In this issue of Blood, Zhang and colleagues show evidence that many thrombocytopenic patients infected with HCV have autoantibodies specific for a defined peptide sequence in platelet membrane glycoprotein IIIa. These autoantibodies appear to be the result of an immune response mounted against one or more peptides present in the HCV core envelope protein 1.
Immune thrombocytopenia is a common complication of infection with the human immunodeficiency (HIV)1 and hepatitis C (HCV)2 viruses. In previous works, the authors showed that many patients with HIV-associated thrombocytopenia have autoantibodies that recognize a restricted peptide sequence (GPIIIa49-66) in platelet membrane glycoprotein IIIa (GPIIIa) and can be recovered from patient plasma in the form of immune complexes consisting of autoantibody and platelet fragments.3 A unique feature of these antibodies is their ability to induce reactive oxygen species through activation of 12-lipoxygenase and NADPH oxidase, leading to complement-independent platelet fragmentation.4
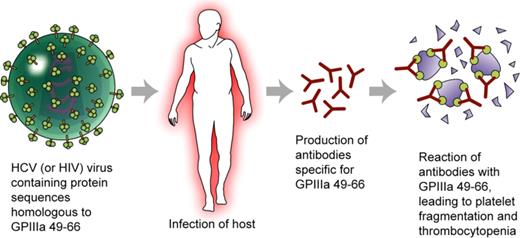
HCV (and HIV) contain proteins with peptide sequences homologous to GPIIIa49-66. Infection with either (or both) of these viruses leads to presentation of these peptides to the host immune system and production of antibodies specific for GPIIIa49-66 by some individuals. The antibodies react with GPIIIa on autologous platelets, leading to platelet fragmentation and thrombocytopenia. Defective immune surveillance associated with HIV infection may explain breakage of immune tolerance to the autologous peptides. Professional illustration by Kenneth X. Probst.
HCV (and HIV) contain proteins with peptide sequences homologous to GPIIIa49-66. Infection with either (or both) of these viruses leads to presentation of these peptides to the host immune system and production of antibodies specific for GPIIIa49-66 by some individuals. The antibodies react with GPIIIa on autologous platelets, leading to platelet fragmentation and thrombocytopenia. Defective immune surveillance associated with HIV infection may explain breakage of immune tolerance to the autologous peptides. Professional illustration by Kenneth X. Probst.
In the new work, Zhang et al used a GP49-66–specific antibody to screen a phage-expression peptide library in order to identify peptides that mimic the antibody binding site and might share homology with HCV proteins.5 It was found that sera from patients coinfected with HCV and HIV reacted with 4 peptides present in nonconserved regions of the HCV core envelope 1 protein. Antibodies raised against one of these peptides (PHC09) caused severe thrombocytopenia when injected into wild-type mice whose GPIIIa is more than 80% identical to that of humans. Moreover, patient IgG eluted from the PHCO9 peptide caused oxidative fragmentation of human platelets comparable to that induced by antibodies from patients infected with HIV. Immunization of wild-type mice with HCV core envelope protein 1 had no effect on platelet count. However, NZB/W F1 mice, a strain in which immune surveillance is defective, produced antibodies specific for PHC09 and became thrombocytopenic. The titer of PHC09-specific antibody in patients coinfected with HCV and HIV correlated with both the incidence of thrombocytopenia and its severity.
The authors conclude that humans and immunodeficient mice immunized with HCV core envelope protein 1 often produce antibodies that recognize GPIIIa49-66 through molecular mimicry and are capable of causing clinically significant thrombocytopenia.
These remarkable observations provide further evidence for the role of molecular mimicry in the pathogenesis of autoimmune thrombocytopenia in patients infected with HCV. The new observations complement similar findings made previously in patients with HIV-associated immune thrombocytopenia3,4 and may have implications for various autoimmune conditions described in patients infected with HCV,6 other viruses,7 and bacteria.8,9
It seems extraordinary that that both HCV (a member of the flavivirus family) and HIV-1 (a retrovirus) each contain structural elements that mimic peptide sequences in the same region of GPIIIa and that both induce GPIIIa-specific antibodies capable of causing thrombocytopenia. As the authors note, mimicry of host proteins is one mechanism by which viruses escape host surveillance. Location of the epitopes identified in this study in nonconserved regions of the 2 viruses is consistent with the possibility that they evolved as part of this process.
A second and quite unexpected property of antibodies specific for GP49-66 is their ability to induced oxidative platelet fragmentation in vitro (and presumably in vivo). GPIIb/IIIa is by far the most immunogenic of platelet proteins and a large number of auto-, allo-, and drug-dependent antibodies reactive with this target have been studied. Yet, no others have been shown to evoke this type of platelet response. Peterson et al previously studied a group of IgG antibodies from patients with quinine-induced immune thrombocytopenia that recognize the very peptide sequence (GPIIIa49-66) identified by Zhang et al as a target for HCV and HIV autoantibodies.10,11 Yet the quinine-dependent antibodies appear not to be capable of inducing platelet fragmentation. Why the autoantibodies identified by Zhang et al have this unusual property is not fully understood.12 Further studies of this process at a molecular level could lead to characterization of a new mechanism for immune-mediated cytopenia.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal