Abstract
Dose density during early induction has been demonstrated to be one of the prime determinants for treatment efficacy in acute myeloid leukemia (AML). The German AML Cooperative Group has therefore piloted a dose-dense induction regimen sequential high-dose AraC and mitoxantrone followed by pegfilgrastim (S-HAM) in which 2 induction cycles are applied over 11 to 12 days instead of 25 to 29 days as used in conventional double induction, thereby increasing dose density 2-fold. Of 172 de novo AML patients (excluding acute promyelocytic leukemia), 61% reached a complete remission, 22% a complete remission with incomplete peripheral recovery, 7% had persistent leukemia, 10% died (early death) resulting in an overall response rate of 83%. Kaplan-Meier estimated survival at 2 years was 61% for the whole group (patients with unfavorable karyotypes, 38%; patients with favorable karyotypes, 69%; patients with intermediate karyotypes, 75%) after S-HAM treatment. Importantly, the compression of the 2 induction cycles into the first 11 to 12 days of treatment was beneficial for normal hematopoiesis as demonstrated by a significantly shortened duration of critical neutropenia of 31 days compared with 46 days after conventionally timed double induction. (European Leukemia Trial Registry LN_AMLINT_2004_230.)
Introduction
The overall prognosis of patients with acute myeloid leukemia (AML) has steadily improved over the last 3 decades. Nowadays, complete remissions (CRs) are achieved in 60% to 70% of all patients with long-term disease-free survival and potential cure in 25% to 40% of cases. A more detailed analysis indicates that this progress has mainly been achieved in patients younger than 60 years, whereas in older patients little improvement has been obtained.1-5
When analyzing the approaches that underlie the progress in AML therapy, 2 major developments appear essential: the intensification of therapy and the improvement of supportive care.
In the last few years, increasing insights into the biology of AML have been gained. It has become clear that AML is a not a homogeneous disease but rather a group of different subtypes. These subtypes differ not only in their biology but also in their prognosis. Therefore, genetic markers have become mandatory to discriminate prognostic subgroups and to adjust treatment according to distinct risk groups.6-12 However, the definition of distinct subgroups of AML is still mainly descriptive, and the major challenge for clinicians and translational researchers remains how to treat these different subgroups most effectively and how to improve on their current outcome. Except for acute promyelocytic leukemia, the better understanding of AML biology and the development of “targeted” therapies so far have not resulted in significant improvements in overall survival.
The standard induction therapy for AML is still a “3 + 7” type regimen comprising 3 days of daunorubicin and 7 days of standard-dose cytosine arabinoside (AraC) as continuous infusion.13,14 Based on cell biologic data, the German AML Cooperative Group (AMLCG) modified the 3 + 7 regimen and established the TAD-9 regimen, which is the combination of thioguanine, AraC, and daunorubicin.15 The TAD-9 regimen resulted in a high CR rate and has been part of the induction strategies of the German AMLCG since 1979. For the dosing of daunorubicin in particular, an improvement in overall survival was demonstrated for full dosing compared with reduced dosing in patients older than 59 years.16,17
In an attempt to improve the long-term prognosis of patients with AML, the AMLCG introduced the concept of “double induction.” This strategy is focused primarily on patients younger than 60 years. It consists of 2 courses of chemotherapy irrespective of the degree of cytoreduction in the bone marrow after the first course, with the second course starting on day 21 unless severe complications prohibit its application.
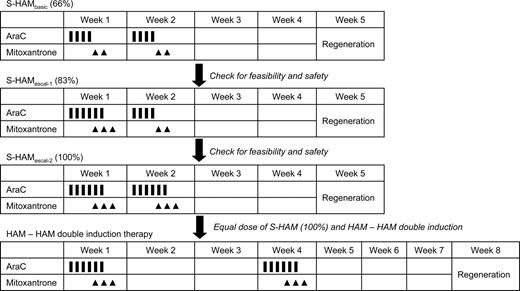
This strategy resulted in a significantly longer remission duration and overall survival compared with standard induction.18 To further improve on these results, double induction with 2 courses of TAD-9 was compared with a first course of TAD-9 followed by high-dose AraC (HD-AraC) plus mitoxantrone (HAM) as second course. Although no significant differences in outcome were observed for the overall group of patients, a favorable effect of HAM was seen in the subgroup of high-risk patients as defined by unfavorable karyotype and/or elevated lactate dehydrogenase level and/or residual day 16 bone marrow blasts with an overall survival at 5 years of 25% versus 18% (P = .012).19 The subsequently performed comparison of 2 courses of HAM (HAM-HAM) versus the TAD-9/HAM sequence, however, showed no significant differences between HAM-HAM and TAD-HAM in terms of CR rate (71% vs 65%), recurrence-free survival at 5 years (35% vs 29%), and overall survival at 5 years (32% vs 30%).20 Although the escalation of drug doses thus obviously has reached a limit, further intensification of therapy by shortening the time interval between induction cycles appeared as a promising new approach. This strategy was first evaluated in patients with relapsed and refractory AML. Based on prior studies by Burke et al21 and Archimbaud et al,22 the HAM regimen was modified into a sequential application of 2 HAM courses (sequential high-dose AraC and mitoxantrone followed by pegfilgrastim [S-HAM]). S-HAM comprises HD-AraC twice a day on days 1 and 2, and mitoxantrone on days 3 and 4; after a rest period of only 3 days, the identical sequence is repeated on days 8 and 9 (HD-AraC) and 10 and 11 (mitoxantrone), respectively (Figure 1).
Schema of the S-HAM regimen and of the planned 3-step escalation of S-HAM within the AMLCG 2004 study.
Schema of the S-HAM regimen and of the planned 3-step escalation of S-HAM within the AMLCG 2004 study.
The S-HAM protocol was highly effective in patients with advanced disease (primary refractory or relapsed AML) with a CR rate of more than 50% but was complicated by a high early death (ED) rate from infections.23-25 Subsequent supportive therapy with granulocyte colony-stimulating factor, however, reduced the duration of critical neutropenia from 40 to 36 days (P = .008) and the ED rate from 30% to 21% (not significant).26 First results of dose-dense therapy in first-line therapy of de novo AML were gained by a prospective randomized comparison of conventional vs dose-dense therapy in children with AML. In the Children Oncology Group Study 2891, dose-dense therapy comprising dexamethasone, cytarabine, thioguanine, etoposide, and rubidomycin (DCTER) given on days 0 to 4 and 10 to 14 regardless of response was compared with the standard DCTER regimen given on days 0 to 4 and 14 to 18 or later, depending on response. Dose-dense treatment resulted in a significantly longer disease-free and overall survival after 3 years of 55% versus 37% (P = .001) and 52% plus or minus 6% versus 42% plus or minus 6% (overall survival), respectively.27 In adult patients, a French study showed that a similar sequential approach (however, not involving high-dose AraC) resulted in a surprisingly low hematologic toxicity and a lower cumulative incidence of relapse compared with conventional induction.28
These results prompted the AMLCG to assess the efficacy and feasibility of dose-dense therapy with S-HAM in newly diagnosed de novo AML in a phase 2 study. Supportive therapy with pegfilgrastim was mandatory.29 In addition, a 3-step escalation of treatment days was planned to obtain total doses of AraC and mitoxantrone equivalent to the HAM-HAM arm of double induction therapy (Figure 1).
Methods
Patients and entry criteria
Adult patients (age ≥18 years) with first diagnosis of de novo AML (except acute promyelocytic leukemia) were eligible for the study. There was no upper age limit. Patients were ineligible in case of severe organ dysfunction not explained by leukemia.
Cytogenetic subgroups
For exact characterization of leukemia, bone marrow samples underwent a standardized processing procedure, including central sample registration, preparation, and evaluation by cytomorphology, cytochemistry, multiparameter immunophenotyping, cytogenetics, fluorescence in situ hybridization, and molecular genetics.
Favorable cytogenetics were defined as t(8;21) and inv(16). Unfavorable cytogenetics were defined as complex chromosomal aberrations [≥ 3 numerical or structural chromosomal aberrations without involvement of t(15;17), t(8;21), or inv(16)], aberrations of chromosomes 5 or 7, inv(3), and involvement of 11q23. Intermediate cytogenetics were defined as normal karyotype or aberrations not qualifying for favorable or unfavorable cytogenetics.
Treatment protocol
In the initial phase of the study, S-HAM was given as previously used in advanced AML.23,26 This S-HAMbasic (66%) regimen, which was identical in dose and schedule to the regimen used in the advanced studies, included AraC 3 g/m2 as a 3-hour continuous infusion, twice a day on days 1 to 2 and days 8 to 9 (1 g/m2 in patients ≥ 60 years) and mitoxantrone 10 mg/m2, 30-minute infusion, days 3 to 4 and days 10 to 11 (Figure 1). A prephase with AraC 100 mg/m2 as continuous infusion over 24 hours for up to 7 days was allowed to reach a peripheral leukocyte count less than 30 000/μL to avoid tumor lysis. Seven days after the completion of treatment, a bone marrow aspirate was performed. If less than or equal to 5% blasts were seen in the aplastic marrow, pegfilgrastim (6 mg) was applied subcutaneously. Pegfilgrastim was repeated every 10 to 12 days until leukocyte recovery (> 1000/μL leukocytes/ > 500/μL neutrophils). Supportive care was provided according to the standards of the local centers.
A dose escalation was planned to reach identical doses of AraC and mitoxantrone in the S-HAM regimen compared with the HAM-HAM regimen of conventional double induction (100%). Patients were to be treated initially with the S-HAM regimen as known from the advanced AML studies. The doses of this so-called S-HAMbasic (66%) regimen amount to 66% of the doses of HAM-HAM. The planned escalation schedule is shown in Figure 1. After the S-HAMbasic (66%) regimen, the second dose level (S-HAMescal, 83%) involved one more day of AraC and mitoxantrone on days 3 and 4, respectively, resulting in 83% of the HAM-HAM dose. For the third dose level (S-HAM, 100%), one further day of AraC and mitoxantrone was planned to be added on days 9 and 10, respectively, resulting in 100% of the HAM-HAM dose (Figure 1).
Postremission therapy was identical to that of the AMLCG 1999 trial with TAD-9 consolidation and monthly myelosuppressive maintenance for 3 years with alternating 5-day cycles of AraC plus thioguanine, daunorubicin, or cyclophosphamide (AD-AT-AD-AC…). Patients younger than 65 years with a high or intermediate risk of relapse according to cytogenetics or molecular genetics and an available donor (family or matched unrelated) were to receive an allotransplant.
Evaluation and response criteria
Response criteria were used as proposed by Cheson et al.30 Patients dying within the first 65 days or while still cytopenic beyond this time were considered EDs, including those that had residual leukemia. Treatment-related side effects were assessed by World Health Organization criteria.
Data description and statistical analysis
The aim of the present study was to test the feasibility of a dose-dense regimen in adult patients with de novo AML. Therefore, overall toxicity and the ED rate were of particular relevance. Given an ED rate of 21.3% in 1238 patients with de novo AML treated within the AMLCG 1999 trial20 and given the potential increase of efficacy achievable by dose-dense S-HAM induction therapy, the present trial was to be stopped prematurely if an excessive death rate was observed (>16% at 60 days; >23% at 90 days).
To put the results of the S-HAM regimen (dose-dense induction) into perspective, a descriptive comparison of the pertinent parameters with data of the AMLCG 1999 trial (conventional double induction) was made. From the large cohort of the AMLCG 1999 trial, the following subgroup was selected as a comparator: patients with de novo AML who received HAM-HAM double induction. In this group, a comparable distribution of cytogenetically defined prognostic subgroups and of the Eastern Cooperative Oncology Group (ECOG) Performance Status was found compared with the S-HAM patient cohort. Because the current phase 2 study was meant only to demonstrate the feasibility of the dose-dense approach to allow a future randomized comparison, no formal statistical comparison with the historical data was attempted.
Conduct of the study
The study was carried out in accordance with the Declaration of Helsinki. All patients gave their consent after having been informed about the purpose and the investigational nature of the trial. Before initiation, the study received approval of the responsible institutional review board and the ethics committees of the participating institutions. The clinical study is an official study of the Kompetenznetz Akute und Chronische Leukämien and is registered in the European Leukemia Trial Registry as #LN_AMLINT_2004_230. A list of AML Cooperative Study Group centers and investigators is contained in an online supplement to this article (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results
Patient characteristics
From August 2004 until January 2008, 172 patients with de novo AML were included in the study and were evaluable for treatment response after induction treatment. Patient characteristics are given in Table 1. The median age was 54 years, which is lower than the median age of 58 years in the AMLCG 1999 trial; 40% of patients were 60 years of age or older and 12% were older than 70 years.
Patient characteristics
| Characteristic . | Value . |
|---|---|
| No. of patients | 172 (100%) |
| Male | 95 (55.2%) |
| Female | 77 (44.8%) |
| Median age, y (range) | 54 (18-83) |
| < 60 y | 104 (60.5%) |
| ≥ 60 y | 68 (39.5%) |
| ≥ 70 y | 20 (11.6%) |
| Induction per protocol | 168 (97.6%) |
| ECOG performance status (n = 141) | |
| 0 | 50 (35%) |
| 1 | 68 (48%) |
| 2 | 21 (15%) |
| 3 | 2 (1%) |
| Cytogenetic subgroup (n = 151) | |
| Favorable | 14 (9%) |
| Intermediate | 104 (69%) |
| Unfavorable | 33 (22%) |
| Characteristic . | Value . |
|---|---|
| No. of patients | 172 (100%) |
| Male | 95 (55.2%) |
| Female | 77 (44.8%) |
| Median age, y (range) | 54 (18-83) |
| < 60 y | 104 (60.5%) |
| ≥ 60 y | 68 (39.5%) |
| ≥ 70 y | 20 (11.6%) |
| Induction per protocol | 168 (97.6%) |
| ECOG performance status (n = 141) | |
| 0 | 50 (35%) |
| 1 | 68 (48%) |
| 2 | 21 (15%) |
| 3 | 2 (1%) |
| Cytogenetic subgroup (n = 151) | |
| Favorable | 14 (9%) |
| Intermediate | 104 (69%) |
| Unfavorable | 33 (22%) |
The majority of patients (83%) had a good or only slightly decreased Performance Status (ECOG 0 and 1; Table 1). The cytogenetically defined prognostic subgroups were as follows: favorable, 9%; intermediate, 69%; and unfavorable, 22% (Table 1). Compared with the historical control of the AMLCG 1999 trial, there was no difference in the distribution (favorable, 12%; intermediate, 67%; and unfavorable, 21%).
Treatment delivery
Of 172 patients recruited into the study, 168 (98%) patients received their induction treatment as planned. Four patients did not receive the full treatment resulting from prohibitive toxicity within the first week of treatment (splenic rupture, pulmonary infiltrates, liver toxicity). Three of these patients received only the first block of treatment (ie, the equivalent of 1 cycle of HAM); 1 patient had also received 1 day of the second block when his treatment was stopped. These 4 patients are included into the following response analyses on an intention-to-treat basis.
After the feasibility of the S-HAMbasis (66%) regimen had been demonstrated in the first 68 patients, the following patients were to receive the S-HAMescal (83%) regimen. However, when a significantly longer duration of neutropenia was observed after the S-HAMescal (83%) regimen (see “Nonhematologic toxicity” below), this regimen was restricted to patients younger than 60 years, whereas patients 60 years or age or older went on to receive the S-HAMbasis (66%) regimen to spare older patients from a prolonged duration of neutropenia. In total, 113 patients received S-HAMbasis (66%) and 59 patients S-HAMescal (83%).
After observing a 6-day prolongation of critical neutropenia by the addition of one more day of AraC and mitoxantrone after the S-HAMescal (83%) regimen compared with the S-HAMbasis (66%) regimen, it was decided that the dose-limiting toxicity was reached and a further escalation to S-HAM (100%) was not performed.
Of 143 responding patients, 98 patients have a follow-up of more than 150 days, which allows an estimation of the feasibility of postremission therapy. Of those patients, 75 (77%) received their postremission therapy as scheduled. A total of 63 patients (64%) received TAD-9 consolidation, with a median interval between the start of S-HAM induction and start of TAD-9 consolidation of 61 days (range, 39-154 days). Fifteen patients (15%) received an allotransplant as the sole consolidation therapy, with a median interval between the start of S-HAM induction and allogeneic transplantion of 127 days (range, 64-286 days). Another 5 patients received their allotransplant in CR1 after having had TAD-9 consolidation therapy resulting in a total rate of 20% of patients with allogeneic transplantation in CR1. Reasons for transplantation were cytogenetic high risk (n = 9), molecular genetic high risk (mostly FLT3-ITD positivity; n = 3), CR with incomplete peripheral recovery, thereby making chemo-consolidation impossible (n = 3), availability of a human leukocyte antigen (HLA)–identical donor in the cytogenetically intermediate risk group (in which case allotransplantation is regularly offered to patients in the current AMLCG studies; n = 3), and other (n = 2).
Nonhematologic toxicity
Nonhematologic grade 3 and 4 toxicities during or after S-HAM induction treatment are given in Table 2. As a historical comparison, the toxicity data of the AMLCG 1999 trial20 with double induction treatment are also listed. The major toxicities were serious infections in 40% of patients after S-HAM (56% after double induction in the AMLCG 1999 trial). There was no toxicity that was more frequent during S-HAM than during standard double induction.
Nonhematologic toxicities (grades 3 and 4) of S-HAM compared with double induction in the AML-CG 99 trial (de novo AML, age <60 years, HAM-HAM double induction)
| Toxicity . | S-HAM AML-CG 2004, % . | HAM-HAM AML-CG 1999, % . |
|---|---|---|
| Nausea | 9 | 16 |
| Stomatitis | 3 | 9 |
| Diarrhea | 14 | 14 |
| Hemorrhage | 8 | 8 |
| Infection | 40 | 56 |
| Cardiac event | 6 | 5 |
| Central nervous system toxicity | 5 | 5 |
| Toxicity . | S-HAM AML-CG 2004, % . | HAM-HAM AML-CG 1999, % . |
|---|---|---|
| Nausea | 9 | 16 |
| Stomatitis | 3 | 9 |
| Diarrhea | 14 | 14 |
| Hemorrhage | 8 | 8 |
| Infection | 40 | 56 |
| Cardiac event | 6 | 5 |
| Central nervous system toxicity | 5 | 5 |
Comparing the 2 evaluable dose levels of S-HAM, serious infections were more frequent after S-HAMescal (83%) than after S-HAMbasis (66%) (54% vs 32%; P < .01) as was lung toxicity (34% vs 15%; P < .01).
Hematologic toxicity
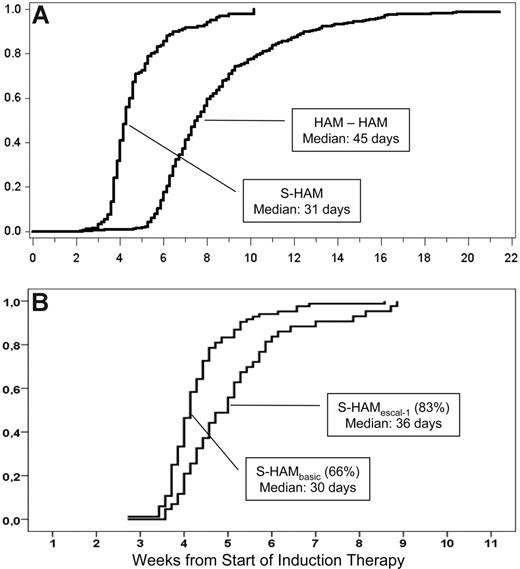
The median time to leukocyte recovery (> 1000/μL) was 31 days after the start of treatment with S-HAM (Figure 2A). Compared with de novo AML patients treated with double induction within the AMLCG 1999 trial (median time to leukocyte recovery, 46 days), the total duration of critical leukopenia was more than 2 weeks shorter after S-HAM (Figure 2A). Comparing the 2 dose levels, leukopenia after S-HAMescal (83%) was 6 days longer than after S-HAMbasis (66%; Figure 2B, 36 days vs 30 days; P < .01).
Duration of critical leukopenia. (A) Duration of critical leukopenia (< 1000/μL) after S-HAM induction and subsequent pegfilgrastim compared with conventional double induction (HAM-HAM),% recovery. (B) According to dose level (left, S-HAMbasis, 66%; right, S-HAMescal, 83% induction).
Duration of critical leukopenia. (A) Duration of critical leukopenia (< 1000/μL) after S-HAM induction and subsequent pegfilgrastim compared with conventional double induction (HAM-HAM),% recovery. (B) According to dose level (left, S-HAMbasis, 66%; right, S-HAMescal, 83% induction).
Response to treatment
A bone marrow aspirate was successfully performed 7 days after the completion of treatment to morphologically assess the early blast clearance (EBC) in 159 of 172 patients. The median percentage of residual blasts was 0% for the whole group. The EBC was higher after the S-HAMescal (83%) regimen compared with S-HAMbasis (66%) with 97% (range, 0%-20%) of patients having less than or equal to 10% residual blasts compared with 86% (range, 0%-70%; P < .05). In the AMLCG 1999 trial, only 63% of patients had less than or equal to 10% blasts 7 days after the completion of the (first of 2) induction cycles.31
Response rates for the whole group are given in Table 3. An overall response rate (CR + CRi) of 83% was reached. Treatment failure occurred (ED 7%, PL 10%) in 17%. Responses according to dose level, age, or cytogenetic subgroup are given in Table 3. For comparison, the results of the AMLCG 1999 trial are also provided (Table 4).
Antileukemic efficacy of the S-HAM regimen for the whole group, according to the respective dose level, age, and cytogenetics
| . | CR . | CRi . | PL . | ED . | Total . |
|---|---|---|---|---|---|
| Whole group | 105 (61) | 38 (22) | 12 (7) | 17 (10) | 172 (100) |
| Dose | |||||
| 66% | 72 (64) | 24 (21) | 10 (9) | 7 (7) | 113 (100) |
| 83% | 33 (56) | 14 (24) | 2 (3) | 10 (17) | 59 (100) |
| Age | |||||
| < 60 y | 69 (66) | 17 (16) | 7 (7) | 12 (11) | 105 (100) |
| ≥ 60 y | 36 (54) | 21 (31) | 5 (7) | 5 (7) | 67 (100) |
| Cytogenetics | |||||
| Favorable | 8 (53) | 6 (40) | 0 (0) | 1 (7) | 15 (100) |
| Intermediate | 68 (68) | 20 (20) | 5 (5) | 7 (7) | 100 (100) |
| Unfavorable | 18 (55) | 5 (15) | 6 (18) | 4 (12) | 33 (100) |
| . | CR . | CRi . | PL . | ED . | Total . |
|---|---|---|---|---|---|
| Whole group | 105 (61) | 38 (22) | 12 (7) | 17 (10) | 172 (100) |
| Dose | |||||
| 66% | 72 (64) | 24 (21) | 10 (9) | 7 (7) | 113 (100) |
| 83% | 33 (56) | 14 (24) | 2 (3) | 10 (17) | 59 (100) |
| Age | |||||
| < 60 y | 69 (66) | 17 (16) | 7 (7) | 12 (11) | 105 (100) |
| ≥ 60 y | 36 (54) | 21 (31) | 5 (7) | 5 (7) | 67 (100) |
| Cytogenetics | |||||
| Favorable | 8 (53) | 6 (40) | 0 (0) | 1 (7) | 15 (100) |
| Intermediate | 68 (68) | 20 (20) | 5 (5) | 7 (7) | 100 (100) |
| Unfavorable | 18 (55) | 5 (15) | 6 (18) | 4 (12) | 33 (100) |
Data are expressed as n (%).
66% indicates S-HAMbasis (66%); 83%, S-HAMescal (83%); CR, complete remission; CRi, complete remission with incomplete peripheral recovery; PL, persistant leukemia; and ED, early death
Comparison of responses after S-HAM in the AML-CG 2004 study and standard double induction in the AML-CG 1999 study in de novo AML according to age and cytogenetics
| . | ORR, % . | PL, % . | ED, % . | |||
|---|---|---|---|---|---|---|
| 1999 . | 2004 . | 1999 . | 2004 . | 1999 . | 2004 . | |
| Age | ||||||
| < 60 y | 72 | 82 | 11 | 7 | 17 | 11 |
| ≥ 60 y | 59 | 85 | 15 | 7 | 26 | 7 |
| Cytogenetics | ||||||
| Favorable | 74 | 93 | 6 | 0 | 20 | 7 |
| Intermediate | 71 | 88 | 12 | 5 | 17 | 7 |
| Unfavorable | 45 | 70 | 25 | 18 | 30 | 12 |
| . | ORR, % . | PL, % . | ED, % . | |||
|---|---|---|---|---|---|---|
| 1999 . | 2004 . | 1999 . | 2004 . | 1999 . | 2004 . | |
| Age | ||||||
| < 60 y | 72 | 82 | 11 | 7 | 17 | 11 |
| ≥ 60 y | 59 | 85 | 15 | 7 | 26 | 7 |
| Cytogenetics | ||||||
| Favorable | 74 | 93 | 6 | 0 | 20 | 7 |
| Intermediate | 71 | 88 | 12 | 5 | 17 | 7 |
| Unfavorable | 45 | 70 | 25 | 18 | 30 | 12 |
ORR indicates overall response rate; PL, persistent leukemia; and ED, early death.
Overall survival
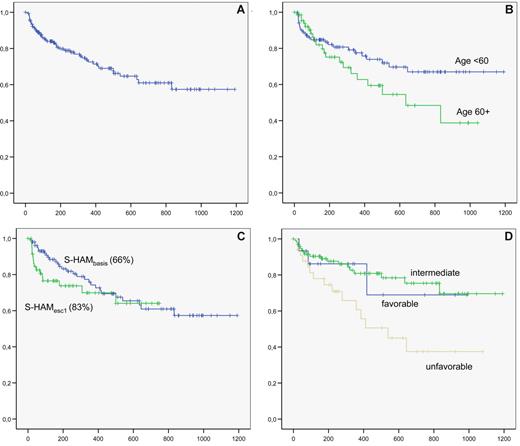
The mean follow-up for the 172 patients is 13 months. The overall survival of the whole group is shown in Figure 3A. Overall survival according to age, dose level, and cytogenetic subgroup is shown in Figure 3B, C, and D, respectively.
Overall survival. (A) overall survival of the whole group. (B) Overall survival according to age ( <60 years vs ≥60 years). (C) Overall survival according to dose level (S-HAMbasis, 66%; vs S-HAMescal, 83%). (D) Overall survival according to karyotype (blue represents favorable; green, intermediate; and brown, unfavorable).
Overall survival. (A) overall survival of the whole group. (B) Overall survival according to age ( <60 years vs ≥60 years). (C) Overall survival according to dose level (S-HAMbasis, 66%; vs S-HAMescal, 83%). (D) Overall survival according to karyotype (blue represents favorable; green, intermediate; and brown, unfavorable).
Survival of patients younger than 60 years at 2 years after S-HAM was 67%, of patients 60 years or older, 45%. In the AMLCG 1999 trial, a 2-year survival of 55% and 30% was reached for patients younger than 60 years and 60 years or older, respectively. No impact of CRi versus CR on overall survival was noted.
Discussion
The current study indicates that the novel strategy of dose-dense intensive induction therapy for de novo AML with the S-HAM regimen is feasible, is associated with a short period of critical neutropenia, and appears highly active. Hence, an ED rate of only 10%, no increase in nonhematologic toxicity compared with the preceding AMLCG study 1999, a median duration of critical neutropenia of only 31 days, and an overall remission rate of 83% were observed in 172 consecutive patients entering this phase 2 trial.
The approach to investigate a dose-dense therapy for de novo AML emerged from several preceding findings: (1) A historic comparison of different AMLCG studies clearly demonstrated that a shorter time interval between 2 induction cycles as applied during double induction therapy resulted in a higher CR rate, a longer CR duration, and a longer overall survival compared with the application of the second induction cycle after hematologic recovery.32 (2) In a prospective randomized comparison, the intensively timed DCTER regimen as applied twice with an interval of 6 to 10 days was superior in long-term outcome to the same regimens as applied with a 16-day interval in children with AML.27 (3) Two consecutive studies of the AMLCG in relapsed and refractory AML revealed a high antileukemic efficacy of S-HAM and a reduction of the duration of critical neutropenia by the supportive use of filgrastim.26
Although the present phase 2 study primarily tested the feasibility of S-HAM in first-line therapy, it was tempting to compare the results with the preceding AMLCG 1999 study, in which 2 different forms of conventional double induction therapy were evaluated. This comparison reveals a lower median age of 54 years in the current study compared with 58 years in the AMLCG 1999 trial but a comparable distribution of cytogenetic and molecular genetics (not shown) as well as of ECOG performance status.
This comparison strongly suggests that S-HAM is obviously not associated with a higher treatment-related mortality with an ED rate of only 10% compared with 21% in the AMLCG 1999 trial. It appears comparable with the preceding study in the frequency and degree of nonhematologic toxicities. After S-HAM, critical neutropenia was substantially shorter, reduced by 15 days compared with standard double induction (31 days vs 46 days). One component of this beneficial effect might be the mandatory application of pegfilgrastim in patients with adequate blast clearance 7 days after the completion of treatment; whereas after double induction, the use of filgrastim was optional and was applied in only 60% of patients. Most probably, however, this effect results from the earlier and potentially more appropriate timing of the second block of treatment. In double induction, the second block of treatment is applied on day 21, at a time when normal hematopoiesis is often about to regenerate and might therefore be particularly vulnerable to cytotoxic agents. In contrast, in the S-HAM regimen, the second block of treatment is applied only 3 days after the first one, at a time when the normal hematopoiesis is still suppressed and less vulnerable to cytotoxic cell damage. Clearly, a shorter (by 2 weeks) period of critical neutropenia is of substantial clinical relevance and most probably explains the lower rate of grade 3/4 infections in S-HAM treated patients versus the historical control (40% vs 55%).
Appropriate timing between cycles appears to be of crucial relevance for treatment-related toxicities as well. Hence, the aforementioned intensively timed DCTER-DCTER regimen that was effective in children with AML was too toxic for adult patients when given with an interval of 6 to 10 days.33 A very similar experience was made by the AMLCG when 2 cycles of TAD-9 were given with a 7-day interval, which resulted in an excessive rate of intestinal toxicity (W.H. and T.B., unpublished data, August 1992). Both experiences indicate that an interval of approximately 7 days between treatment blocks leads to intolerable toxicity in adults. In accordance with our findings, the Acute Leukemia French Association (ALFA) 9000 study demonstrated that a 4-day interval between 2 intensively timed induction cycles in adult patients was associated with surprisingly low toxicity.28
The notion that shortening the time interval between treatment cycles might be associated with a good tolerability of therapy is also supported by experiences with the fludarabin Ara-C amsacrin (FLAMSA) protocol in allogeneic transplantation. In this setting, an intensive 4-day course of cytarabine, amsacrine, and fludarabine is followed after an identical interval of 3 days by reduced intensity conditioning treatment with total body irradiation and cyclophosphamide with only very moderate nonhematologic toxicities.34
The antileukemic efficacy of the S-HAM regimen was high with an overall response rate of 83% compared with the historical control of 66% after double induction (AMLCG 1999, de novo AML patients) and treatment failure (PL + ED) of 17% compared with 34%. The difference in antileukemic efficacy was especially pronounced in the rate of EBC because in the S-HAM setting 91% of patients had less than 10% residual blast in the bone marrow on day 18 or 19; whereas after double induction, this was only the case in 63% of patients on day 16.
With respect to EBC, there was a significant difference between the 2 dose levels with the S-HAMescal (83%) being successful in 97% of patients compared with 88% after the S-HAMbasis (66%) regimen, indicating the substantial antileukemic efficacy of the S-HAMescal (83%) regimen. However, because there was no superiority in overall survival for the higher dose level but a trend toward higher toxicity, the S-HAMbasis (66%) regimen was chosen as the candidate regimen for a future randomized comparison.
Because of the high response rate, most patients could proceed to their postremission treatment. This was the case in 83% of responders receiving chemo-consolidation after a median of 61 days after the start of S-HAM. Patients allocated to allogeneic transplantation in CR1 underwent this approach after a median of 127 days. This proportion of patients successfully receiving their postremission treatment and the median duration until its initiation also compare favorably to the experiences after double induction.
As a result, the overall survival after S-HAM also looked promising, with a 2-year survival of 67% for patients younger than 60 years and 45% for patients 60 years or older. This also compares favorably with the historical control of de novo AML patients treated with double induction with overall survival at 2 years of 55% for patients younger than 60 years and 30% for patients 60 years or older.
In conclusion, the dose-dense intensive S-HAM regimen is a highly effective treatment regimen with a response rate of 83% and a low ED rate of 10% in the first 65 days, which is probably the result of the short duration of critical neutropenia. Despite these promising results, a prospective randomized comparison of such a dose-reduced but intensely timed therapy versus conventional double induction is required to prove the potential superiority of the new approach. This trial has been initiated within the next generation of the AMLCG studies (AMLCG 2008).
Presented in part in abstract form at the American Society of Hematology Annual Meeting in Atlanta, GA, December 10, 2007.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all colleagues and centers of the AMLCG who have contributed to this study either by diagnosing or treating patients included in this study.
This work was supported in part by research funding from Amgen (Munich, Germany).
Authorship
Contribution: J.B. designed research, analyzed and interpreted data, and wrote the manuscript; K.S. and W.H. analyzed and interpreted data and wrote the manuscript; P.S., A.G., A.R., R.P., D.O., C. Schmid, X.S., M.H., M.F., W.K., C.B., S.B., and H.B. collected data; B.W. and H.S. collected data and analyzed and interpreted data; W.-D.L. and D.W.B. analyzed and interpreted data; C. Sauerland, M.U., and A.H. performed statistical analysis; and W.E.B. and T.B. designed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan Braess, Department of Internal Medicine III, Laboratory for Leukemia Diagnostics, Klinikum Grosshadern, Ludwig-Maximilians University, 81377 Munich, Germany; e-mail: jan.braess@med.uni-muenchen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal