Published results of clinical trials in AML are only applicable to a select group of patients and are not representative of the entire population.
In the United States, only a small minority of patients with cancer are enrolled in clinical studies.1 It has been estimated that only about 5% to 10% of adults with acute myeloid leukemia (AML) enroll in a clinical trial. The information from such trials is clearly important but limited in its applicability only to patients who do not differ from the study populations.2 Clinical studies in AML almost always require patients to have an adequate performance status as determined by cardiac, renal, and hepatic function. Patients with a prior malignancy, an antecedent hematologic disorder, or myelodysplasia are often excluded from AML trials.3 Prior exposure to cytotoxic chemotherapy such as, for example, cyclophosphamide for rheumatoid arthritis or vasculitis, is often prohibited. Above all else, the fundamental decision to enter a patient in a clinical trial is dependent on the patient's and, more importantly, the physician's attitude. The problem is more marked among older adults in whom AML is more common, given the median age of 70 years. Only a minority of older patients are referred to tertiary care centers and even within such centers, only a select minority enter clinical trials.4-7 In contrast to young adults, the selection of therapy for older patients with AML is exceedingly heterogeneous, ranging from an aggressive remission-inducing regimen to a nihilistic approach leading to palliative care only. In this group, therapy is often compounded by opinion rather than data. When does a patient become “old”? When can an older person not “tolerate” standard therapy? When does the risk of therapy outweigh any benefit?
In this issue of Blood Juliusson and colleagues report, on behalf of the Swedish Acute Leukemia Registry, a remarkably comprehensive analysis of 2767 patients diagnosed from 1997 to 2005, comprising 98% of all patients with AML in Sweden, excluding only those with acute promyelocytic leukemia.8 In this cohort, the median age was 72 years with a peak incidence at 80 to 85 years and a subsequent decrease in the very old. The authors assume this distribution is real and speculate as to the biologic reasons for this. Just as likely, this may be due to a lack of willingness by the physicians to make this diagnosis in the very old.
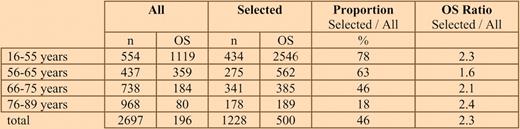
Median overall survival (OS) in days according to age for all patients versus those selected for de novo AML, fit for intensive treatment, and performance status 0-II.
Median overall survival (OS) in days according to age for all patients versus those selected for de novo AML, fit for intensive treatment, and performance status 0-II.
The strength of the Swedish Registry for AML is its compulsory reporting, including a physician assessment at diagnosis as to whether a patient is eligible for chemotherapy or not. Performance status (PS) at diagnosis was available in more than 97% of patients. Selecting for patient criteria usually applied in clinical trials, such as de novo AML, lack of significant morbidity, and PS 0-II, resulted in more than doubling of the median survival in almost all age groups compared with the entire patient population (see table).
The most important analysis relates to older patients. Most patients up to 80 years of age benefit from standard induction therapy and, critically, standard intensive therapy decreases rather than increases early death rate. The latter, at first, may appear as a self-fulfilling prophecy, given a physician's choice to withhold therapy among those not likely to survive. However, to bolster their analysis the authors have compared 6 regions in Sweden that had populations with similar health conditions and similar availability of advanced health care, without external referrals. The early treatment-related mortality (16%) was identical in regions where 75% of the patients between 70 to 79 years received intensive induction therapy compared with regions where only 41% of patients received similar intensive treatment. Importantly, the response to therapy, as determined by the complete remission rate, was not lower in the region where 75% of patients were treated intensively.
The implications from this report are far reaching. There is a common bias not to treat many older patients. Performance status remains the most important predictor of early death at all ages. Outcome is also likely to be affected by cytogenetic and molecular determinants — not included in this analysis.
Nevertheless, this is a sobering, real-world analysis that, while not perfect, leads us much closer to an understanding of the true outcome of patients with AML, and hopefully a reexamination of prior concepts in the management of older patients with AML.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal