Abstract
In this study, we analyzed the long-term outcome of a risk-adapted transplantation strategy for mantle cell lymphoma in 121 patients enrolled in sequential transplantation protocols. Notable developments over the 17-year study period were the addition of rituximab to chemotherapy and preparative regimens and the advent of nonmyeloablative allogeneic stem cell transplantation (NST). In the autologous transplantation group (n = 86), rituximab resulted in a marked improvement in progression-free survival for patients who received a transplant in their first remission (where a plateau emerged at 3-8 years) but did not change the outcomes for patients who received a transplant beyond their first remission. In the NST group, composed entirely of patients who received a transplant beyond their first remission, durable remissions also emerged in progression-free survival at 5 to 9 years. The major determinants of disease control after NST were the use of a peripheral blood stem cell graft and donor chimerism of at least 95%, whereas the major determinant of death was immunosuppression for chronic graft-versus-host disease. Our results show that long-term disease-free survival in mantle cell lymphoma is possible after rituximab-containing autologous transplantation for patients in first remission and after NST for patients with relapsed or refractory disease.
Introduction
Mantle cell lymphoma (MCL) is an incurable B-cell malignant neoplasm with a median survival of 3 to 5 years.1-3 The results of CHOP (cyclophosphamide, adriamycin, vincristine, and prednisone) and similar regimens as frontline therapy are poor, with complete remissions (CRs) being achieved in less than 25% of patients4-9 and responses lasting a median of 1 to 2 years.5-9 These results led to the widespread exploration of autologous stem cell transplantation (SCT) in first remission, which improved the CR rate and median remission duration of 60% to 100% and 3 to 4 years, respectively.4,6,7,10-16 However, relapses continued to occur in a continuous fashion and no cured fractions were apparent on long-term follow-up.4,11,13-15
Two recent major therapeutic advances have substantially altered the outlook of patients with MCL. The first is the introduction of the chimeric anti-CD20 antibody rituximab,17 which in combination with chemotherapy has improved the results of both frontline and salvage treatments for MCL.9,18,19 The most successful combination is the rituximab and hyper–cyclophosphamide, vincristine, adriamycin, and dexamethasone program (R-hyper-CVAD),20,21 which is capable of achieving CR rates of up to 90% in the frontline setting, with a prolonged 5-year failure-free survival of 60% in younger patients.22 These results appear at least equivalent to that of autologous SCT in the era before rituximab. What is not known, however, is whether rituximab has also improved the outcome of autologous stem cell transplantation in a similar manner. The other major therapeutic advance is the use of nonmyeloablative stem cell transplantation (NST).23 Allogeneic SCT may be curative in patients with lymphoma, but it is associated with a prohibitive transplant-related mortality (TRM) of up to 40%.24,25 The use of a nonmyeloablative preparative regimen ameliorates this toxicity, while preserving the graft-versus-lymphoma (GVL) effect, and broadens the applicability of allogeneic transplantation to older patients. Investigators from our center had previously reported the safety and efficacy of NST in patients with advanced lymphoid malignancies,23 including promising preliminary results in a small number of patients with relapsed or refractory MCL.26 However, long-term follow-up to assess the durability of disease control was not available at the time of our previous report.
To determine whether rituximab has improved the outcome of patients undergoing autologous SCT and to establish the effectiveness and durability of disease control in patients undergoing NST, we analyzed the mature results of 17 years of transplantation experience in patients with MCL at our cancer center. Our results show that cured fractions may be emerging in patients who had received rituximab-containing autologous transplants in first remission and in patients who had received NST for relapsed or refractory disease.
Methods
Patient population and synopsis of transplantation strategy
In this retrospective study, we included all patients with MCL who had undergone transplantation in sequential phase 2 protocols of autologous SCT or NST at the University of Texas M. D. Anderson Cancer Center (MDACC) from February 1990 to June 2007. The protocols had been approved by the MDACC Institutional Review Board, and informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Eligible patients had a biopsy-proven diagnosis of MCL as defined by the World Health Organization criteria. The diagnosis was based on histologic and immunophenotypic criteria and included either immunohistochemical analysis for cyclin D1, cytogenetic analysis by either conventional karyotyping or fluorescence in situ hybridization (FISH) for the t(11;14)(q13;q32), or both.
The transplantation strategy used was a risk-adapted approach based primarily on the patient's treatment status. For patients in the first remission after chemotherapy, autologous SCT was performed as consolidation therapy between 1990 and 2001.10 From 2001 onward, the favorable clinical outcomes found with R-hyper-CVAD chemotherapy led to autologous SCT being performed in first remission only in patients not in CR after R-hyper-CVAD and to patients who had received less-intensive induction chemotherapy (eg, R-CHOP). For patients with relapsed or primary refractory MCL, autologous SCT was performed before the use of NST in 1997. After 1997, NST was performed whenever a histocompatible donor was available.26 Patients generally underwent autologous SCT up to the age of 70 years and NST up to the age of 65 years. Since 2004, patients up to the age of 75 years were allowed to receive an autologous SC transplant. Patients were categorized as having undergone autologous SCT in first complete or partial remission (AUTO1), autologous SCT for relapsed or refractory disease (AUTO2), or NST for relapsed or refractory disease (NST).
Preparative regimens and stem cell infusion
Details of preparative regimens, supportive care, infection, and graft-versus-host disease (GVHD) prophylaxis (where applicable) and early results have been previously published.10,11,26 The predominant autologous SCT preparative regimens were high-dose cyclophosphamide and total body irradiation (Cy/TBI) between 1990 and 1999, rituximab (375 mg/m2 the day before mobilization and again at 1000 mg/m2 7 days later and on days +1 and +8 after autologous SCT)27 and Cy/TBI (R-Cy/TBI) between 1999 and 2001, and rituximab and BEAM (R-BEAM; rituximab, carmustine, etoposide, cytarabine, and melphalan) between 2001 and 2007 (Table 1). Five patients who received an autologous SC transplant received other preparative regimens (BEAM, n = 3; busulfan and melphalan, n = 1; thiotepa, busulfan, and cyclophosphamide, n = 1). Peripheral blood stem cells (PBSCs) were the preferred stem cell source whenever sufficient numbers (≥ 2 × 106 CD34/kg) were available.
Preparative regimens
| Preparative regimen . | SCT . | n . | Period . | Doses . |
|---|---|---|---|---|
| Cy/TBI | Auto | 43 | 1990-1999 | Cyclophosphamide 60 mg/kg per day (days −7, −6); TBI 12.0 Gy in 4 fractions (days −4 to −1). |
| R-Cy/TBI | Auto | 15 | 1999-2001 | Cyclophosphamide 60 mg/kg per day (days −7, −6); TBI 12.0 Gy in 4 fractions (days −4 to −1). |
| Rituximab 375 mg/m2 day 1 and 1000 mg/m2 day 8 of chemomobilization of stem cells. | ||||
| Rituximab 1000 mg/m2 days 1 and 8 after transplantation. | ||||
| R-BEAM | Auto | 23 | 2001-2007 | Carmustine 300 mg/m2 day −6; etoposide 200 mg/m2 twice daily (days −5 to −2); cytarabine 200 mg/m2 twice daily (days −5 to −2); melphalan 140 mg/m2 day −1. |
| Rituximab 375 mg/m2 day 1 and 1000 mg/m2 day 8 of chemomobilization of stem cells. | ||||
| Rituximab 1000 mg/m2 days 1 and 8 after transplantation. | ||||
| PFA | NST | 5 | 1997-2000 | Cisplatin 25 mg/m2 days −6 to −3; fludarabine 30 mg/m2 days −4 and −3; cytarabine 1 g/m2 days −4 and −3. |
| FCR | NST | 30 | 2000-2007 | Fludarabine 30 mg/m2 days −5 to − 3; cyclophosphamide 750 mg/m2 days −5 to −3; rituximab 375 mg/m2 day −13 and 1 g/m2 days −6, 1, 8. |
| Preparative regimen . | SCT . | n . | Period . | Doses . |
|---|---|---|---|---|
| Cy/TBI | Auto | 43 | 1990-1999 | Cyclophosphamide 60 mg/kg per day (days −7, −6); TBI 12.0 Gy in 4 fractions (days −4 to −1). |
| R-Cy/TBI | Auto | 15 | 1999-2001 | Cyclophosphamide 60 mg/kg per day (days −7, −6); TBI 12.0 Gy in 4 fractions (days −4 to −1). |
| Rituximab 375 mg/m2 day 1 and 1000 mg/m2 day 8 of chemomobilization of stem cells. | ||||
| Rituximab 1000 mg/m2 days 1 and 8 after transplantation. | ||||
| R-BEAM | Auto | 23 | 2001-2007 | Carmustine 300 mg/m2 day −6; etoposide 200 mg/m2 twice daily (days −5 to −2); cytarabine 200 mg/m2 twice daily (days −5 to −2); melphalan 140 mg/m2 day −1. |
| Rituximab 375 mg/m2 day 1 and 1000 mg/m2 day 8 of chemomobilization of stem cells. | ||||
| Rituximab 1000 mg/m2 days 1 and 8 after transplantation. | ||||
| PFA | NST | 5 | 1997-2000 | Cisplatin 25 mg/m2 days −6 to −3; fludarabine 30 mg/m2 days −4 and −3; cytarabine 1 g/m2 days −4 and −3. |
| FCR | NST | 30 | 2000-2007 | Fludarabine 30 mg/m2 days −5 to − 3; cyclophosphamide 750 mg/m2 days −5 to −3; rituximab 375 mg/m2 day −13 and 1 g/m2 days −6, 1, 8. |
Five patients underwent autologous SCT received regimens not listed in the table: BEAM (n = 3); busulfan and melphalan (n = 1); and thiotepa, busulfan, and cyclophosphamide (n = 1).
SCT indicates stem cell transplantation; Cy, cyclophosphamide; TBI, total body irradiation; R, rituximab; BEAM, carmustine, etoposide, cytarabine, and melphalan; NST, nonmyeloablative stem cell transplantation; PFA, cisplatin, fludarabine, and cytarabine; and FCR, fludarabine, cyclophosphamide, and rituximab.
Among patients who received an NST, the preparative regimens used were PFA (cisplatin, fludarabine, and cytarabine) between 1997 and 2000, and FCR (fludarabine, cyclophosphamide, and rituximab)26 between 2000 and 2007 (Table 1). Twenty-two patients (63%) received a transplant from a histocompatible sibling, 11 (31%) from a histocompatible unrelated donor, and 2 (6%) from a single-antigen mismatched parent (n = 1) or offspring (n = 1). PBSCs were used as the stem cell source in all 22 siblings (100%) and in 5 (38%) of 13 nonsibling transplantations. Patients who underwent nonsibling transplantation also received additional rejection prophylaxis with antithymocyte globulin (n = 5, as previously described28 ) or alemtuzumab (n = 8, total dose 45 mg) during conditioning. Prophylaxis for GVHD consisted of tacrolimus for 6 months (adjusted to trough levels of 5-10 ng/mL) and methotrexate (5 mg/m2 on days +1, +3, and +6, with an additional dose on day +11 for nonsibling transplantations). Immunomanipulation after transplantation (withdrawal of immunosuppression ± step-wise donor lymphocyte infusions [DLI]) was performed in patients with progressive or residual disease with the use of previously established methods.28,29
Statistical analysis
Standard definitions were used to assess disease response, treatment complication, and GVHD severity.30-32 Because many of the transplantations occurred before the widespread availability of 18FDG positron emission tomography (PET) scanning, these scans were not used as part of the disease response criteria. The Mantle Cell International Prognostic Index (MIPI) and Hematopoietic Stem Cell Transplantation-Comorbidity Index (HSCT-CI) were calculated according to published methods.33,34 Comparisons of categorical and continuous variables were made with the use of the Fisher exact or χ2 tests, and the t or F tests, as appropriate. Actuarial estimates of overall survival (OS) and progression-free survival (PFS) were calculated with the use of the method of Kaplan and Meier and were dated from the day of transplantation to the time of progression, death, or last follow-up. The effect of patient and transplantation characteristics on PFS and OS were examined with the use of the log-rank test and Cox regression analysis as appropriate. Multivariate analysis of significant univariate factors was performed with the use of Cox regression analysis with backward selection. All P values were 2-sided.
Results
Patient characteristics
Our study included 121 patients with MCL: 50 AUTO1, 36 AUTO2, and 35 NST. The patients' pretransplantation characteristics are summarized in Table 2. The ages at transplantation were similar in all 3 groups (median, 57 years [range, 38-73 years] for AUTO1; median, 59 years [range, 42-76 years] for AUTO2; and median, 58 years [range, 43-68 years] for NST; P = NS for all comparisons). AUTO2 patients were more likely than AUTO1 patients to be chemorefractory at transplantation (14% vs 0%; P = .01), to have positive PET/gallium scan (29% vs 3%; P = .01), and to have elevated lactate dehydrogenase levels (31% vs 14%; P = .06). The distribution of conditioning regimens was similar between the 2 autologous groups (Table 2).
Patient characteristics
| Characteristic . | Autologous SCT in first remission (AUTO1, n = 50) . | Autologous SCT for relapsed / refractory MCL (AUTO2, n = 36) . | Nonmyeloablative allogeneic SCT (NST, n = 35) . |
|---|---|---|---|
| Median age, y (range) | 57 (38-73) | 59 (42-76) | 58 (43-68) |
| Male, n (%) | 44 (88) | 29 (81) | 27 (77) |
| Female, n (%) | 6 (12) | 7 (19) | 8 (23) |
| Median time from initial diagnosis, mo (range) | 7 (4-63) | 27 (5-84)* | 44 (11-119)† |
| Median no. of prior chemotherapy regimens (range) | 1 | 2 (2-4)* | 3 (1-10)† |
| Performance status ≥ 1, n (%) | 23 of 49 (47) | 15 of 31 (48) | 14 (40) |
| Stage 3 or 4, n (%) | 46 (92) | 33 (92) | 35 (100) |
| Prior B symptoms, n (%) | 14 (28) | 8 (22) | 6 (17) |
| Prior GI involvement, n (%) | 21 (42) | 12 (33) | 13 (37) |
| Prior blood involvement, n (%) | 8 (16) | 7 (19) | 8 (23) |
| Blastic morphology, n (%) | 5 (10) | 2 (6) | 1 (3) |
| β2-microglobulin ≥ 3 mg/L, n (%) | 12 of 44 (27) | 9 of 30 (30) | 7 (20) |
| Elevated LDH, n (%) | 7 (14) | 11 (31) | 10 (29) |
| PET/gallium positive before SCT | 1 (3) | 6 (29)* | 11 (33) |
| Bone marrow stem cell source, n (%) | 6 (12) | 5 (14) | 8 (23) |
| Chemosensitive disease at transplantation, n (%) | 50 (100) | 31 (86) | 29 (83) |
| CR/CRu at SCT, n (%) | 23 (46) | 25 (69) | 16 (46)† |
| PR at SCT, n (%) | 27 (54) | 6 (17) | 13 (37) |
| Refractory disease at SCT, n (%) | 0 (0) | 5 (14) | 6 (17) |
| MIPI low risk, n (%) | 16 (40) | 7 (47) | 9 (56) |
| MIPI intermediate risk, n (%) | 13 (33) | 2 (13) | 1 (6) |
| MIPI high risk, n (%) | 11 (28) | 6 (40) | 6 (38) |
| HSCT-CI < 3, n (%) | 28 (56) | 18 (50) | 21 (60) |
| HSCT-CI ≥ 3, n (%) | 22 (44) | 18 (50) | 14 (40) |
| Prior hyper-CVAD, n (%) | 29 (58) | 16 (44) | 1 (3)† |
| Prior R-hyper-CVAD, n (%) | 13 (26) | 6 (17) | 23 (66)† |
| Failed prior auto-SCT, n (%) | 0 (0) | 0 (0) | 6 (17)† |
| Rituximab during induction, and/or at transplantation, n (%) | 21 (42) | 19 (53) | 32 (91)† |
| Conditioning regimen, n (%) | |||
| Cy/TBI | 28 (56) | 15 (42) | |
| R-Cy/TBI | 9 (18) | 6 (17) | |
| R-BEAM | 11 (22) | 12 (33) | |
| Other | 2 (4) | 3 (8) | |
| PFA | 5 (14) | ||
| FCR | 30 (86) |
| Characteristic . | Autologous SCT in first remission (AUTO1, n = 50) . | Autologous SCT for relapsed / refractory MCL (AUTO2, n = 36) . | Nonmyeloablative allogeneic SCT (NST, n = 35) . |
|---|---|---|---|
| Median age, y (range) | 57 (38-73) | 59 (42-76) | 58 (43-68) |
| Male, n (%) | 44 (88) | 29 (81) | 27 (77) |
| Female, n (%) | 6 (12) | 7 (19) | 8 (23) |
| Median time from initial diagnosis, mo (range) | 7 (4-63) | 27 (5-84)* | 44 (11-119)† |
| Median no. of prior chemotherapy regimens (range) | 1 | 2 (2-4)* | 3 (1-10)† |
| Performance status ≥ 1, n (%) | 23 of 49 (47) | 15 of 31 (48) | 14 (40) |
| Stage 3 or 4, n (%) | 46 (92) | 33 (92) | 35 (100) |
| Prior B symptoms, n (%) | 14 (28) | 8 (22) | 6 (17) |
| Prior GI involvement, n (%) | 21 (42) | 12 (33) | 13 (37) |
| Prior blood involvement, n (%) | 8 (16) | 7 (19) | 8 (23) |
| Blastic morphology, n (%) | 5 (10) | 2 (6) | 1 (3) |
| β2-microglobulin ≥ 3 mg/L, n (%) | 12 of 44 (27) | 9 of 30 (30) | 7 (20) |
| Elevated LDH, n (%) | 7 (14) | 11 (31) | 10 (29) |
| PET/gallium positive before SCT | 1 (3) | 6 (29)* | 11 (33) |
| Bone marrow stem cell source, n (%) | 6 (12) | 5 (14) | 8 (23) |
| Chemosensitive disease at transplantation, n (%) | 50 (100) | 31 (86) | 29 (83) |
| CR/CRu at SCT, n (%) | 23 (46) | 25 (69) | 16 (46)† |
| PR at SCT, n (%) | 27 (54) | 6 (17) | 13 (37) |
| Refractory disease at SCT, n (%) | 0 (0) | 5 (14) | 6 (17) |
| MIPI low risk, n (%) | 16 (40) | 7 (47) | 9 (56) |
| MIPI intermediate risk, n (%) | 13 (33) | 2 (13) | 1 (6) |
| MIPI high risk, n (%) | 11 (28) | 6 (40) | 6 (38) |
| HSCT-CI < 3, n (%) | 28 (56) | 18 (50) | 21 (60) |
| HSCT-CI ≥ 3, n (%) | 22 (44) | 18 (50) | 14 (40) |
| Prior hyper-CVAD, n (%) | 29 (58) | 16 (44) | 1 (3)† |
| Prior R-hyper-CVAD, n (%) | 13 (26) | 6 (17) | 23 (66)† |
| Failed prior auto-SCT, n (%) | 0 (0) | 0 (0) | 6 (17)† |
| Rituximab during induction, and/or at transplantation, n (%) | 21 (42) | 19 (53) | 32 (91)† |
| Conditioning regimen, n (%) | |||
| Cy/TBI | 28 (56) | 15 (42) | |
| R-Cy/TBI | 9 (18) | 6 (17) | |
| R-BEAM | 11 (22) | 12 (33) | |
| Other | 2 (4) | 3 (8) | |
| PFA | 5 (14) | ||
| FCR | 30 (86) |
Patients were divided into those who underwent autologous stem cell transplantation (SCT) in first remission (AUTO1) or beyond first remission (AUTO2) and those who underwent nonmyeloablative SCT beyond first remission (NST).
GI indicates gastrointestinal; LDH, lactate dehydrogenase; CR, complete response; CRu, unconfirmed CR; PR, partial response; MIPI, Mantle Cell International Prognostic Index; and HSCT-CI, Hematopoietic Stem Cell Transplantation-Comorbidity Index.
P < .05 comparing AUTO1 and AUTO2.
P < .05 comparing AUTO2 and NST.
NST patients had more adverse disease features than did AUTO2 patients, including being further from the time of initial diagnosis (median, 44 vs 27 months, respectively; P = .02), having undergone more lines of prior chemotherapy (median, 3 vs 2, respectively; P < .001), and being less likely to be in CR or unconfirmed CR (CRu) at the time of transplantation (46% vs 69%, respectively; P = .04). The proportions of patients with chemorefractory disease at the time of transplantation were similar (17% vs 14% for AUTO2; P = NS). NST patients were more likely to have received rituximab during induction therapy or transplantation (91% vs 53% for AUTO2; P < .001).
R-hyper-CVAD and autologous stem cell collection
Sixteen patients were referred to our center for consideration of autologous stem cell transplantation in first remission after R-hyper-CVAD induction. Three patients did not experience stem cell mobilization and could not undergo transplantation. In 2 patients, we could not collect blood stem cells, but the patients underwent transplantation after bone marrow harvesting. These 5 patients received a median of 6 cycles of R-hyper-CVAD and had active disease at the time of their referral.
AUTO1 patients
Fifty patients received an autologous SC transplant in first remission: 23 (46%) in CR or CRu and 27 (54%) in partial response (PR). The following induction chemotherapy regimens were used: hyper-CVAD in 29 patients (58%), R-hyper-CVAD in 13 patients (26%), R-CHOP in 6 patients (12%), and CHOP in 1 patient (2%). The regimen was not documented in 1 patient (2%). Thirty-four patients underwent functional imaging with either gallium (n = 24) or PET (n = 10) scans before transplantation, of which 33 (97%) were negative for disease. The 1 patient with a positive pretransplantation PET scan was in a partial response after R-hyper-CVAD induction. The major conditioning regimens were Cy/TBI (56%), R-Cy/TBI (18%), and R-BEAM (22%).
All patients had attained primary neutrophil engraftment at a median 10 days (range, 7-29 days) after transplantation. Two patients (4%) had not recovered a transfusion-independent platelet count of at least 20 × 109/L and died of bleeding at 8 and 22 weeks after transplantation. Both patients had received bone marrow grafts because of inadequate PBSC yields. No other transplantation-related deaths occurred. Thus, the TRM rates were 2% at 3 months and 4% at 12 months. Of the 27 patients who had undergone SCT with a PR, 26 (96%) achieved CR or CRu after transplantation. Of the 23 patients who had undergone transplantation with a CR or CRu, one was not evaluable for response because of early TRM, and 21 (95%) remained in CR or CRu after transplantation.
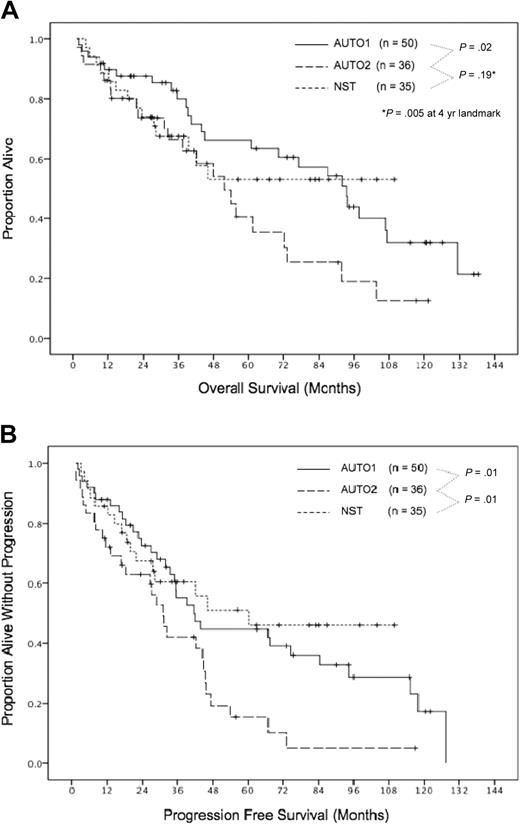
At a median follow-up of 6 years, the actuarial PFS and OS were 39% and 61%, respectively, with median PFS and OS durations of 42 months and 93 months (Figure 1). We determined whether disease status at transplantation (CR or CRu vs PR) was associated with long-term outcome and found no significant differences in the PFS durations (median, 67 vs 42 months, respectively; P = .27) or OS durations (median, 131 vs 93 months, respectively; P = .53). The single patient with PET-positive disease before transplantation remained in ongoing CRu 30 months after transplantation. Other factors evaluated for their relation with PFS and OS are listed in Table 3.
Overall survival and progression-free survival. Overall survival (A) and progression-free survival (B). AUTO1 indicates patients receiving autologous transplant in first remission; AUTO2, patients receiving autologous transplant for relapsed/refractory disease; and NST, patients receiving nonmyeloablative stem cell transplant for relapsed/refractory disease.
Overall survival and progression-free survival. Overall survival (A) and progression-free survival (B). AUTO1 indicates patients receiving autologous transplant in first remission; AUTO2, patients receiving autologous transplant for relapsed/refractory disease; and NST, patients receiving nonmyeloablative stem cell transplant for relapsed/refractory disease.
Univariate and multivariate analyses of pretransplant and posttransplant factors and outcome for patients who underwent autologous transplantation in first remission (AUTO1), autologous transplantation beyond first remission (AUTO2), or nonmyeloablative allogeneic stem cell transplantation (NST)
| Group . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Median (mo) . | P . | Hazard ratio . | P . | |
| Progression-free survival | ||||
| AUTO1 | ||||
| Marrow graft | 18 (vs 67) | < .001 | 8.3 | < .001 |
| B symptoms | 18 (vs 44) | .02 | 3.1 | .006 |
| AUTO2 | No factor identified | |||
| NST | ||||
| Peripheral blood graft and best chimerism ≥ 95% | NR (vs 20) | .02 | 0.25 | .008 |
| Acute GVHD | 22 (vs NR) | .03 | ||
| ≥ 5 prior therapies | 15 (vs NR) | .04 | 4.0 | .02 |
| Overall survival | ||||
| AUTO1 | ||||
| Marrow graft | 24 (vs 98) | < .001 | 7.2 | < .001 |
| B symptoms | 41 (vs 107) | .02 | 4.6 | .002 |
| HSCT-CI ≥ 3 | 70 (vs 131) | .05 | ||
| AUTO2 | No independent factor identified | |||
| B symptoms | 32 (vs 61) | .04 | ||
| β2m ≥ 3 mg/L | 22 (vs 72) | .05 | ||
| Total body irradiation | 42 (vs 104) | .04 | ||
| HSCT-CI ≥ 3 | 48 (vs 72) | .04 | ||
| NST | ||||
| Acute GVHD | 23 (vs NR) | .009 | 3.9 | .02 |
| ≥ 5 prior therapies | 23 (vs NR) | .03 | ||
| Chronic GVHD | 42 (vs NR) | .03 | ||
| Group . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Median (mo) . | P . | Hazard ratio . | P . | |
| Progression-free survival | ||||
| AUTO1 | ||||
| Marrow graft | 18 (vs 67) | < .001 | 8.3 | < .001 |
| B symptoms | 18 (vs 44) | .02 | 3.1 | .006 |
| AUTO2 | No factor identified | |||
| NST | ||||
| Peripheral blood graft and best chimerism ≥ 95% | NR (vs 20) | .02 | 0.25 | .008 |
| Acute GVHD | 22 (vs NR) | .03 | ||
| ≥ 5 prior therapies | 15 (vs NR) | .04 | 4.0 | .02 |
| Overall survival | ||||
| AUTO1 | ||||
| Marrow graft | 24 (vs 98) | < .001 | 7.2 | < .001 |
| B symptoms | 41 (vs 107) | .02 | 4.6 | .002 |
| HSCT-CI ≥ 3 | 70 (vs 131) | .05 | ||
| AUTO2 | No independent factor identified | |||
| B symptoms | 32 (vs 61) | .04 | ||
| β2m ≥ 3 mg/L | 22 (vs 72) | .05 | ||
| Total body irradiation | 42 (vs 104) | .04 | ||
| HSCT-CI ≥ 3 | 48 (vs 72) | .04 | ||
| NST | ||||
| Acute GVHD | 23 (vs NR) | .009 | 3.9 | .02 |
| ≥ 5 prior therapies | 23 (vs NR) | .03 | ||
| Chronic GVHD | 42 (vs NR) | .03 | ||
Factors examined included: (1) age, sex, time from diagnosis, advanced stage (3/4) B symptoms, gastrointestinal involvement, blood involvement, blastic morphology, β2-microglobulin, lactate dehydrogenase; (2) International Prognostic Index (IPI), Mantle Cell IPI (MIPI), number of prior treatments, performance status, rituximab versus no rituximab, hyper-CVAD versus other regimens, disease status at transplantation, PET/Gallium scan at transplantation, conditioning regimen, stem cell source (PB vs BM); and (3) HSCT-CI. The following factors were applicable to nonmyeloablative stem cell transplantation patients only: sibling versus nonsibling donor, CMV status, sex mismatch, donor age, donor chimerism, acute GVHD, chronic GVHD, alemtuzumab exposure, and prior autologous transplantation.
NR indicates not reached; GVHD, graft-versus-host disease; PB, peripheral blood; BM, bone marrow; and HSCT-CI, Hematopoietic Stem Cell Transplantation-Comorbidity Index.
Univariate analysis of baseline characteristics identified marrow stem cell source and the presence of B symptoms (at any time before transplantation) as being significantly associated with inferior PFS and OS (Table 3). In addition, a high HSCT-CI was also associated with an inferior OS. On multivariate analysis, both marrow stem cell source and B symptoms were confirmed to be independent associates of inferior PFS and OS (marrow stem cell source: hazard ratio [HR], 8.3 and P < .001 for PFS, and HR, 7.2 and P < .001 for OS; B symptoms: HR, 3.1 and P = .006 for PFS, and HR, 4.6 and P = .002 for OS).
AUTO2 patients
Of the 36 AUTO2 patients, 11 (31%) did not experience a response to initial chemotherapy but did experience a PR or better to salvage therapy with hyper-CVAD (n = 6), R-hyper-CVAD (n = 4), or methotrexate and ara-C (n = 1). Seventeen patients (47%) were in their second remission, 3 (8%) were in their third or subsequent remission, and 5 (14%) had chemorefractory relapse and were transplanted in less than partial remission. The major conditioning regimens were Cy/TBI (42%), R-Cy/TBI (17%), and R-BEAM (33%).
All patients experienced neutrophil and platelet engraftment. Early deaths as a result of infection occurred at 5, 11, and 12 weeks in 3 patients. Thus, the TRM rates were both 8% at 3 months and 1 year (P = .04 compared with AUTO1). The actuarial 6-year PFS and OS rates were 10% and 35%, respectively (P = .01 and .02 compared with AUTO1; Figure 1), and the median PFS and OS durations were 27 and 52 months, respectively. The inferior results for both PFS and OS compared with AUTO1 patients were maintained in a multivariate analysis that accounted for differences in baseline factors.
No baseline factor was significantly associated with PFS (Table 3). Importantly, PFS durations were similar regardless of disease status at transplantation (medians, 31, 27, and 23 months for CR or CRu, PR, and refractory relapse, respectively; P = NS for all comparisons). The presence of B symptoms, elevated β2m, use of total body irradiation, and HSCT-CI score of 3 or greater were associated with inferior OS on the univariate analysis (Table 3). However, none of these factors was independently prognostic on the multivariate analysis.
Favorable effect of rituximab in autologous transplantation
Rituximab was administered during the treatment sequence in 21 (42%) AUTO1 and 19 (53%) AUTO2 patients. Among frontline patients, 18 (86%) had received rituximab during both the chemotherapy and transplantation phases, 2 (10%) during transplantation only, and 1 (5%) during chemotherapy only. Among patients with relapsed or refractory MCL, 16 (84%) had received rituximab during both the chemotherapy and transplantation phases and 3 (16%) during transplantation only.
The baseline characteristics of patients receiving rituximab in both SCT groups were analyzed (Table 4). Among AUTO1 patients, the characteristics of those who received rituximab were similar to those who did not (P ≥ .10 for all comparisons), with the exception of prior blood involvement (more frequent in the rituximab group, 33% vs 3%; P = .007) and timing of referral for transplantation, whereas all patients in the nonrituximab group received a transplant in CR or PR after 4 cycles of induction chemotherapy, 6 patients (29%) in the rituximab group received a transplant after failing to achieve a CR after 6 to 8 cycles of R-hyper-CVAD. These patients were important because their outcomes were substantially inferior (see next paragraph). The median follow-up times for surviving patients in the rituximab and nonrituximab AUTO1 groups were 37 and 87 months, respectively. Among AUTO2 patients, baseline characteristics were similar between patients in the rituximab and nonrituximab groups except for a trend toward older age in the rituximab group (median, 63 vs 57 years for the nonrituximab group; P = .08) and a greater proportion of patients with β2-microglobulin levels of at least 3 mg/L in the rituximab group (47% vs 8% in the nonrituximab group; P = .04). The median follow-up times for surviving patients in the rituximab and nonrituximab AUTO2 groups were 29 and 37 months, respectively.
Characteristics of patients who underwent an autologous SCT in first remission (AUTO1) or for relapsed/refractory lymphoma (AUTO2), with or without rituximab
| Characteristic . | AUTO1 no rituximab (n = 29) . | AUTO1 with rituximab (n = 21) . | AUTO2 no rituximab (n = 17) . | AUTO2 with rituximab (n = 19) . |
|---|---|---|---|---|
| Median age, y (range) | 57 (42-66) | 56 (38-73) | 57 (42-65) | 63 (45-76) |
| Male, n (%) | 26 (90) | 18 (86) | 14 (82) | 15 (79) |
| Female, n (%) | 3 (10) | 3 (14) | 3 (18) | 4 (21) |
| Median time from initial diagnosis, mo (range) | 7 (4-63) | 8 (6-55) | 27 (5-84) | 26 (7-65) |
| Median no. of prior chemotherapy regimens (range) | 1 | 1 | 2 (2-3) | 2 (2-4) |
| Performance status at least 1, n (%) | 15 (52) | 8 of 18 (44) | 9 (53) | 6 of 14 (43) |
| Stage 3 or 4, n (%) | 28 (97) | 18 (86) | 15 (88) | 18 (95) |
| Prior B symptoms, n (%) | 6 (21) | 8 (38) | 4 (24) | 4 (21) |
| Prior GI involvement, n (%) | 13 (45) | 8 (38) | 3 (18) | 9 (47) |
| Prior blood involvement, n (%) | 1 (3) | 7 (33)* | 2 (12) | 5 (26) |
| Blastic morphology, n (%) | 2 (7) | 3 (14) | 1 (6) | 1 (5) |
| β2-microglobulin 3 mg/L or greater, n (%) | 6 of 24 (25) | 6 of 20 (30) | 1 of 13 (8) | 8 of 17 (47)† |
| Elevated LDH, n (%) | 5 (17) | 2 (10) | 6 (65) | 5 (26) |
| PET/Gallium positive pre-SCT | 0 of 14 (0) | 1 of 20 (5) | 1 of 4 (25) | 5 of 17 (29) |
| Bone marrow stem cell source, n (%) | 5 (17) | 1 (5) | 4 (24) | 1 (5) |
| Chemosensitive disease at transplantation, n (%) | 29 (100) | 21 (100) | 15 (88) | 16 (84) |
| CR/CRu at SCT, n (%) | 11 (38) | 12 (57) | 10 (59) | 15 (79) |
| PR at SCT n (%) | 18 (62) | 9 (43) | 5 (29) | 1 (5) |
| Refractory disease at SCT, n (%) | 0 (0) | 0 (0) | 2 (12) | 3 (16) |
| MIPI low risk, n (%) | 10 of 27 (37) | 6 of 13 (46) | 4 (57) | 3 of 8 (38) |
| MIPI intermediate risk, n (%) | 10 of 27 (37) | 3 of 13 (23) | 1 (14) | 1 of 8 (13) |
| MIPI high risk, n (%) | 7 of 27 (26) | 4 of 13 (31) | 2 (29) | 4 of 8 (50) |
| HSCT-CI less than 3, n (%) | 17 (59) | 11 (52) | 11 (65) | 7 (37) |
| HSCT-CI 3 or greater, n (%) | 12 (41) | 10 (48) | 6 (35) | 12 (63) |
| Characteristic . | AUTO1 no rituximab (n = 29) . | AUTO1 with rituximab (n = 21) . | AUTO2 no rituximab (n = 17) . | AUTO2 with rituximab (n = 19) . |
|---|---|---|---|---|
| Median age, y (range) | 57 (42-66) | 56 (38-73) | 57 (42-65) | 63 (45-76) |
| Male, n (%) | 26 (90) | 18 (86) | 14 (82) | 15 (79) |
| Female, n (%) | 3 (10) | 3 (14) | 3 (18) | 4 (21) |
| Median time from initial diagnosis, mo (range) | 7 (4-63) | 8 (6-55) | 27 (5-84) | 26 (7-65) |
| Median no. of prior chemotherapy regimens (range) | 1 | 1 | 2 (2-3) | 2 (2-4) |
| Performance status at least 1, n (%) | 15 (52) | 8 of 18 (44) | 9 (53) | 6 of 14 (43) |
| Stage 3 or 4, n (%) | 28 (97) | 18 (86) | 15 (88) | 18 (95) |
| Prior B symptoms, n (%) | 6 (21) | 8 (38) | 4 (24) | 4 (21) |
| Prior GI involvement, n (%) | 13 (45) | 8 (38) | 3 (18) | 9 (47) |
| Prior blood involvement, n (%) | 1 (3) | 7 (33)* | 2 (12) | 5 (26) |
| Blastic morphology, n (%) | 2 (7) | 3 (14) | 1 (6) | 1 (5) |
| β2-microglobulin 3 mg/L or greater, n (%) | 6 of 24 (25) | 6 of 20 (30) | 1 of 13 (8) | 8 of 17 (47)† |
| Elevated LDH, n (%) | 5 (17) | 2 (10) | 6 (65) | 5 (26) |
| PET/Gallium positive pre-SCT | 0 of 14 (0) | 1 of 20 (5) | 1 of 4 (25) | 5 of 17 (29) |
| Bone marrow stem cell source, n (%) | 5 (17) | 1 (5) | 4 (24) | 1 (5) |
| Chemosensitive disease at transplantation, n (%) | 29 (100) | 21 (100) | 15 (88) | 16 (84) |
| CR/CRu at SCT, n (%) | 11 (38) | 12 (57) | 10 (59) | 15 (79) |
| PR at SCT n (%) | 18 (62) | 9 (43) | 5 (29) | 1 (5) |
| Refractory disease at SCT, n (%) | 0 (0) | 0 (0) | 2 (12) | 3 (16) |
| MIPI low risk, n (%) | 10 of 27 (37) | 6 of 13 (46) | 4 (57) | 3 of 8 (38) |
| MIPI intermediate risk, n (%) | 10 of 27 (37) | 3 of 13 (23) | 1 (14) | 1 of 8 (13) |
| MIPI high risk, n (%) | 7 of 27 (26) | 4 of 13 (31) | 2 (29) | 4 of 8 (50) |
| HSCT-CI less than 3, n (%) | 17 (59) | 11 (52) | 11 (65) | 7 (37) |
| HSCT-CI 3 or greater, n (%) | 12 (41) | 10 (48) | 6 (35) | 12 (63) |
GI indicates gastrointestinal; LDH, lactate dehydrogenase; CR, complete response; CRu, unconfirmed CR; PR, partial response; MIPI, Mantle Cell International Prognostic Index; and HSCT-CI, Hematopoietic Stem Cell Transplantation-Comorbidity Index.
P < .05 comparing AUTO1 with or without rituximab.
P < .05 comparing AUTO2 with or without rituximab.
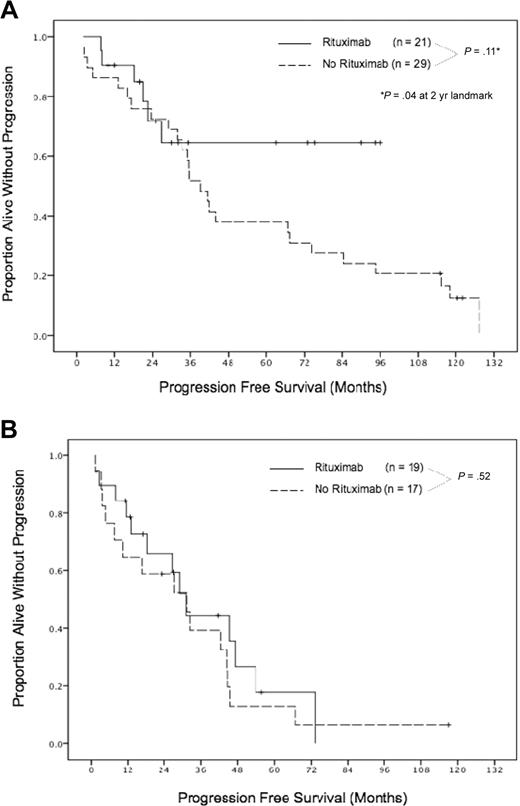
Rituximab use was associated with a significant improvement in outcome among AUTO1 patients, despite the inclusion of 6 patients who failed to achieve a CR after 6 to 8 cycles of R-hyper-CVAD (these later patients constituted 3 of the 6 relapses in the rituximab-AUTO1 group). The split in transplantation outcomes between patients receiving rituximab and not receiving rituximab occurred after 2 years (Figure 2A), although no differences in the PFS curves were evident in the first 24 months. A clear separation then emerged whereby only 1 of 11 patients receiving rituximab subsequently relapsed (at 27 months), with the rest remaining alive and in remission at a median follow-up of 68 months (range, 25-96 months), whereas patients who did not receive rituximab continued to relapse in a continuous pattern (P = .04 compared with patients receiving rituximab at the 2-year landmark). For AUTO1 patients remaining in remission at 2 years, no death had occurred in the rituximab group, whereas 15 of 22 patients not receiving rituximab had died (P = .003 at the 2-year landmark).
Progression-free survival for patients receiving autologous transplantation in first remission and for relapsed/refractory disease. Patients received autologous transplant in first remission (A) and for relapsed/refractory disease (B).
Progression-free survival for patients receiving autologous transplantation in first remission and for relapsed/refractory disease. Patients received autologous transplant in first remission (A) and for relapsed/refractory disease (B).
In contradistinction to the situation with frontline patients, rituximab was not associated with an improved outcome in AUTO2 patients (Figure 2B). These patients experienced a continuous pattern of relapse that mirrored the progression curve of patient not receiving rituximab (P = .52) with similar OS (P = .50).
NST patients
Thirty-five patients underwent NST for relapsed or refractory MCL. Seven patients (20%) did not experience a response to initial chemotherapy but experienced a PR or better to salvage therapy with R-hyper-CVAD. Eleven patients (31%) were in their second remission, 11 (31%) were in their third or subsequent remission, and 6 (17%) had refractory relapse and receiving a transplant in less than partial remission. The preparative regimen was PFA in 5 patients (14%) and FCR in 30 patients (86%).
All patients attained primary engraftment at a median of 11 days (range, 7-15 days) for neutrophils and 0 days (range, 0-19 days) for platelets; 16 patients (46%) did not require platelet support during transplantation. Two patients (6%) were found by chimerism studies on day 90 to have lost their allogeneic graft and to have recovered autologous hematopoiesis. Both of these patients had undergone transplantations from nonsibling donors; one had received a PBSC graft from a single-antigen mismatched offspring, and the other had received a bone marrow graft from an unrelated donor. No patients died within 100 days of transplantation, and 3 patients died at 18, 24, and 34 weeks of infection (n = 2) or cerebral bleeding (n = 1). Thus, the TRM rates were 0% at 3 months and 9% at 1 year.
None of the 16 patients who had undergone transplantation during CR or CRu developed recurrent disease during the transplantation period. Of the 13 patients who had undergone transplantation during PR, all experienced CR or CRu after NST alone (n = 11) or after additional immunomanipulation with rituximab and DLI (n = 2). Of the 6 patients who had undergone transplantation in refractory relapse, 5 (83%) achieved a CR or CRu after NST alone (n = 4) or after rituximab and DLI (n = 1), and 1 patient did not respond. This patient was in his fourth relapse and had received 10 prior lines of chemotherapy.
With a median follow-up of 56 months (range, 19-110 months), the median PFS duration was 60 months, and the median OS has not yet been reached (Figure 1). The 6-year actuarial PFS rate was 46%, and the 6-year actuarial OS rate was 53%. Importantly, plateaus in the survival curves were observed for both PFS and OS, with no relapses or deaths occurring in 9 patients followed between 63 and 110 months. These outcomes were significantly superior to that of AUTO2 patients, whereby relapses and deaths occurred in a continuous fashion (P = .01 for PFS; P = .005 for OS [4-year landmark for OS]). Compared with AUTO1 patients, NST patients had an initially lower OS; however, this reversed at 8 years because of the lack of late deaths among NST patients (Figure 1).
The major determinants of disease control after NST included receipt of a PBSC graft and achievement of a good (≥ 95%) donor chimerism. Among 24 patients meeting both of these criteria, no lymphoma relapses have occurred at a median follow-up of 60 months (range, 19-110 months), compared with a continuous pattern of relapse in patients who had received marrow grafts or did not achieve at least 95% donor chimerism (P < .001; Figure 3). Long-term lymphoma control was similar regardless of disease status (CR/u, PR, or refractory relapse) at the time of transplantation. Factors significant for favorable PFS were peripheral blood graft with best chimerism at least 95%, 4 or fewer prior therapies, and the absence of acute GVHD (Table 3). On multivariate analysis, peripheral blood graft with best chimerism at least 95% and 4 or fewer prior therapies were confirmed to have independent significance for PFS (HR, 0.25 and 0.25; P = .008 and .02, respectively). Factors significant for favorable OS were the absence of acute GVHD, 4 or fewer prior therapies, and the absence of chronic GVHD (Table 3). On multivariate analysis, the absence of acute GVHD was independently prognostic (HR, 0.26; P = .02).
Relapse risk after nonmyeloablative stem cell transplantation by graft source and best chimerism. PBSC indicates peripheral blood stem cell. No relapses have occurred in patients receiving PBSCs and achieving best donor chimerism of 95% or greater.
Relapse risk after nonmyeloablative stem cell transplantation by graft source and best chimerism. PBSC indicates peripheral blood stem cell. No relapses have occurred in patients receiving PBSCs and achieving best donor chimerism of 95% or greater.
Graft-versus-host disease and its effect on survival
The actuarial risk of acute GVHD in NST patients was 37%. All of these were grade 1 (20%) or 2 (17%) in severity, with no patient experiencing grades 3 to 4 acute GVHD. Nevertheless, the occurrence of either grade 1 or 2 acute GVHD was associated with an inferior survival (median OS, 28 and 21 months for grades 1 and 2, respectively; P = .82) and a high risk of evolution to chronic GVHD (86% and 100%, respectively). Surprisingly, the risk of acute GVHD was similar between patients who had received transplants from sibling donors (31%) and those who had received transplants from nonsibling donors (41%; P = .72). This may be attributable to the use of alemtuzumab in the nonsibling transplantation population whereby only 1 of 8 patients developed acute GVHD.
The actuarial risk of chronic GVHD was 60% (limited in 23% of patients and extensive in 37%). Twelve patients (57%) evolved into chronic GVHD from acute GVHD, and 9 patients (43%) developed chronic GVHD without antecedent acute disease (de novo onset). The occurrence of chronic GVHD was a significant determinant of inferior survival irrespective of the disease extent (limited vs extensive) or the pattern of onset (evolving vs de novo). All transplant-related deaths (3 within first year, 4 between 1 and 4 years) occurred in patients with chronic GVHD, 6 of whom had active immunosuppression at the time of death. Chronic GVHD was not significantly different for patients who received a transplant from sibling donors (67%) and from nonsibling donors (45%; P = .20). Chronic GVHD was significantly associated with a lower probability of disease relapse (5% at 6 years compared with 46% for patients without chronic GVHD; P = .05). However, no disease relapse occurred in 9 GVHD-free patients with PBSC grafts and at least 95% chimerism, showing that GVHD was not necessary for durable lymphoma control.
Feasibility of NST in patients who had failed a previous autograft
Six patients who had failed a previous autologous SCT received NST, all of whom survived the procedure and achieved CR or CRu after transplantation. At a median survivor follow-up of 7 years, 3 patients have died at 12, 42, and 46 months (1 patient each from unknown cause after discharging himself against medical advice, invasive fungal disease related to long-term immunosuppression, or pneumonia after pericardectomy for radiation-induced pericarditis), and 3 remained alive in continuous remission at 63, 87, and 110 months. The PFS and OS of these patients were similar to that of NST patients who had not undergone previous autologous transplantation (P = .70 and .93, respectively).
Discussion
The current study provides early evidence that MCL may be curable in both the frontline and salvage settings. In chemotherapy-naive patients, our results showed that rituximab-containing autologous transplantation in first remission may result in long-term disease control, with only 1 relapse occurring among 11 patients followed between 2 and 8 years. This pattern was in stark contrast to that of autologous transplantation without rituximab, whereby relapses continued to occur in a continuous fashion. Our observations were supported by the results of the Nordic Lymphoma Group MCL2 study,35 which evaluated a strategy of rituximab-containing induction chemotherapy followed by autologous transplantation in 160 previously untreated patients with MCL. At a median of 3.8 years of follow-up, 6-year overall, event-free and progression-free survival rates were 70%, 56%, and 66%, respectively. Most impressively, plateaus appeared to be emerging on all 3 curves. These results in combination with that of the current study point to the exciting possibility that an aggressive early treatment strategy incorporating rituximab and high-dose cytotoxic therapy may be capable of effecting complete disease eradication in MCL. However, these results are preliminary, and ongoing follow-up is required to confirm the absence of late relapses. In addition, most patients in the current report and in the MCL2 study were exposed to rituximab during both chemotherapy and transplantation, and it was not possible to determine whether the beneficial effect of rituximab occurred in the chemotherapy phase, in the treatment phase, or in both treatment phases. Therefore, on the basis of current data, rituximab should be included in both the chemotherapy and preparative regimens during frontline autologous transplantation. The dose of rituximab used in autologous SCT for mantle cell lymphoma at our institution was identical to that used in patients with recurrent large cell lymphoma; in the latter group, we had previously reported that the use of rituximab before and after autologous SCT had improved both OS and disease-free survival rates compared with those of a historic group of patients with similar disease characteristics who were not given rituximab.27
With the improvements in conventional chemotherapy and the advent of new therapeutic agents, the application of autologous SCT in patients with MCL in first remission is a subject of an intense debate. In a recent report, Martin et al36 analyzed the outcome in 111 patients with MCL in whom the date of diagnosis was identified and who received various forms of conventional chemotherapy at a single institution. Median OS, as calculated from the time of diagnosis, was reasonably favorable at 7.1 years. The same investigators reported more recently, in an abstract form, that a subset of patients with MCL can be observed and that patients may not need to be subjected to therapy until they become symptomatic.37 These observations, coupled with reports of favorable outcomes after intensive chemoimmunotherapy without transplantation,22 highlight the potential for selection bias in single-center series and underscore the need for multicenter comparative trials to define the optimal frontline treatment strategy in MCL. Our experience with stem cell mobilization failures after prolonged periods of intensive chemoimmunotherapy (eg, R-hyper-CVAD) indicates that stem cell collection should be attempted early (at or before cycle 4) in patients receiving such regimens. The administration of further courses of intensive induction in patients with persistent disease after the fourth cycle may predispose them to an increased risk of mobilization failure and possibly a worse outcome after autologous SCT.
In contradistinction to the situation with frontline transplantation, the outcome of autologous transplantation in patients with relapsed or refractory MCL remained unsatisfactory, with no evidence of a cured fraction on the survival curves. This was probably because of the selection of chemoresistant lymphoma clones during chemotherapy, with these clones subsequently reconstituting the disease bulk at relapse. Disappointingly, the addition of rituximab was not able to overcome this chemoresistance, and after transplantation response duration and survival for patients with relapsed or refractory MCL in the rituximab era were identical to that of the era before rituximab. For these patients, the GVL effect imparted by nonmyeloablative allogeneic transplantation may provide an alternative, non–cross-resistant mechanism of lymphoma killing. Indeed, the results of autologous transplantation and NST in patients with relapsed or refractory MCL were markedly different, with patients receiving an NST showing significantly superior lymphoma control and a disease-free plateau extending between 5 and 9 years. In comparison, patient who received an autologous transplant had a median remission of 2 years and experienced a continuous pattern of relapse. Therefore, NST may be capable of salvaging a cure in patients who were no longer curable with maximum cytotoxic strategies. Interestingly, the efficacy of NST was most marked in patients who had received a PBSC graft and who achieved a donor chimerism of 95% or better, with none of 24 patients with these characteristics relapsing after a median follow-up of 5 years. This may be related to the number of graft-derived cytotoxic cells, because the presence of chronic GVHD (as a barometer of graft-vs-antigenic activity) was also strongly associated with a low risk of disease recurrence. Indeed, one possible explanation for why patients receiving marrow grafts experienced inferior disease control may be related to the dose of CD3 cells delivered in the marrow stem cell product, which was 1 log lower than equivalent PBSC grafts (data not shown).
In a disease in which the median age at diagnosis was older than 60 years, the tolerability of a treatment strategy was a major determinant of its broad applicability and clinical relevance. In this regard, the tolerability of NST in the current study was encouraging, with 1-year TRM of less than 10% despite the patients being older than most allogeneic transplantation studies. Importantly, the transplantation procedure was safe and effective even in heavily pretreated patients, such as those who had failed previous autologous transplantation and those who had received up to 4 lines of prior therapies. The major cause of transplant-related mortality was chronic GVHD and its attendant requirement for long-term immunosuppression. Although acute GVHD was significantly correlated with decreased survival in the univariate and multivariate analyses, it was limited to grade 1 to 2 in severity, and affected patients did not die until a median of 2 years after transplantation. Therefore, patients with acute GVHD were at increased risk of dying because of their high propensity to evolve into chronic GVHD. These observations suggest that better GVHD prophylaxis may be an effective means of improving after transplantation survival. Indeed, there was some suggestion in the present study that those patients who had received alemtuzumab may be at a lower risk of acute GVHD, although the absolute numbers of patients in the subgroups were too small for statistical comparison. Other investigators had explored the use of a higher dose of alemtuzumab in reduced-intensity transplantations, whereby it appeared to ameliorate GVHD at the expense of inferior disease control.38-40 Patients at the MDACC received a lower total dose of alemtuzumab (45 mg) as GVHD prophylaxis for nonsibling transplantations. This strategy needs to be investigated in a larger number of patients to address its effect on disease control and survival.
The results of the current study support the ongoing exploration of stem cell transplantation as a potentially curative method in the treatment of patients with MCL. In particular, our findings and that of the Nordic Lymphoma Group suggest that the window of opportunity for long-term disease control with autologous transplantation may only be available in the frontline setting, giving substantial impetus to a renewal of interest in studies of early transplantation in younger patients. For patients with relapsed or refractory disease, the results of autologous transplantation remain unsatisfactory, whereas the timely application of a nonmyeloablative allogeneic transplantation may be curative.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank our patients for trusting us with their care.
This work was supported by the Lymphoma Research Foundation (New York, NY).
Authorship
Contribution: I.F.K. designed and performed the study, analyzed the results, helped write the paper, and reviewed the paper for final approval; C.S.T. analyzed the results and wrote the paper; M.K., A.A., C.H., P.K., S.A.G., P.A., U.P., B.P., F.H., and R.E.C. provided clinical care to patients, assisted in the analysis of data, and reviewed and helped write the paper; B.S. interpreted radiologic studies and reviewed and helped write the paper; L.J.M. interpreted lymphoma diagnostic studies and reviewed and helped write the paper; R.B. and R.H. analyzed data and helped write the paper; and C.L. and G.R. collected and verified patient information, analyzed data, and helped write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Issa F. Khouri, Department of Stem Cell Transplantation and Cellular Therapy, Unit 423, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: ikhouri@mdanderson.org.