Abstract
Internal tandem duplication mutations of FLT3 (FLT3/ITD mutations) are common in acute myeloid leukemia (AML) and confer a poor prognosis. This would suggest that FLT3 is an ideal therapeutic target, but FLT3 targeted therapy has produced only modest benefits in clinical trials. Due to technical obstacles, the assessment of target inhibition in patients treated with FLT3 inhibitors has been limited and generally only qualitative. KW-2449 is a novel multitargeted kinase inhibitor that induces cytotoxicity in Molm14 cells (which harbor an FLT3/ITD mutation). The cytotoxic effect occurs primarily at concentrations sufficient to inhibit FLT3 autophosphorylation to less than 20% of its baseline. We report here correlative data from a phase 1 trial of KW-2449, a trial in which typical transient reductions in the peripheral blast counts were observed. Using quantitative measurement of FLT3 inhibition over time in these patients, we confirmed that FLT3 was inhibited, but only transiently to less than 20% of baseline. Our results suggest that the failure to fully inhibit FLT3 in sustained fashion may be an underlying reason for the minimal success of FLT3 inhibitors to date, and stress the importance of confirming in vivo target inhibition when taking a targeted agent into the clinical setting. The clinical studies are registered on www.clinicaltrials.gov as NCT00346632.
Introduction
Activating mutations of the receptor tyrosine kinase (RTK) FLT3 are some of the most common molecular abnormalities found in acute myeloid leukemia (AML) and are found in about 30% of newly diagnosed patients.1,2 The presence of a FLT3/ITD mutation in a patient with AML implies a poor prognosis,3-10 and for the last several years efforts have been underway throughout the world to develop a targeted therapy for this subtype of AML.11 Following the success of imatinib for the treatment of Ph+ acute lymphocytic leukemia (ALL), several compounds with inhibitory activity against FLT3 are currently in clinical development. FLT3 inhibitors are cytotoxic to acute myeloid leukemia cells, but generally only if those cells harbor FLT3 activating mutations. All of the compounds in clinical development have been shown to induce apoptosis in FLT3-dependent cell lines. Several compounds (lestaurtinib, midostaurin, tandutinib, sorafenib, sunitinib, and 2 new compounds, KW-2449 and AC220) have been or are currently being investigated as FLT3 inhibitors in clinical trials, with even more in early development.12-18
Clinical trials of FLT3 inhibitors have thus far not resulted in clinical responses comparable to those seen with imatinib in Ph+ ALL. One explanation for this could be that FLT3 mutations are not as fundamentally important to the development of AML as the t(9;22) translocation is to ALL, and as such, inhibition of FLT3 might simply lead to the selection of AML subclones that are less dependent on FLT3 signaling for survival. However, another possible explanation is pharmacokinetic failure. In any clinical trial of a kinase inhibitor, it is of paramount importance to confirm that the target is being inhibited. Preclinical studies of FLT3 inhibitors demonstrated that these agents had cytotoxic effects against FLT3-mutant cell lines and primary blasts, but only when the leukemia cells were subject to continuous exposure at concentrations of drug sufficient to inhibit FLT3 autophosphorylation to 10% to 20% of its baseline level. Of the several FLT3 inhibitors that have advanced beyond phase 1 of development, none were evaluated in this context. That is, the phase 1 trials of these agents tended to focus on safety and tolerability. A number of technical obstacles have prevented anything more than a cursory examination of in vivo FLT3 inhibition in isolated patients in these studies.14,15,19-23 There has thus far been no study in which a quantitative correlation has been obtained for the dose of an inhibitor and the degree of FLT3 inhibition achieved in vivo.
We present here the correlative studies of a phase 1 clinical trial of KW-2449, a small molecule tyrosine kinase inhibitor with known activity against FLT3, aurora kinase, FGFR-1, and Abl kinase.24 This is the first phase 1 study of a FLT3 inhibitor specifically designed to establish in a quantitative fashion the degree of FLT3 inhibition achieved in patients at each dose level. Our results suggest that pharmacokinetic obstacles (such as a short drug half-life) may be responsible for the limited responses to FLT3 inhibitors in general. Specifically, while transient inhibition of FLT3 autophosphorylation is readily achievable, this is insufficient both in vitro and in vivo for achieving significant cytotoxicity in leukemia cells. FLT3 inhibition needs to be sustained in order to effect killing of FLT3-dependent AML cells. Our study highlights the importance of using a phase 1 study of a kinase inhibitor to determine not just a safe and tolerable dose of a drug, but rather a kinase inhibitory dose that is safe, tolerable, and sustainable.
Methods
Inhibitors
KW-2449 and its metabolite M1 were provided by Kyowa Hokko Kogyo (Mishima, Japan). Compounds were dissolved in DMSO and stored at −80°C as 10 mM stock solutions. Working stocks of 100 μM were prepared by diluting DMSO stock solutions into RPMI/0.05% bovine serum albumin (BSA). All samples in any given experiment contained identical concentrations of DMSO. Plasma experiments typically contained 0.5%, while all others contained less than 0.01%.
Cell culture
All cell lines and primary blast samples were cultured in RPMI/10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) at 37°C in 5% CO2. Molm-14 cells were obtained from the DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany). Mononuclear cells from patients with AML were isolated from whole blood or marrow using density gradient centrifugation with Ficoll-Hypaque (Amersham, Piscataway, NJ), as described.25
FLT3 phosphorylation
Leukemia cells were washed in phosphate-buffered saline (PBS), then lysed by resuspending the cells in lysis buffer (20 mM Tris pH 7.4, 100 mM NaCl, 1% Igepal (Sigma, St Louis, MO), 1 mM EDTA, 2 mM NaVO4, plus Complete protease inhibitor [Roche, Indianapolis, IN]) for 30 minutes while rocking. The extract was clarified by centrifugation at 16 000 g and the supernatant was assayed for protein (Bio-Rad, Richmond, CA). A 50-μg aliquot was removed as a whole-cell lysate for analysis of STAT5, and the remainder was used for immunoprecipitation with anti-FLT3. Anti-FLT3 antibody (S18; Santa Cruz Biotechnology, Santa Cruz, CA) was added to the extract for overnight incubation, then protein A sepharose (Upstate Biotechnology, Lake Placid, NY) was added for 2 additional hours. Separate sodium dodecyl sulfate–polyacrylamide electrophoresis (SDS-PAGE) gels for whole-cell lysate and immunoprecipates were run in parallel. After transfer to Immobilon membranes (Millipore, Bedford, MA), immunoblotting was performed with antiphosphotyrosine antibody (4G10; Upstate Biotechnology) to detect phosphorylated FLT3 or, for the whole-cell lysate gels, with a rat monoclonal antibody against phosphorylated STAT5 (residue Y694) then stripped and reprobed with anti-FLT3 antibody to measure total FLT3. Proteins were visualized using chemiluminescence (ECL; Amersham), exposed on Kodak BioMax XAR film, developed, and scanned using a Bio-Rad GS800 densitometer (Hercules, CA). The concentration of drug for which the phosphorylation of FLT3 or STAT5 was inhibited to 50% of its baseline (IC50) was determined using linear regression analysis of the dose response curves.
For direct analysis of FLT3 and STAT5 in circulating blasts, peripheral blood was collected in heparinized tubes and promptly chilled on ice. Samples were centrifuged for 10 minutes at 900g, at 4°C. The plasma was removed and stored frozen at −80°C. The buffy coat was carefully transferred to ice-cold PBS, layered onto chilled Ficoll-Hypaque, and centrifuged for 5 minutes at 600g, at 4°C. All subsequent steps were carried out at 4°C. Mononuclear cells were collected and washed rapidly once in red blood cell lysis buffer (0.155 M NH4Cl, 0.01 M KHCO3, 0.1 mM EDTA), then washed once in PBS. Cells were then lysed as described for FLT3 and STAT5 analysis.
PIA assay
Frozen plasma samples were thawed and clarified by centrifugation at 16 000 g for 2 minutes. All assays described herein were performed within 6 months of collection. For each time point, 6 × 106 Molm-14 were incubated with 1 mL plasma at 37°C for 1 hour. The cells were washed twice with ice-cold PBS then lysed. A 50-μg aliquot was removed as a whole-cell lysate for analysis of STAT5, and the remainder was used for immunoprecipitation with anti-FLT3. Separate gels for whole-cell lysate and immunoprecipates were run in parallel, and, following transfer to membrane, immunoblots for phosphotyrosine and phosphorylated STAT5 were performed as described in “FLT3 phosphorylation.” After immunoblotting, densitometric analysis was performed on the bands and the plasma inhibitory activity (PIA) for a given plasma sample was calculated by expressing the density of its corresponding band as a percentage of the density of the baseline band (which was arbitrarily set at 100%).
Cytotoxicity assays
Cytotoxicity was assessed using an MTT (dimethyl-thiazol diphenyl tetrazolium bromide) assay. In selected cases, we also used an annexin V–binding apoptosis assay to confirm that the cytotoxic effect observed (or lack thereof) was in fact accompanied by an equivalent degree of apoptosis. MTT (Roche) and annexin V (Pharmingen, San Diego, CA) assays were performed as described.25 Cell lines were seeded into 96-well plates with the indicated reagents at a concentration of 10 000 cells per well. Primary blasts were seeded at 250 000 cells per well.
Clinical trial design
Kyowa Pharmaceutical Trial 2449-US-001 was an open-label first-in-human dose escalation study using a 3 × 3 design. Three centers in the United States participated. This study was approved by the institutional review board of Johns Hopkins University and informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Inclusion criteria included documented relapsed or refractory, AML, ALL, or chronic myelogenous leukemia (CML) no longer responsive to imatinib or dasatinib; Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2; age more than 18 years old; and serum creatinine level of less than 2.0. Exclusion criteria included systemic chemotherapy within 14 days of initiation (except imatinib or dasatinib to be discontinued within 48 hours of initiation), active central nervous system leukemia, uncontrolled systemic infection, and treatment with another FLT3 inhibitor in development within 28 days of initiation. Standard response criteria were used.26 Patients were dosed orally twice daily with KW-2449 and pharmacokinetic and pharmacodynamic samples drawn at defined time points and evaluated at a central facility. Arm A was dosed days 1 to 14 followed by a clinician-determined 7- to 28-day break period, and Arm B was dosed days 1 to 28 with a clinician-determined 7- to 28-day break period. Arm B was discontinued by amendment after dose level 3 to shorten study accrual time. If a dose-limiting toxicity (DLT) was obtained the dose level was expanded to 6 patients. If a second DLT was obtained at that dose level this was defined as the maximally tolerated dose and the trial would conclude. Dosing ranged from 25 mg per day (dose level 1) to 500 mg per day (dose level 7). The primary endpoints of 2449-US-001 were safety and tolerability of KW-2449 and the determination of a maximum tolerated dose. An important secondary end point was determination of FLT3 inhibition in vivo. Based on the preliminary results from this secondary end point (which we are presenting here), it was concluded that the dosing schedule used in 2449-US-001 would not lead to sustained inhibition of FLT3 in vivo. The trial was therefore discontinued before the primary endpoints were reached. A new trial, with a modified dose and schedule based on the data presented herein, is planned for early 2009. Preliminary safety and tolerability results from 2449-US-001 have been presented elsewhere, and will be the subject of a separate publication.17
Patient samples
Peripheral blood samples from patients with leukemia enrolled in 2449-US-001 were obtained at time points 0 hour, 1 hour, 2 hours, 4 hours, 8 hours, and 12 hours, on days 1, 14, 21, and 28 (Arm B only) of cycle 1, and 0 hour and 2 hours on day 1 and the last day of the cycle for subsequent cycles. Peripheral blood samples in heparinized tubes were sent via overnight carrier on cold packs from 2 locations in the United States (New York, NY, and Houston, TX) to Baltimore, MD, for pharmacodynamic analysis. Stability studies performed with KW-2449 added to peripheral blood confirmed stability for 48 hours in the PIA assay (data not shown). Samples received more than 48 hours after acquisition were not included in pharmacodynamic analysis. The whole-blood samples were centrifuged at 4°C for 10 minutes to separate the plasma from the cellular fraction. The plasma was used for the PIA assay, while any leukocytes presented were purified using Ficoll-Hypaque. The leukocytes, if viable, were used for cytotoxicity assays and FLT3 genotyping.
Pharmacokinetic analysis
Peripheral blood samples were sent from each clinical location via overnight carrier to Kyowa Pharmaceuticals (Princeton, NJ). The analysis of KW-2449 and M1 in plasma samples was conducted at Taylor Technologies (Princeton, NJ) using a validated high performance liquid chromatographic method. The analytes were extracted from plasma using liquid/liquid extraction, and quantified using liquid chromatography with tandem mass spectrometry using a heated nebulizer interface (LC/APCI/MS/MS).
Results
KW-2449 is rapidly absorbed and converted to a major metabolite M1
The clinical trial in humans has progressed through 7 dose levels (25, 50, 100, 200, 300, 400, and 500 mg total daily dose divided twice daily). Dose levels 3 and 7 were expanded following the report of a dose limiting toxicity (grade 3 atrial fibrillation at 100 mg daily and grade 3 vomiting at 500 mg daily). Preclinical studies revealed that KW-2449 is converted by monoamine oxidase-B (MAO-B) and aldehyde oxidase into its major metabolite M1.24 Pharmacokinetic analysis revealed metabolism of the parent compound led to higher overall levels of M1 (Table 1). KW-2449 was rapidly absorbed with peak levels achieved around 2 hours after the dose. The half-life of the parent compound, KW-2449 and M1, is approximately 2.5 to 3.5 hours.
Pharmacokinetic summary for patients treated with KW-2449
| Dose level . | Daily dose, mg . | No. of patients . | KW-2449, 2 hours, nM . | M1, 2 hours, nM . | KW-2449, 12 hours, nM . | M1, 12 hours, nM . |
|---|---|---|---|---|---|---|
| 1 | 25 | 6 | 20.5 | 165.7 | 1.2 | 76.1 |
| 2 | 50 | 7 | 24.5 | 204.4 | 2.5 | 68.6 |
| 3 | 100 | 9 | 74.1 | 693 | 7.1 | 241.4 |
| 4 | 200 | 3 | 111.3 | 593.2 | 12.6 | 290.3 |
| 5 | 300 | 3 | 264 | 1865 | 17.6 | 413.8 |
| 6 | 400 | 3 | 387.2 | 2130 | 21.1 | 491.7 |
| 7 | 500 | 5 | 515.0 | 2867 | 102.5 | 420.3 |
| Dose level . | Daily dose, mg . | No. of patients . | KW-2449, 2 hours, nM . | M1, 2 hours, nM . | KW-2449, 12 hours, nM . | M1, 12 hours, nM . |
|---|---|---|---|---|---|---|
| 1 | 25 | 6 | 20.5 | 165.7 | 1.2 | 76.1 |
| 2 | 50 | 7 | 24.5 | 204.4 | 2.5 | 68.6 |
| 3 | 100 | 9 | 74.1 | 693 | 7.1 | 241.4 |
| 4 | 200 | 3 | 111.3 | 593.2 | 12.6 | 290.3 |
| 5 | 300 | 3 | 264 | 1865 | 17.6 | 413.8 |
| 6 | 400 | 3 | 387.2 | 2130 | 21.1 | 491.7 |
| 7 | 500 | 5 | 515.0 | 2867 | 102.5 | 420.3 |
Mean levels of KW-2449 and the metabolite, M1, are shown at 2 and 12 hours after dosing on day 1. The daily dose shown was administered in 2 divided doses, 12 hours apart (ie, 25 mg = 12.5 mg twice daily).
KW-2449 mediates cytotoxicity thru inhibition of FLT3/ITD
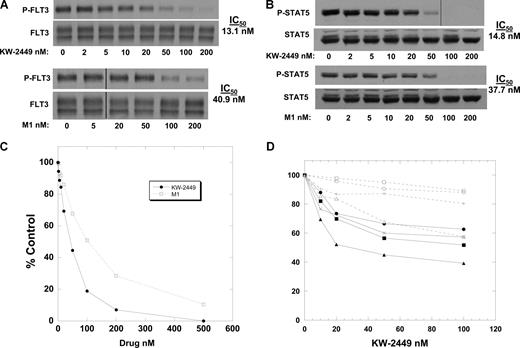
To confirm that KW-2449 is a direct inhibitor of FLT3 and induces inhibition of its downstream target STAT5, we prepared dose response curves of phosphorylated FLT3 and STAT5 using Molm-14 cells in culture medium (with 10% fetal bovine serum [FBS]). Immunoblot analysis revealed an IC50 of 13.1 nM for inhibition of phosphorylated FLT3 (Figure 1A). Under the same conditions, M1 was found to have an IC50 of 40.9 nM. We then confirmed the inhibitory activity of each compound on FLT3 signaling by determining the IC50 for inhibition of phosphorylated STAT5, an important second messenger for FLT3 signaling.27 Similar IC50 results were noted for each compound (Figure 1B). To examine the cytotoxicity of KW-2449 and M1, a series of MTT assays were performed (Figure 1C). In Figure 1A, the densities of the bands corresponding to 50 nM and 100 nM KW-2449 were 20% and 10%, respectively, of control. Comparing this to the data in Figure 1C, we concluded that KW-2449 cytotoxicity is most evident at drug levels achieving an 80% or greater reduction of baseline FLT3 signaling. These results are similar to previously studied FLT3 inhibitors using these assays.28 While cell line cytotoxicity is easily achieved for many compounds in development, cytotoxicity in primary leukemia samples may be a better model for determining the potential efficacy against leukemia seen clinically. We performed MTT assays on 8 primary leukemia samples obtained in the clinical trial and noted dose-dependent cytotoxicity in 5 of the samples. All 4 patients with an FLT3/ITD and one patient with a nonmutated FLT3 displayed this dose-dependent cytotoxicity (Figure 1D).
KW-2249 and its metabolite inhibit FLT3. (A) Molm14 cells in culture medium were incubated for 1 hour in increasing concentrations of KW-2449 (top blots) and M1 (bottom blots). The cells were lysed, and FLT3 was immunoprecipitated from the extracts and resolved by SDS-PAGE. After probing the blots with antiphosphotyrosine (4G10), the blots were stripped and reprobed with anti-FLT3 to confirm equal lane loading. The blots were analyzed by densitometry, and the IC50 values were calculated using linear regression analysis. Vertical lines have been inserted to indicate a repositioned gel lane. (B) Extracts from the experiment in panel A were resolved directly with SDS-PAGE and the blots were probed with anti–phospho-STAT5, then stripped and reprobed with anti-STAT5 to confirm equal lane loading. Densitometry was performed as in panel A. Vertical lines have been inserted to indicate a repositioned gel lane. (C) Molm14 cells were incubated in culture medium with increasing concentrations of KW-2449 (solid line) or M1 (dashed line) for 48 hours. The MTT assay was then performed, with results plotted as percent untreated control. (D) Primary AML cells isolated from the peripheral blood of patients on the clinical trial prior to beginning treatment with KW-2449 were incubated for 48 hours with increasing concentrations of KW-2449. After 48 hours, the MTT assay was performed and the results plotted as percent untreated control. Four of the patients harbored FLT3/ITD mutations (solid lines), and 4 did not (dashed lines).
KW-2249 and its metabolite inhibit FLT3. (A) Molm14 cells in culture medium were incubated for 1 hour in increasing concentrations of KW-2449 (top blots) and M1 (bottom blots). The cells were lysed, and FLT3 was immunoprecipitated from the extracts and resolved by SDS-PAGE. After probing the blots with antiphosphotyrosine (4G10), the blots were stripped and reprobed with anti-FLT3 to confirm equal lane loading. The blots were analyzed by densitometry, and the IC50 values were calculated using linear regression analysis. Vertical lines have been inserted to indicate a repositioned gel lane. (B) Extracts from the experiment in panel A were resolved directly with SDS-PAGE and the blots were probed with anti–phospho-STAT5, then stripped and reprobed with anti-STAT5 to confirm equal lane loading. Densitometry was performed as in panel A. Vertical lines have been inserted to indicate a repositioned gel lane. (C) Molm14 cells were incubated in culture medium with increasing concentrations of KW-2449 (solid line) or M1 (dashed line) for 48 hours. The MTT assay was then performed, with results plotted as percent untreated control. (D) Primary AML cells isolated from the peripheral blood of patients on the clinical trial prior to beginning treatment with KW-2449 were incubated for 48 hours with increasing concentrations of KW-2449. After 48 hours, the MTT assay was performed and the results plotted as percent untreated control. Four of the patients harbored FLT3/ITD mutations (solid lines), and 4 did not (dashed lines).
KW-2449 is highly protein bound
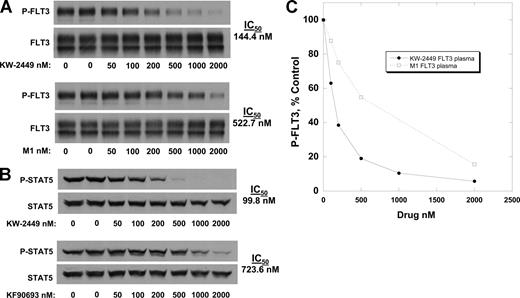
Previous work with tyrosine kinase inhibitors has shown most inhibitors in development are highly protein bound, such that the IC50 values for these agents in plasma are typically at least 1 to 2 orders of magnitude higher than the values obtained from experiments in culture medium.28,29 We therefore determined the IC50 values of KW-2449 and M1 for FLT3 and STAT5 using Molm14 cells, substituting normal human plasma for culture medium. In plasma, the IC50 for P-FLT3 inhibition shifts approximately 10-fold to 144 nM for KW-2449 (Figure 2A,B). From the dose response curves derived from the plasma experiments, M1 is calculated to be 3.6-fold less potent at inhibition of FLT3 by comparing IC50 values (144 nM vs 522 nM). Similar results were noted in inhibition of STAT5 signaling in plasma (Figure 2C). Therefore, based on these data, the FLT3 inhibitory activity of KW-2449 and its metabolite M1 in plasma can be estimated by using the following formula: [KW-2449] + [M1] / 3.6, in which [KW-2449] is the concentration of the parent drug in plasma and [M1] is the concentration of the metabolite in plasma. To confirm that this effect applied to cytotoxicity as well as to FLT3 inhibition, MTT assays were performed with KW-2449 in combination with M1 at a ratio of 1:4. This ratio approximates the difference in potency, and also is reflective of typical levels seen in patients receiving KW-2449 (Table 1). The MTT assays (data not shown) demonstrated cytotoxity that was additive to synergistic.
Inhibition of FLT3 in plasma. Molm14 cells were incubated for 1 hour in plasma containing increasing concentrations of KW-2449 (solid lines) and M1 (dashed lines). The cells were then lysed and analyzed for phosphorylated FLT3 (A) and STAT5 (C) as in Figure 1A and B. Densitometry was performed and the results plotted as percent untreated control in panels B and D. IC50 values were calculated using linear regression analysis.
Inhibition of FLT3 in plasma. Molm14 cells were incubated for 1 hour in plasma containing increasing concentrations of KW-2449 (solid lines) and M1 (dashed lines). The cells were then lysed and analyzed for phosphorylated FLT3 (A) and STAT5 (C) as in Figure 1A and B. Densitometry was performed and the results plotted as percent untreated control in panels B and D. IC50 values were calculated using linear regression analysis.
The PIA assay correlates with FLT3 inhibition in circulating blasts
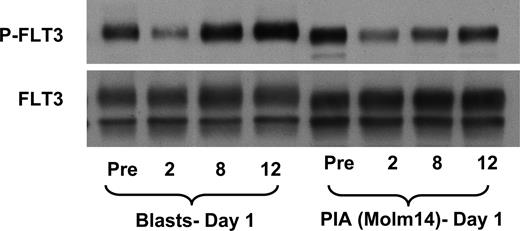
One approach to determining the degree of target inhibition by a kinase inhibitor is to assay the target directly in the malignant cells. Even in patients with leukemia, however, this represents a considerable technical challenge, as trial patients frequently have little or no circulating leukemia cells, or those that do have widely fluctuating levels of circulating blasts. Reliable correlation between the degree of inhibition of a target such as FLT3 and plasma drug levels is therefore difficult to achieve using conventional methods. We developed the PIA assay as a surrogate means of quantifying FLT3 inhibition over time in a consistent fashion for large numbers of patients.28 We wished to confirm that the results of this assay are an accurate reflection of the FLT3 inhibition occurring in the actual circulating blasts of the treated patient. Figure 3 documents consistent inhibitory effects seen in both FLT3 phosphorylation in circulating blasts and FLT3 phosphorylation in our PIA assay. By using the PIA assay as a surrogate for direct FLT3 inhibition, we are able to follow the activity of KW-2449 for all patients at all time points during dosing, regardless of whether or not they have circulating blasts.
The PIA assay is a valid surrogate of in vivo target inhibition for KW-2449. The immunoblot shows phospho-FLT3 (top) and total FLT3 (blot stripped and reprobed; bottom). A patient enrolled on the clinical trial of KW-2449 was administered a dose of 200 mg orally. Whole blood was obtained prior to the dose and at 2, 8, and 12 hours afterward, and separated into plasma and cellular fractions. Circulating blasts were isolated and analyzed for phospho-FLT3 as described in “Methods.” To perform the PIA assay, Molm14 cells were incubated for 1 hour in the plasma from the same time points, and likewise analyzed for phospho-FLT3 (as described in “Methods”). The first 4 lanes of the blot are from the circulating blasts, whereas the next 4 lanes show FLT3 from the Molm14 cells exposed to the plasma from which those blasts were isolated.
The PIA assay is a valid surrogate of in vivo target inhibition for KW-2449. The immunoblot shows phospho-FLT3 (top) and total FLT3 (blot stripped and reprobed; bottom). A patient enrolled on the clinical trial of KW-2449 was administered a dose of 200 mg orally. Whole blood was obtained prior to the dose and at 2, 8, and 12 hours afterward, and separated into plasma and cellular fractions. Circulating blasts were isolated and analyzed for phospho-FLT3 as described in “Methods.” To perform the PIA assay, Molm14 cells were incubated for 1 hour in the plasma from the same time points, and likewise analyzed for phospho-FLT3 (as described in “Methods”). The first 4 lanes of the blot are from the circulating blasts, whereas the next 4 lanes show FLT3 from the Molm14 cells exposed to the plasma from which those blasts were isolated.
While the degree of FLT3 inhibition observed via the PIA assay correlated, in general, with the inhibition found in the circulating blasts at the same time points, one interesting and notable difference was seen. As shown in Figure 3, the phosphorylation of FLT3 in circulating blasts 12 hours after a dose of KW-2449 seemed to recover to a level greater than the level prior to dosing. This “flare” of activity, seen in more than one patient, suggests that a compensatory up-regulation of FLT3 signaling may occur following elimination of the FLT3 inhibitor. The significance of this finding is unclear, but may represent a means for leukemia cells to recover from any proapoptotic signals induced by the temporary inhibition of FLT3. The flare effect was not seen in the PIA assay for the same time points. This indicates that it is intrinsic to the circulating blasts, as a uniform aliquot of Molm14 cells are used for each time point examined in the PIA assay.
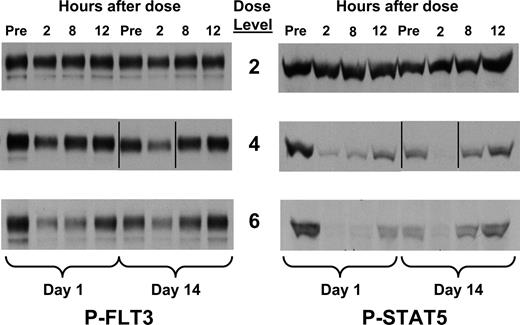
The PIA assay correlates with the pharmacokinetics of KW-2449
Each patient's plasma samples were analyzed by the PIA assay 2, 8, and 12 hours after dosing on day 1 and day 14 of the first cycle of therapy. At daily doses of 100 mg and higher, most patients had some degree of inhibition of FLT3 at the 2 hour time point, but in most cases this inhibition waned by the 8 hour time point (Figure 4). Quantification of degree of inhibition in the PIA assay was performed on each time point via densitometry and indexed by the pretreatment controls. Each value was then plotted against the pharmacokinetic values obtained for both KW-2449 and M1 (Figure 5A). These 2 values were combined to represent a “KW-2449-equivalent” level by our estimate of M1 being 3.6-fold less potent than KW-2449 in plasma (using the formula [KW-2449] + [M1] / 3.6, found in the section “KW-2449 is highly protein bound,” generated in the experiment described in Figure 2). To correlate the degree of FLT3 inhibition determined by the PIA assay with the pharmacokinetic data, we took the standard curve for FLT3 inhibition in plasma (Figure 5B) and superimposed this plot on the PIA-pharmacokinetic dot plot (Figure 5C). This plot of individual PIA-pharmacokinetic points follows the standard curve plot closely. Using the same approach, the PIA-pharmacokinetic results for STAT5 correlate with the standard curve for STAT5 (Figure 5D). This allows us to use pharmacokinetic data (with the formula [KW-2449] + [M1] / 3.6) to predict the degree of target inhibition across a broad range of drug levels. From this data we can conclude that the “KW-2449-equivalent” level needed to achieve 80% inhibition (the level needed to achieve a consistent cytotoxic effect; Figure 2B) of FLT3 is approximately 500 nM.
PIA results for patients receiving KW-2449. Plasma was isolated from whole-blood samples obtained from patients receiving increasing doses of KW-2449 on the clinical trial. Samples were obtained immediately prior to dosing, and at 2, 8, and 12 hours after dosing on days 1 and 14 of cycle 1. Dose levels 2, 4, and 6 correspond to total daily doses of 50, 200, and 400 mg, respectively (Table 1). Shown are the results from 3 representative patients on successively higher dose levels using the PIA assay for phosphorylated FLT3 (left) and STAT5 (right). Vertical lines have been inserted to indicate a repositioned gel lane.
PIA results for patients receiving KW-2449. Plasma was isolated from whole-blood samples obtained from patients receiving increasing doses of KW-2449 on the clinical trial. Samples were obtained immediately prior to dosing, and at 2, 8, and 12 hours after dosing on days 1 and 14 of cycle 1. Dose levels 2, 4, and 6 correspond to total daily doses of 50, 200, and 400 mg, respectively (Table 1). Shown are the results from 3 representative patients on successively higher dose levels using the PIA assay for phosphorylated FLT3 (left) and STAT5 (right). Vertical lines have been inserted to indicate a repositioned gel lane.
PIA results compared with standard curve for KW-2449. (A) Plasma was collected from patients receiving KW-2449 on the clinical trial 2, 8, and 12 hours after ingesting drug. The plasma samples underwent conventional pharmacokinetic analysis for concentrations of KW-2449 and the metabolite, M1. In parallel, plasma from the same time points was used in PIA assays for FLT3. On the x axis, the concentration of active drug is plotted for individual time point by the following formula: [KW-2449] + [M1] / 3.6. On the y axis, the degree of FLT3 inhibition is plotted as percent control. The pretreatment plasma sample for each patient was used as the control for each individual PIA assay. Thus, each circle on the graph represents a single time point for which the concentration of KW-2449 plus metabolite in a plasma sample is plotted against how well that plasma inhibited FLT3 phosphorylation in Molm14 cells (the PIA assay). (B) This graph is the standard curve of KW-2449 inhibiting FLT3 in plasma shown in Figure 2. (C) This graph shows the graph from panel A overlying the standard curve from panel B, demonstrating how the results of the PIA assay, using the concentrations of KW-2449 and M1 divided by 3.6, correspond to the standard curve. (D) The same experiment as in panel C, but using the PIA assay for STAT5 rather than FLT3.
PIA results compared with standard curve for KW-2449. (A) Plasma was collected from patients receiving KW-2449 on the clinical trial 2, 8, and 12 hours after ingesting drug. The plasma samples underwent conventional pharmacokinetic analysis for concentrations of KW-2449 and the metabolite, M1. In parallel, plasma from the same time points was used in PIA assays for FLT3. On the x axis, the concentration of active drug is plotted for individual time point by the following formula: [KW-2449] + [M1] / 3.6. On the y axis, the degree of FLT3 inhibition is plotted as percent control. The pretreatment plasma sample for each patient was used as the control for each individual PIA assay. Thus, each circle on the graph represents a single time point for which the concentration of KW-2449 plus metabolite in a plasma sample is plotted against how well that plasma inhibited FLT3 phosphorylation in Molm14 cells (the PIA assay). (B) This graph is the standard curve of KW-2449 inhibiting FLT3 in plasma shown in Figure 2. (C) This graph shows the graph from panel A overlying the standard curve from panel B, demonstrating how the results of the PIA assay, using the concentrations of KW-2449 and M1 divided by 3.6, correspond to the standard curve. (D) The same experiment as in panel C, but using the PIA assay for STAT5 rather than FLT3.
Twice daily dosing schedule results in intermittent FLT3 inhibition
Having established that a KW-2449–equivalent level of 500 nM is needed for inhibition of FLT3 to 20% baseline, we examined these values plotted over time for all trial patients (Figure 6). On day 1, patients on dose level 7 achieved levels sufficient for therapeutic effect through 8 hours (Figure 6A). However, by day 14 the total drug levels were maintained above the 500 nM threshold for only 4 to 6 hours (Figure 6B). This suggests that, by day 14, inhibition of the production of M1 resulted in lower total drug levels. According to this data, therefore, patients receiving KW-2449 twice daily were achieving target inhibition for 4- to 6-hour periods twice daily.
Plasma levels of KW-2449 and the metabolite M1. Plasma was obtained 2, 4, 8, and 12 hours after dosing on day 1 and day 14 of cycle 1 of the clinical trial for all patients enrolled. A “composite” drug level was determined by adding the concentration of KW-2449 with the concentration of the metabolite divided by 3.6 ([M1] / 3.6). The 3.6 divisor was derived from the standard curve experiments described in Figure 2. The graphs display the means values of these composite numbers at each of 7 dose levels (25, 50, 100, 200, 300, 400 and 500 mg per day), with error bars representing the standard error of the mean. The dashed line at 500 nM represents the level of drug estimated to be necessary in order to achieve a significant cytotoxic effect via FLT3 inhibition. (A) Day 1. (B) Day 14.
Plasma levels of KW-2449 and the metabolite M1. Plasma was obtained 2, 4, 8, and 12 hours after dosing on day 1 and day 14 of cycle 1 of the clinical trial for all patients enrolled. A “composite” drug level was determined by adding the concentration of KW-2449 with the concentration of the metabolite divided by 3.6 ([M1] / 3.6). The 3.6 divisor was derived from the standard curve experiments described in Figure 2. The graphs display the means values of these composite numbers at each of 7 dose levels (25, 50, 100, 200, 300, 400 and 500 mg per day), with error bars representing the standard error of the mean. The dashed line at 500 nM represents the level of drug estimated to be necessary in order to achieve a significant cytotoxic effect via FLT3 inhibition. (A) Day 1. (B) Day 14.
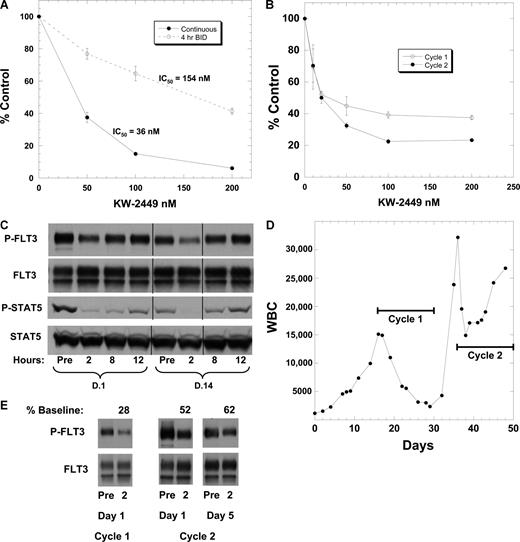
Preclinical studies with virtually all FLT3 inhibitors thus far studied report cytotoxicity in response to continuous exposure only.23,30-33 Given the short duration of FLT3 inhibition for patients treated with twice daily KW-2449, we decided to confirm that such an exposure pattern would result in decreased cytotoxicity. We treated the FLT3/ITD cell line Molm14 with KW-2449 in continuous versus intermittent (4 hours twice daily) fashion (Figure 7A). Intermittent FLT3 inhibition resulted, as expected, in a 4.3-fold reduction in cytotoxicity.
Intermittent inhibition of FLT3 in vivo. (A) Parallel flasks of Molm14 cells were incubated in culture medium containing escalating doses of KW-2449. For one set (4 hours, twice daily; dashed line), cells were exposed to KW-2449 for 4 hours, then washed in warm drug-free medium and resuspended in DMSO control medium. This was repeated twice daily for a total of 52 hours (5 total “doses”). Control cells (continuous; solid line) were treated in continuous fashion. The continuously treated cells were likewise washed twice daily, but then resuspended in KW-2449. One set of cells had drug incubated on the cells for 4-hour periods twice daily and the other set had continuous exposure to KW-2449. At the 52-hour time point each flask was analyzed by Trypan blue, MTT, and annexin V (data not shown for Trypan blue or annexin V). The results display reduced cytotoxicity with intermittent exposure to KW-2449. (B) Peripheral blood blasts were isolated by Ficoll gradient centrifugation (“Methods”) from a 60-year-old woman with relapsed/refractory AML harboring an FLT3/ITD mutation and incubated in culture medium containing increasing concentrations of KW-2449. After 48 hours, the MTT assay was performed. Blasts were isolated at the beginning of cycle 1 and at the beginning of cycle 2. (C) PIA results for cycle 1 for this patient, showing phosphorylated FLT3 and STAT5 on days 1 and 14 for different time points. Vertical lines have been inserted to indicate a repositioned gel lane. (D) Graph of WBCs over time. The horizontal black lines denote the time period during which the patient received treatment with KW-2449. The trial dictated a 5- to 7-day break from therapy between cycles. The patient was taken off study for progressive disease after completing the second cycle. (E) FLT3 phosphorylation in circulating blasts. These immunoblots show P-FLT3 immediately prior to, and 2 hours after, dosing with KW-2449 during the first and second cycles. Densitometry was performed and the density of the 2-hour time point was expressed as a percent of the pretreatment sample.
Intermittent inhibition of FLT3 in vivo. (A) Parallel flasks of Molm14 cells were incubated in culture medium containing escalating doses of KW-2449. For one set (4 hours, twice daily; dashed line), cells were exposed to KW-2449 for 4 hours, then washed in warm drug-free medium and resuspended in DMSO control medium. This was repeated twice daily for a total of 52 hours (5 total “doses”). Control cells (continuous; solid line) were treated in continuous fashion. The continuously treated cells were likewise washed twice daily, but then resuspended in KW-2449. One set of cells had drug incubated on the cells for 4-hour periods twice daily and the other set had continuous exposure to KW-2449. At the 52-hour time point each flask was analyzed by Trypan blue, MTT, and annexin V (data not shown for Trypan blue or annexin V). The results display reduced cytotoxicity with intermittent exposure to KW-2449. (B) Peripheral blood blasts were isolated by Ficoll gradient centrifugation (“Methods”) from a 60-year-old woman with relapsed/refractory AML harboring an FLT3/ITD mutation and incubated in culture medium containing increasing concentrations of KW-2449. After 48 hours, the MTT assay was performed. Blasts were isolated at the beginning of cycle 1 and at the beginning of cycle 2. (C) PIA results for cycle 1 for this patient, showing phosphorylated FLT3 and STAT5 on days 1 and 14 for different time points. Vertical lines have been inserted to indicate a repositioned gel lane. (D) Graph of WBCs over time. The horizontal black lines denote the time period during which the patient received treatment with KW-2449. The trial dictated a 5- to 7-day break from therapy between cycles. The patient was taken off study for progressive disease after completing the second cycle. (E) FLT3 phosphorylation in circulating blasts. These immunoblots show P-FLT3 immediately prior to, and 2 hours after, dosing with KW-2449 during the first and second cycles. Densitometry was performed and the density of the 2-hour time point was expressed as a percent of the pretreatment sample.
KW-2449, administered on a twice daily schedule, is therefore predicted to fail for pharmacokinetic rather than pharmacodynamic reasons. The correlative studies from a FLT3/ITD patient enrolled in dose level 4 (100 mg twice daily) serves as a useful illustration of this. The patient's blasts displayed a cytotoxic response to KW-2449 in vitro (Figure 7B), suggesting that if an adequate drug level could be achieved in vivo there would be a clinical response. During cycle 1, the PIA assay data showed significant FLT3 inhibition at the 2-hour time point only (Figure 7C). The patient, who started therapy with a peripheral WBC count of more than 15 000 cells/cubic millimeter (consisting almost entirely of blasts) displayed a steady reduction in peripheral blasts during the first cycle of therapy. Following a 5-day break in treatment (dictated by the clinical protocol), during which time the blast count rose, the patient resumed KW-2449 at the same dose. During the second cycle, the blast count initially fell, but then increased again, and the patient was taken off protocol. Direct examination of FLT3 in the patient's circulating blasts (Figure 7E) revealed that phospho-FLT3 was reduced to 28% of baseline at 2 hours on day 1 of cycle 1, but during cycle 2 the inhibition was considerably less effective, likely due to decreased M1 production after repeat dosing. It is interesting to note that even this modest degree of FLT3 inhibition resulted in biologic activity resembling that observed for virtually all other FLT3 inhibitors previously studied in clinical trials.
The trial was terminated during accrual to dose level 7. While a single dose-limiting toxicity (grade 3 vomiting) was observed in one patient on this dose, the maximum tolerated dose (MTD) was not formally reached. The reason for not continuing the trial to the MTD was that the pharmacokinetic and correlative analysis presented here strongly suggested that effective, sustained FLT3 inhibition with KW-2449 could be better achieved using a different dosing scheme than the one used in this trial. No clinical responses were seen, although the majority of patients enrolled lacked FLT3 mutations. In those patients who did harbor FLT3/ITD mutations, peripheral blast reductions of greater than 50% were observed in 5 of 11 patients at day 14.
Discussion
In summary, KW-2449 is a multitargeted kinase inhibitor that potently inhibits FLT3 and is cytotoxic to FLT3 mutant AML cells. The drug is metabolized in humans to metabolite M1, which is 3.6-fold less potent than the parent drug. The combination of KW-2449 and metabolite can successfully inhibit FLT3 in patients, and FLT3 mutant patients treated with KW-2449 on a twice daily schedule display the typical, short-lived reduction in peripheral blasts that has been seen with the other FLT3 inhibitors in development. What is unique about our study, however, is that we have been able to establish through correlative data that we are failing for pharmacokinetic reasons to inhibit the target in a manner that models the preclinical data. It is possible that pharmacokinetic failure underlies the disappointing lack of sustained clinical activity of FLT3 inhibitors in general. At this time, we are planning a trial of KW-2449 using a new dose and schedule, one that takes into account both the shorter-than-predicted half-life as well as the decrease in active drug concentrations in the plasma noted with repeated dosing. This trial should allow us to determine the true clinical activity of sustained FLT3 inhibition by this drug.
There has been a general consensus that FLT3 inhibition will most likely be effective as a therapy only in AML cases harboring FLT3 activating mutations.11,34,35 However, occasional primary samples and even cell lines have been noted by other investigators to respond in vitro to FLT3 inhibitors, and there is at least one report of modest clinical responses in patients with wild-type FLT3 AML.30,36,37 In the present study, we noted one primary sample (Figure 1D) which seemed to display in vitro sensitivity to KW-2449. The patient in question did not show any clinical response, but was not receiving a dose of KW-2449 that would be expected to significantly inhibit FLT3 in vivo. The mechanism of the observed cytotoxicity in this case (and in other cases) is not known, but may be due to off-target effects of these compounds. KW-2449 has activity against Aurora kinases, FGFR1, and (albeit at higher concentrations) KIT, JAK2, and SRC, and inhibition of these or other unknown targets may account for the response observed in the wild-type FLT3 sample.24 Alternately, others have reported that overexpression of wild-type FLT3 can result in constitutive activation and potential dependence on FLT3 signaling.38 Finally, autocrine stimulation of the FLT3 wild-type receptor may lead to dependence on FLT3 signaling that can be interrupted by FLT3 targeted therapy.39
The oncogenic signaling of FLT3 is, at least in part, mediated through STAT5.40 Our results using the FLT3/ITD-dependent cell line Molm14 in standard curves (Figure 2) and the PIA assays (Figure 7) suggest that STAT5 activation in Molm14 cells is almost exclusively dependent on FLT3, and that there is a threshold of FLT3 activation required to activate STAT5. STAT5 phosphorylation in isolated primary leukemia samples may represent a useful surrogate for responsiveness to FLT3-targeted therapy, as it has been noted by others that constitutive STAT5 phosphorylation is present in a large percentage of isolated samples, both mutant and wild-type FLT3.41 Nonetheless, it seems more likely that the single most important parameter to monitor in vivo for FLT3 inhibitors is the target itself, FLT3.
This is the first phase 1 clinical trial of an FLT3 inhibitor in which target inhibition has been tightly linked to dose and pharmacokinetic data throughout the treatment cycle. On the basis of the pharmacodynamic studies reported here, this clinical trial was closed before the traditional endpoints were actually reached. While the endpoints of safety and tolerability are obviously vital, the principal target of the therapy is equally important. Through the use of the PIA assay, real-time determination of target kinase inhibition in FLT3-mutated AML is feasible. As more and more targeted therapies enter development, the establishment of in vivo target inhibition, or lack thereof, should be a critical component of early phase clinical trials. At the present time, it is not clear that the clinical efficacy of sustained, effective FLT3 inhibition, by any drug, has been determined.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.W.P. conducted the experiments, contributed to the study design, and wrote the manuscript; N.R. supervised the pharmacokinetic studies, helped organize the data, and edited the manuscript; J.C., G.J.R., Y.S., A. Shudo, S.A., D.S., and J.E.K. all contributed to the study design and edited the manuscript; O.A. and A. Stine helped conduct the experiments; and M.L. conducted the experiments, designed the study, and helped write the manuscript.
Conflict-of-interest disclosure: N.R., Y.S., A. Shudo, and S.A. are employees of Kyowa Hakko Kogyo, a company whose product is featured in this report. M.L. received research funding from Kyowa Hakko Kogyo. The remaining authors declare no competing financial interests.
Correspondence: Mark Levis, Kimmel Cancer Center at Johns Hopkins, 1650 Orleans St, Rm 243, Baltimore, MD 21231; e-mail: levisma@jhmi.edu.

![Figure 5. PIA results compared with standard curve for KW-2449. (A) Plasma was collected from patients receiving KW-2449 on the clinical trial 2, 8, and 12 hours after ingesting drug. The plasma samples underwent conventional pharmacokinetic analysis for concentrations of KW-2449 and the metabolite, M1. In parallel, plasma from the same time points was used in PIA assays for FLT3. On the x axis, the concentration of active drug is plotted for individual time point by the following formula: [KW-2449] + [M1] / 3.6. On the y axis, the degree of FLT3 inhibition is plotted as percent control. The pretreatment plasma sample for each patient was used as the control for each individual PIA assay. Thus, each circle on the graph represents a single time point for which the concentration of KW-2449 plus metabolite in a plasma sample is plotted against how well that plasma inhibited FLT3 phosphorylation in Molm14 cells (the PIA assay). (B) This graph is the standard curve of KW-2449 inhibiting FLT3 in plasma shown in Figure 2. (C) This graph shows the graph from panel A overlying the standard curve from panel B, demonstrating how the results of the PIA assay, using the concentrations of KW-2449 and M1 divided by 3.6, correspond to the standard curve. (D) The same experiment as in panel C, but using the PIA assay for STAT5 rather than FLT3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/17/10.1182_blood-2008-09-177030/4/m_zh80070931040005.jpeg?Expires=1769093313&Signature=CME8AeXT4NTDrBtkqV0NekBADU54R1Idjak~9gR3LP-kpjrCfXofOpX-6exEsfkya0QCt1b76m2VR~Hv93Affz8UL9DWEoGHm8wrQipbJbNd~7p1HEkgBzSg7JnhmCtEQD23sm8kH8F~6SHeelts0xS50o5bDh1SMrlLB0Z6PhYxPj39pTlNhc0fmYZcOZTbpVI-NwsbXTgYMXQ1K5SuHMANCEQwUpdSP6Kh35I50nVMHxJlp7paue9Z~8C6i9BhIPgbRxIr1fau3-3g30ZjDOVVFc5UhtTqBDImNgvXEnJsA9HeW7AeI7AbouqlPPb09x6OPzGXtXfYvdh6QDIBpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Plasma levels of KW-2449 and the metabolite M1. Plasma was obtained 2, 4, 8, and 12 hours after dosing on day 1 and day 14 of cycle 1 of the clinical trial for all patients enrolled. A “composite” drug level was determined by adding the concentration of KW-2449 with the concentration of the metabolite divided by 3.6 ([M1] / 3.6). The 3.6 divisor was derived from the standard curve experiments described in Figure 2. The graphs display the means values of these composite numbers at each of 7 dose levels (25, 50, 100, 200, 300, 400 and 500 mg per day), with error bars representing the standard error of the mean. The dashed line at 500 nM represents the level of drug estimated to be necessary in order to achieve a significant cytotoxic effect via FLT3 inhibition. (A) Day 1. (B) Day 14.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/17/10.1182_blood-2008-09-177030/4/m_zh80070931040006.jpeg?Expires=1769093313&Signature=Q8OChkzCGEgK9QahMOjMwxCULjLIL4egPKkkJyLYyAkxvm45tDhVEuwQStZJQbqcwb1x4f3ono-djc9W6~qaD2yf0HnSr51J1BeJyRerRY3zC9DKSmpEPMHjB-EWk6k4Z-1weKt7kEC1z7LD9yU1cotOQqPqul~U1DyOCymb8PuoFjIJ~mog6maOxUPJTlg0o8nFind3WrGhh8dxL3aNuxNs7GkozwiwAeIf~kVnLtLnO8-oPWoqp5y3mfHbklmZQ1-1GlhJd3O9jDRivFCWwPZHa7kkFjgNeP-NmQhAm7rN3W7HmU0SGb7MZ9hdAPgb0XhWXq1lWQgj8Oth0tZMsg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal