T cells. (A) Experimental design for these studies: A/J mice were treated as depicted in Figure 1A, some mice were treated with in vivo–depleting anti-CD8 or anti-CD4 mAb (250 μg intraperitoneal) on days −1, 2, 5, and 8 before and after HSCT. (B) Depletion of CD8 cells in hosts given adoptive T-cell transfer with tumor-sensitized T cells resulted in significantly decreased tumor-free survival rates (P < .0001) compared with historical treatment groups from Figure 1D (Sen T and Sen T + Vac). (C) Depletion of CD4 cells in hosts given naive T-cell transfer resulted in significantly worse tumor-free survival compared with a historical group from Figure 1D (Naive T + Vac; P = .035). Depletion of CD4 cells in hosts given tumor-sensitized T cells resulted in better tumor-free survival compared with historical treatment groups from Figure 1D (Sen T and Sen T + Vac). The survival differences between the nonvaccinated and vaccinated groups did not reach statistical significance. The data for the antibody-treated groups represents the combined results of 2 to 3 independent experiments, except for the Naive T + anti-CD4 + Vac group, which is from 1 experiment. Each group consisted of 5 to 15 total mice.
CD8 T cells were required for antitumor efficacy, while the role of CD4 T cells depended upon the source of adoptively transferred T cells. (A) Experimental design for these studies: A/J mice were treated as depicted in Figure 1A, some mice were treated with in vivo–depleting anti-CD8 or anti-CD4 mAb (250 μg intraperitoneal) on days −1, 2, 5, and 8 before and after HSCT. (B) Depletion of CD8 cells in hosts given adoptive T-cell transfer with tumor-sensitized T cells resulted in significantly decreased tumor-free survival rates (P < .0001) compared with historical treatment groups from Figure 1D (Sen T and Sen T + Vac). (C) Depletion of CD4 cells in hosts given naive T-cell transfer resulted in significantly worse tumor-free survival compared with a historical group from Figure 1D (Naive T + Vac; P = .035). Depletion of CD4 cells in hosts given tumor-sensitized T cells resulted in better tumor-free survival compared with historical treatment groups from Figure 1D (Sen T and Sen T + Vac). The survival differences between the nonvaccinated and vaccinated groups did not reach statistical significance. The data for the antibody-treated groups represents the combined results of 2 to 3 independent experiments, except for the Naive T + anti-CD4 + Vac group, which is from 1 experiment. Each group consisted of 5 to 15 total mice.
When the role of CD4 cells was assessed using the same antibody depletion approach, the source of transferred T cells (naive vs tumor-sensitized) impacted the results in surprisingly different ways. When naive T cells were adoptively transferred to the graft, CD4 depletion of vaccinated mice resulted in significantly decreased antitumor efficacy compared with a historical group of non–CD4-depleted/vaccinated mice (Figure 4C; P = .035). These results were similar to what we had previously observed after HSCT in tumor challenge experiments,28 confirming that CD4 T cells are important for generating antitumor immunity in this model. In contrast, when tumor-sensitized T cells were adoptively transferred and the AGN2a-4P vaccine administered, tumors regressed in all CD4-depleted mice and the tumor-free survival rate increased to 100% (Figure 4C). Increased survival of CD4-depleted mice was also observed when tumor-sensitized T cells were transferred without vaccination (Figure 4C). These data suggest that once the antitumor T-cell response has been generated, the presence of CD4 T cells early after HSCT has a negative impact on antitumor immunity.
CD4 T-cell depletion enhanced the expansion and phenotypic differentiation of transferred tumor-sensitized CD8 T cells
It has been suggested that homeostatic expansion and activation of T cells are responsible for enhanced antitumor responses that have been observed after adoptive cell transfer into irradiated hosts.5 To evaluate the mechanism(s) for the increased antitumor response we observed in CD4-depleted hosts, we analyzed the expansion and activation status of transferred tumor-sensitized CD8 T cells in vivo. Using CFSE cell labeling, we evaluated the proliferation of transferred T cells in mice treated with or without anti-CD4 mAb. Four days after transfer into HSCT recipient mice, 44% and 51% of tumor-sensitized CD8 T cells in recipient spleens and tumor-draining lymph nodes (LNs), respectively, had divided at least once (Figure 5A, Sen T). A single vaccine (day 2 after HSCT) had only a modest effect on proliferation of the transferred CD8 T cells in peripheral lymphoid tissues (Figure 5A, Sen T + Vac). However, CD4 cell depletion (Sen T + anti-CD4) had a dramatic effect on the proliferative capacity of transferred CD8 T cells, and vaccination (Sen T + Vac + anti-CD4) again had only a modest impact on further increasing T-cell proliferation.
CD4 cell depletion enhanced the expansion and differentiation of transferred tumor-sensitized CD8 T cells. (A) CFSE staining profiles of gated CD8 cells isolated from the spleens (top panels) or lymph nodes (LN; bottom panels) from groups of tumor-bearing mice treated with 6 × 106 CFSE-labeled tumor-sensitized CD8 T cells (Sen T), AGN2a-4P vaccine (Vac), or anti-CD4 mAb treatment. The anti-CD4 mAb was administered 1 day before HSCT, and the AGN2a-4P vaccine was given 2 days after HSCT. Cells were harvested for flow cytometric analysis 4 days after HSCT. (B) Splenocytes (top panels) or LN (bottom panels) were collected from recipient mice 14 days after adoptive transfer and stained with fluorescently labeled anti-CD8, anti-CD44, and anti-CD62L mAbs. Shown are 2-color flow cytometric histograms depicting the CD44 (x-axis) and CD62L (y-axis) expression on gated CD8 T cells. Cells from normal, nontransplanted mice were included for comparison. (C) Spleens were collected 11 days after HSCT, CD8 T cell numbers counted (left panel), and absolute numbers of tumor-reactive IFN-γ–secreting CD8 T cells (right panel) were determined. **P < .01 compared with the other treatment groups. (D) Immunohistochemical staining of tumors for infiltrating CD8 T cells. Frozen sections of tumors from the indicated experimental groups were stained with CD8-specific antibody (200× magnification). All results are representative of 2 or 3 separate experiments in which splenocytes and LN cells were pooled from 3 or 4 individual mice.
CD4 cell depletion enhanced the expansion and differentiation of transferred tumor-sensitized CD8 T cells. (A) CFSE staining profiles of gated CD8 cells isolated from the spleens (top panels) or lymph nodes (LN; bottom panels) from groups of tumor-bearing mice treated with 6 × 106 CFSE-labeled tumor-sensitized CD8 T cells (Sen T), AGN2a-4P vaccine (Vac), or anti-CD4 mAb treatment. The anti-CD4 mAb was administered 1 day before HSCT, and the AGN2a-4P vaccine was given 2 days after HSCT. Cells were harvested for flow cytometric analysis 4 days after HSCT. (B) Splenocytes (top panels) or LN (bottom panels) were collected from recipient mice 14 days after adoptive transfer and stained with fluorescently labeled anti-CD8, anti-CD44, and anti-CD62L mAbs. Shown are 2-color flow cytometric histograms depicting the CD44 (x-axis) and CD62L (y-axis) expression on gated CD8 T cells. Cells from normal, nontransplanted mice were included for comparison. (C) Spleens were collected 11 days after HSCT, CD8 T cell numbers counted (left panel), and absolute numbers of tumor-reactive IFN-γ–secreting CD8 T cells (right panel) were determined. **P < .01 compared with the other treatment groups. (D) Immunohistochemical staining of tumors for infiltrating CD8 T cells. Frozen sections of tumors from the indicated experimental groups were stained with CD8-specific antibody (200× magnification). All results are representative of 2 or 3 separate experiments in which splenocytes and LN cells were pooled from 3 or 4 individual mice.
Next, we performed a phenotypic analysis of the transferred T cells by flow cytometry and analyzed the influence of lethal irradiation, vaccination, and CD4 cell depletion on percentages of CD8 cells with an activated CD44highCD62Llow phenotype. Before adoptive transfer, splenic, tumor-sensitized CD8 T cells had increased percentages of CD44highCD62Llow T cells compared with CD8 T cells collected from naive mice (12% vs 6% [data not shown]). Fourteen days after BMT and adoptive T-cell transfer, mice given tumor-sensitized T cells had elevated percentages of splenic CD8CD44highCD62Llow cells (19.2%; Figure 5B). Vaccination increased the percentages of CD8CD44highCD62Llow cells to 28.2%. In CD4-depleted hosts, there was a dramatic increase in percentages of CD8 T cells with the CD44highCD62Llow effector phenotype in both the spleen and vaccine-draining LN (Figure 5B).
The next experiments were designed to address how CD4 T-cell depletion affected the quantity and function of tumor-reactive CD8 T cells. Specifically, we determined, by IFN-γ ELISPOT assays, the absolute numbers of tumor-reactive CD8 T cells in the spleens of treated mice. On day 11 after HSCT and adoptive transfer of tumor-sensitized T cells, we found that AGN2a-4P vaccination increased the number of tumor-reactive CD8 T cells (IFN-γ spots) by 8.7-fold, and anti-CD4 treatment increased numbers by 6.5-fold (Figure 5C). The combination of anti-CD4 treatment and vaccination resulted in the highest numbers of tumor-reactive CD8 T cells, a 27-fold increase compared with mice given tumor-sensitized T cells only. Similar increases were also observed in total CD8 T cells (Figure 5C left panel). These results suggest that CD4 cell depletion may result in a preferential expansion of transferred tumor-reactive CD8 T cells and/or promote the differentiation of tumor-reactive CD8 T cells into IFN-γ–producing effectors.
Lastly, we harvested tumor masses from experimental and control groups of mice and performed immunohistochemistry to look for the presence of infiltrating CD8 T cells. Tumors from control mice given naive T cells (no vaccine) had few, if any, infiltrating CD8 T cells (Figure 5D). The greatest numbers of infiltrating CD8 T cells were seen in tumors from mice that had been given tumor-sensitized T cells, AGN2a-4P vaccination, and CD4 cell depletion, followed by mice that had been treated with tumor-sensitized T cells and vaccination, followed by mice given sensitized T cells and CD4 T-cell depletion or sensitized T cells only (Figure 5D). Together, these data indicate that the combination of adoptively transferred tumor-sensitized T cells, depletion of CD4 cells, and posttransplantation tumor vaccination results in the complete elimination of established tumors by enhancing the proliferation of tumor-reactive CD8 T cells and facilitating their migration into the tumor microenvironment.
Depletion of CD4 T cells impaired development of T-cell memory
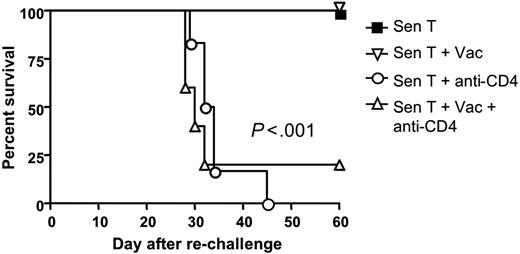
CD4 T-helper function has been demonstrated to be critical for the generation and maintenance of T-cell memory in the non-HSCT setting.17 However, the role of CD4 T cells in generating CD8 T-cell memory to tumor antigens after HSCT has not been well described. To determine whether depletion of CD4 T cells affected the generation of memory to tumor antigens, the survivors shown in Figure 4C that had been given tumor-sensitized T-cell transfer were rechallenged 70 days after HSCT with 105 wild-type AGN2a cells and followed for survival. All of the non–CD4-depleted mice survived the rechallenge, indicating the presence of T-cell memory to tumor antigens. In contrast, only 1 of 11 mice that had been depleted of CD4 T cells early after HSCT survived the rechallenge (Figure 6), indicating that T-cell memory to tumor antigens had been severely compromised. This loss of memory occurred even though the CD8 T cells transferred to the mice at the time of HSCT were from donors that had been vaccinated to tumor antigens in the presence of CD4 T cells. This suggests that the continued presence of CD4 cells after HSCT is necessary for the generation of long-lasting CD8 memory.
CD4 T cell depletion severely impaired the development of antitumor T-cell memory. Seventy days after HSCT, tumor-free survivors in Figure 4C were rechallenged with 105 wild-type AGN2a tumor cells injected subcutaneously, and followed for survival (ie, ability to reject the tumor cells). Survival curves are shown, and the data represent the combined results of 2 or 3 separate experiments. Each group consisted of 5 to 9 total mice.
CD4 T cell depletion severely impaired the development of antitumor T-cell memory. Seventy days after HSCT, tumor-free survivors in Figure 4C were rechallenged with 105 wild-type AGN2a tumor cells injected subcutaneously, and followed for survival (ie, ability to reject the tumor cells). Survival curves are shown, and the data represent the combined results of 2 or 3 separate experiments. Each group consisted of 5 to 9 total mice.
Accelerated loss of tumor-reactive CD8 T cells and diminished recall CD8 T-cell responses in CD4-depleted hosts
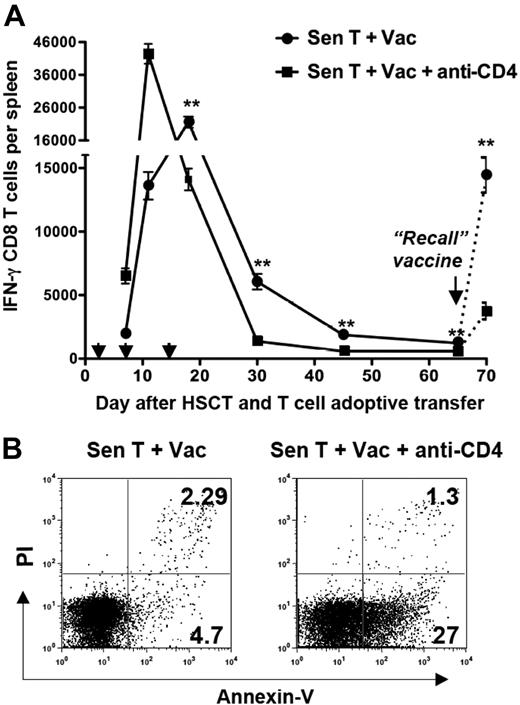
To explore the loss of antitumor T-cell memory in CD4-depleted hosts, we monitored absolute numbers of tumor-reactive CD8 T cells in the spleens of experimental mice over time by conducting IFN-γ ELISPOT assays. Numbers of tumor-reactive CD8 T cells were higher and peaked more quickly in CD4-depleted hosts than in non–CD4-depleted hosts, but the earlier peak in CD4-depleted hosts was accompanied by an accelerated contraction of tumor-reactive CD8 T cells (Figure 7A). After the third vaccine, tumor-reactive CD8 cell numbers in the CD4-depleted mice had fallen below those in non–CD4-depleted mice, and only a small fraction of the peak number of tumor-reactive CD8 T cells (1%-2%) remained detectable 30 days after HSCT in these mice. The presence of CD8 memory was assessed by revaccinating mice on day 65 after HSCT and examining the “recall” CD8 ELISPOT response to tumor 5 days later. Although a recall CD8 antitumor response was detected in the CD4-depleted hosts, the response was significantly diminished (P < .01) compared with the recall response in non–CD4-depleted hosts (Figure 7A).
An accelerated loss of tumor-reactive CD8 T cells in CD4-depleted hosts correlated with the failure to generate antitumor T-cell memory. Tumor-bearing mice were treated with TBI, HSCT, tumor-sensitized T-cell transfer, vaccination, and anti-CD4 mAb as depicted in Figure 4A. (A) Spleens were collected on days 7, 11, 18, 30, 45, and 65 after HSCT. The arrowheads indicate the vaccination times (2, 7, and 14 days after HSCT). Some mice were revaccinated (“Recall” vaccine) on day 65 after HSCT, and spleens collected 5 days later. Splenic CD8 T cells were isolated by immunomagnetic sorting and tested for tumor reactivity in IFN-γ ELISPOT assays. The figure shows the absolute numbers of tumor-reactive IFN-γ–secreting CD8 T cells (± SD) per spleen. Experimental values on day 70 after HSCT were adjusted by subtracting background values that were determined from mice given naive T cells at the time of HSCT and a single vaccine 65 days after HSCT. **P < .01. (B) Annexin V staining of CD8 T cells isolated 21 days after HSCT from the indicated groups of mice. The experiment is representative of 3 separate experiments in which splenocytes were pooled from 3 individual mice.
An accelerated loss of tumor-reactive CD8 T cells in CD4-depleted hosts correlated with the failure to generate antitumor T-cell memory. Tumor-bearing mice were treated with TBI, HSCT, tumor-sensitized T-cell transfer, vaccination, and anti-CD4 mAb as depicted in Figure 4A. (A) Spleens were collected on days 7, 11, 18, 30, 45, and 65 after HSCT. The arrowheads indicate the vaccination times (2, 7, and 14 days after HSCT). Some mice were revaccinated (“Recall” vaccine) on day 65 after HSCT, and spleens collected 5 days later. Splenic CD8 T cells were isolated by immunomagnetic sorting and tested for tumor reactivity in IFN-γ ELISPOT assays. The figure shows the absolute numbers of tumor-reactive IFN-γ–secreting CD8 T cells (± SD) per spleen. Experimental values on day 70 after HSCT were adjusted by subtracting background values that were determined from mice given naive T cells at the time of HSCT and a single vaccine 65 days after HSCT. **P < .01. (B) Annexin V staining of CD8 T cells isolated 21 days after HSCT from the indicated groups of mice. The experiment is representative of 3 separate experiments in which splenocytes were pooled from 3 individual mice.
To examine the mechanism(s) responsible for the accelerated contraction of tumor-reactive CD8 T cells in CD4-depleted hosts, vaccine-draining lymph nodes were harvested during the contraction phase (21 days after HSCT; Figure 7A), and the CD8 T-cell viability was analyzed. There were increased percentages of annexin V–positive, PI-negative preapoptotic CD8 T cells in CD4-depleted mice compared with non–CD4-depleted mice (27% vs 4.7%; Figure 7B). These data suggest that increased apoptosis of tumor-reactive CD8 T cells in the absence of CD4 help contributes to the loss of CD8 memory.
Discussion
We previously demonstrated that administration of the AGN2a-4P cell-based neuroblastoma vaccine could elicit a tumor-protective immune response early after HSCT that required adoptive T-cell transfer and involved both CD8 and CD4 T cells.28 However, to our disappointment, this tumor vaccine was unable to eliminate established tumors in nontransplanted mice (unpublished data). In the current study, we combined myeloablative conditioning, syngeneic HSCT, adoptive T-cell transfer, and vaccination in an effort to treat established neuroblastoma. Adoptive transfer of naive T cells at the time of transplantation resulted in elimination of established tumors in 27% of vaccinated mice, which was the first time we had observed elimination of established tumors in the AGN2a model. It was clear that each component of this multifaceted approach to immunotherapy contributed to the generation of antitumor immunity. Notably, although the mice in these experiments were genetically identical, and the same immunotherapy administered, tumors were eliminated in some mice but not in others. Mice with “regressing” tumors had higher frequencies of tumor-reactive CD8 T cells in their peripheral lymphoid tissues than mice with “progressing” tumors, indicating that there is heterogeneity in the immune response. We found that antitumor efficacy could be increased by adoptive transfer with T cells presensitized to tumor antigens (ie, T cells from tumor vaccinated donors), but vaccination was still important for generating the “optimal” antitumor response, reemphasizing the benefits of combining different forms of immunotherapy. Our results differed from the pmel-1 TCR-Tg transgenic melanoma tumor model, where vaccination was not required for antitumor immunity when tumor antigen-reactive CD8 T cells were adoptively transferred to myeloablated hosts.11 It is possible that if sufficient numbers of tumor-reactive T cells are administered in this setting, vaccination is not needed to obtain an optimal antitumor effect.
It has been reported that combined adoptive transfer of CD4 and CD8 effector T cells can provide synergistic antitumor effects in mice with established tumors.24 Some studies demonstrated that CD4 T cells were capable of protecting the host against tumor challenge, and that they could mediate complete tumor regressions independent of CD8 T cells.18,20,30,31 However, most of these studies administered purified helper CD4 cells or monoclonal populations of tumor-specific CD4 T cells as adoptive immunotherapy, and none of these experiments were done in the HSCT setting. In this study, one intriguing result is that antitumor efficacy could be increased, albeit not significantly, in hosts given tumor-sensitized T cells by depleting CD4 T cells (Figure 4C, 100% survival). The increased antitumor response in these mice was reflected by increased T-cell proliferation, increased expansion of CD8 T cells with a CD44highCD62low activated phenotype, significantly increased numbers of tumor-reactive CD8 T cells, and increased tumor infiltration by CD8 T cells. These results suggest that although CD4 T cells are important for the generation of effective antitumor immunity, previously generated tumor-reactive CD8 T cells are inhibited by the presence of CD4 T cells. The mechanisms of how depletion of CD4 T cells enhanced activation of adoptively transferred tumor reactive CD8 T cells are not clear. One possible explanation is that depletion of both endogenous and transferred CD4 T cells removes competition for lymphoid space32 or homeostatic cytokines.9,10 We found that the majority of host T cells that survive total body irradiation were CD4+, and that the splenic CD4:CD8 ratio was increased from the pretransplantation ratio of 2:1 up to 20:1, 4 days after HSCT (Figure S1; available on the Blood website; see the Supplemental Materials link at the top of the online article). Another explanation is the depletion of CD4+CD25+Foxp3+ regulatory T cells. We have found that percentages of CD4+CD25+Foxp3+ cells in lymphoid tissues were elevated in mice that had undergone HSCT compared with normal mice28 when the recipients were also given adoptive T-cell transfer (Figure S2). We hypothesize that the inhibitory effect of CD4 T cells in our model (when tumor-sensitized T cells are adoptively transferred) may involve the activation and/or expansion of CD4+CD25+Foxp3+ regulatory T cells. The negative influences mediated by the regulatory T cells after HSCT may temporarily outweigh the beneficial effects provided by the CD4 helper T cells. Thus, the removal of all CD4 cells results in a net beneficial antitumor response after HSCT.
Some studies have shown that CD4 T-cell help is important during primary immune responses to deliver signals necessary for the differentiation, function, and survival of CD8 memory T cells.33,34 Other work demonstrated that the presence of CD4 T cells is required during the postprogramming phase of the CD8 T-cell response to generate memory,35 suggesting that CD8 memory T cells are not irreversibly programmed and CD4 T cells are important for the maintenance of memory. However, little information is available about the role of CD4 T cells in the generation of CD8 memory after HSCT. In the current study, we found that some mice given just tumor-sensitized T cells at the time of HSCT (no vaccine) could completely eliminate established tumors. These mice were able to resist a second tumor challenge 65 days after HSCT, suggesting that some of the sensitized effector T cells differentiate into antitumor memory cells when CD4 T cells are present. When tumor-sensitized T cells were adoptively transferred, tumor-reactive CD8 T cells could still be detected in the spleen, LNs and BM of mice 90 days after adoptive transfer (data not shown). It is possible that these tumor-reactive cells proliferate, persist, and differentiate into tumor-specific central memory cells, or perhaps central memory precursor cells are present in the adoptively transferred T cells.36 However, even though the adoptively transferred T cells harvested from donor mice were primed in the presence of CD4 T cells, CD8 memory to tumor antigens failed to develop in HSCT recipients if CD4 T cells were depleted. In addition, administration of the AGN2a-4P vaccine did not restore the antitumor memory response when CD4 T cells were depleted. Therefore, this study demonstrates that the continued presence of CD4 T cells is necessary for the generation of persistent CD8 T-cell memory after HSCT.
In summary, we have demonstrated that a multifaceted approach to immunotherapy, involving HSCT, adoptive T-cell transfer and cell-based tumor vaccination, can be used to eliminate established neuroblastoma. When we examined the role of CD4 T cells in this HSCT-based immunotherapy, we unexpectedly found that in vivo depletion of CD4 T cells enhanced antitumor efficacy when the adoptively transferred T cells were pre-sensitized to tumor antigens. Despite enhancement of the early antitumor response, depletion of CD4 T cells resulted in impaired T-cell memory. Because CD4 T cells can promote or inhibit antitumor responses, depending upon the balance of CD4 subsets present (helper or regulatory), manipulation of these subsets may offer a strategy for designing approaches that can generate increased and sustained antitumor immunity. Such strategies might involve selective depletion of regulatory T cells from the transferred T cells or restoration of memory by giving additional tumor vaccines to activate new thymus-derived CD4 T cells. These approaches are currently being investigated in our laboratory.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jamie Zernicke for expert technical assistance and William Hallett for help in editing the manuscript.
This work was supported by US Public Health Service Grant CA100030 and the Midwest Athletes Against Childhood Cancer (MACC Fund Inc, Milwaukee, WI).
Authorship
Contribution: W.J. designed and performed all of the experiments, analyzed the results, produced the figures, and wrote the manuscript; J.G. aided in data analysis and helped write the manuscript; and B.D.J. assisted in experimental design, aided in data analysis, and assisted in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bryon D. Johnson, Department of Pediatrics, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: bjohnson@mcw.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal