Abstract
CD19 and CD20 are B cell–specific antigens whose expression is heterogeneous when analyzed by flow cytometry (FCM). We determined the association between CD20 expression and clinical outcome in patients with diffuse large B-cell lymphoma (DLBCL). The mean fluorescence intensity of CD20 and CD19 was determined by FCM, and the cytoplasmic expression of CD20 was determined by immunohistochemistry (IHC) on 272 diagnostic DLBCL samples. Exon 5 of the MS4A1 gene coding for the extracellular component of the CD20 antigen was sequenced in 15 samples. A total of 43 of 272 (16%) samples had reduced CD20 expression by FCM; of these, 35 (13%) had bright CD19 expression. The latter had a markedly inferior survival when treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or rituximab-CHOP (R-CHOP; median survival of 1.2 and 3.0 years vs not reached for the others, P < .001 and P = .001), independent of the International Prognostic Index. A total of 41 of 43 samples with reduced CD20 expression by FCM had strong staining for CD20 by IHC. There were no mutations in exon 5 of the MS4A1 gene to explain the discrepancy between FCM and IHC. CD20 and CD19 expression by FCM should be determined on all biopsies of patients with DLBCL because reduced CD20 expression cannot be reliably detected by IHC.
Introduction
Diffuse large B-cell lymphoma (DLBCL) represents 40% of the non-Hodgkin lymphomas and expresses the classic B-cell markers found on normal B lymphocytes, that is, CD19, CD20, and CD79a.1 The CD20 antigen is a membrane-bound protein that is thought to play a role in B-cell activation, differentiation, and cell-cycle progression.2,3 Rituximab (R) is a monoclonal antibody directed against the CD20 antigen, and its addition to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) has dramatically improved the survival of patients with DLBCL.4,5 However, not all patients are cured by this primary therapy, and insight into the mechanisms of treatment failure may guide the development of better therapy in the future.
CD20 protein expression, as determined by flow cytometry (FCM), is very heterogeneous between and within different lymphoma subtypes.6 For instance, CD20 expression in small lymphocytic lymphoma (SLL)/chronic lymphocytic leukemia (CLL) is usually lower (dim CD20) than in follicular lymphoma (FL), and this difference may correlate with clinical responses to rituximab.6 In the pivotal trial conducted by McLaughlin et al,7 only 13% of patients with SLL/CLL compared with 60% of patients with FL (P < .01) responded to rituximab. Olejniczak et al found that CD20 expression in DLBCL also showed marked variability and that some samples had “dim” CD20, similar to that of SLL/CLL.6 We hypothesized that such patients would have an inferior response to R-CHOP compared with patients with “bright” CD20 expression on their lymphoma cells. The goal of this study was to determine the frequency of reduced (dim) CD20 expression relative to CD19 expression in DLBCL samples at diagnosis and to correlate this finding with clinical outcome in patients treated with CHOP with or without rituximab. Furthermore, we compare CD20 protein expression by FCM to CD20 expression determined by immunohistochemistry (IHC).
Methods
Patient selection
Patients with de novo DLBCL, diagnosed by experienced hematopathologists (R.D.G., M.C.) according to the World Health Organization criteria who had FCM analysis performed on their diagnostic biopsies between 1997 and 2007 were included in this study.1 Patients were older than 18 years, HIV-negative, and treated with curative intent with CHOP with or without rituximab. Their baseline clinical characteristics, including the international prognostic index (IPI) variables, pathology of their staging bone marrow, and clinical outcomes were recorded. All patients treated with CHOP-R at the British Columbia Cancer Agency were required to have CD20+ DLBCL by IHC. Ethical approval to conduct this retrospective review was granted by the University of British Columbia–British Columbia Cancer Agency Research Ethics Board, and informed consent was obtained in accordance with the Declaration of Helsinki.
Monoclonal antibodies
Cell suspensions from freshly disaggregated lymph node biopsies were stained according to the manufacturer's recommendations with monoclonal antibodies conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), or PE-Cy5. The routine diagnostic panel comprised the following 7 tubes. Tube 1 contained anti-CD45–FITC, anti-CD14–PE, and anti-CD19–PE-Cy5. Tube 2 contained isotype controls IgG1-FITC, IgG1/IgG2a-PE, and IgG1–PE-Cy5. Tube 3 contained anti-CD10–FITC, anti-CD11c–PE, and anti-CD20–PE-Cy5. Tube 4 contained anti-CD5–FITC, anti-CD19–PE, and anti-CD3 PE-Cy5. Tube 5 contained anti-CD7–FITC, anti-CD4–PE, and anti-CD8–PE-Cy5. Tube 6 contained anti-FMC7–FITC, anti-CD23–PE, and anti-CD19–PE-Cy5. Tube 7 contained anti–κ-FITC, anti-λ–PE, and anti-CD19–PE-Cy5. The anti-CD20 antibody was directed against the B1 epitope, clone B9E9. All antibodies were obtained from Beckman Coulter (Fullerton, CA) except CD23, κ, λ, and CD19–PE-Cy 5 (in tube 7), which were obtained from Dako North America (Carpinteria, CA).
Cell preparation
The cell suspensions were generated by disaggregating cells from fresh tissue and suspending them in phosphate-buffered solution (Dulbecco PBS; StemCell Technologies, Vancouver, BC) to a lymphocyte concentration approximating 107/mL. Cell concentration and viability was assessed using Trypan blue exclusion dye (Invitrogen, Carlsbad, CA). A total of 500 000 live cells were stained with the appropriate antibody combinations (in “Monoclonal antibodies”) and incubated at 4°C for 30 minutes. Cells were treated with 250 μL Opti-Lyse C containing 1.5% formaldehyde (Beckman Coulter) to deplete red cells and fix the lymphocytes. The remaining cells were then washed once with IsoFlow sheath fluid (Beckman Coulter) before FCM analysis. Peripheral blood (PB) lymphocytes taken from 67 patients without lymphoma were counted using the Bayer Advia 120 hematology system cell counter and diluted in PBS to a concentration of 1 to 10 × 109/mL. A total of 500 × 106 cells were then treated using the same method as described above except that PB lymphocytes were incubated with antibody combinations at room temperature for 15 minutes.
FCM analysis
Quantitative fluorescence analysis was performed using a Beckman Coulter Cytomics FC500 equipped with a single 488-nm argon laser source. FITC/PE/PE-Cy5 emission was collected in FL1/2/4 channels using 525/575/675 nm bandpass filters, respectively. Daily instrument calibration was performed using Flow-Set/Flow-Check beads (Beckman Coulter).
We noted that the voltage settings of the cytometer were changed significantly twice between 1997 and 2007 as analysis protocols evolved in the laboratory, however, within the 3 time windows (1997-2002 [February], 2002 [March] to 2004 [November], 2004 [December] to 2007), these settings remained constant. Thus, the mean fluorescence intensity (MFI) for specific antigens in samples studied within each time window could be compared with each other.
Data analysis
List mode files were analyzed using FlowJo software, version 8.7.1 (TreeStar, Ashland, OR). A minimum of 5000 events were analyzed for all gated populations presented. Live cells were gated using forward and side scatter criteria. The MFI, variance, and SD were recorded for each cell population of interest. The samples within each of the 3 time frames (1997-2002, 2002-2004, 2004-2007) were then rank ordered by MFI. In each of the 3 time frames, a natural bimodal distribution was apparent, which allowed an MFI cutoff value to be defined, separating the samples into “dim” versus “bright” subpopulations. Staining for the T-cell marker CD3 allowed discrimination between “dim” CD20 B cells and CD20-negative T cells. The CD19 MFI distribution was also rank ordered and “dim” CD19 defined in a similar fashion as “dim” CD20. A sample was considered CD5+ if the CD19+ events (determined in tube 1) also stained positive for CD5 (CD5+). The threshold for calling a CD5+ event was determined using the CD5 fluorescent intensity of T cells, which coexpressed CD3 and CD5 in tube 4.
Immunohistochemistry
CD20 protein expression using formalin-fixed, paraffin-embedded tissue (FFPET) was assessed using routine methods of staining (Ventana Medical Systems, Tucson, AZ) with the L26 antibody (Dako North America) directed against a cytoplasmic epitope of the CD20 antigen.8 Cyclin D1 (Dako North America) staining was performed on all cases that coexpressed CD19 and CD5 by FCM.
Determination of DLBCL subtypes
A total of 69 patients had sufficient tissue available at diagnosis that a portion of the biopsy was frozen in liquid nitrogen and stored at −80°C, whereas the remaining tissue was used for FCM. A total of 200 μm of this fresh frozen tissue was sectioned in a cryostat, and total RNA was extracted using the ALL PREP kit (QIAGEN, Valencia, CA). Total RNA was reversed transcribed (one cycle) and hybridized to U133-2 Plus arrays according to the manufacturer's protocol (Affymetrix, Santa Clara, CA). CEL files were normalized using robust multichip analysis.9 Cell of origin was calculated using model scores for activated B-cell type (ABC) and germinal B-cell type (GCB) derived from the 100 gene model described by Dave et al10 and the Bayesian formula described by Wright et al.11 A subset of 61 patients had FFPET available for staining for Bcl-6 protein, MUM1, and CD10. Cell of origin was determined as GCB and non-GCB according to Hans criteria.12
Sequencing of exon 5 of the MS4A1 (CD20) gene
Fifteen samples that were considered discordant CD20 (“dim” CD20 and “bright” CD19) had sufficient remaining frozen tissue to allow extraction of DNA using the ALL PREP kit (QIAGEN). We amplified exon 5 of MS4A1 with the following PCR primers: 5′-TGTAAAACGACGGCCAGTTTGGAATTCCCTCCCAGATT-3′ and 5′-CAGGAAACAGCTATGACGGATCCAGAGTTCATGCTCA-3′; −21M13F and M13R were used as sequence tag extensions (italics) to facilitate sequencing with standardized M13 primers.13 The purified 431-bp PCR product was bidirectionally sequenced using BigDye Terminator, version 3.1 (Applied Biosystems, Foster City, CA) and an ABI 3730 XL sequencer (Applied Biosystems). The forward and reverse sequence reads were assembled together and analyzed using PolyPhred and displayed using Consed.14,15
Statistical analysis
Univariate survival analysis was performed using the log-rank test and Kaplan-Meier method (SPSS software, version 11). The Cox proportional hazard model was used to determine the relationship between survival and the known covariates in this study. The Fisher exact test was used to determine the association between CD20 expression and CD5 expression. Two-sided P values of .05 were considered significant.
Results
A total of 272 patients with newly diagnosed DLBCL had CD20 expression by FCM performed on their primary biopsy and had complete clinical information to be included in this analysis. The baseline clinical characteristics were similar in both CHOP- and R-CHOP–treated patients (Table 1). R-CHOP–treated patients had a superior overall survival (OS) than CHOP-treated patients (P = .03) over a median follow-up time of 3.2 and 6.0 years, respectively. Thus, both patient groups were analyzed separately when assessing the association of CD20 expression with clinical outcome. Each sample was reanalyzed for CD3, CD19, CD20, and FMC7 expression. We found that the tumor content across samples was variable, and contaminating T cells represented a significant portion of the cells present. The average T-cell content was 37%, and one-third of the samples had a T-cell content of more than 50%.
Patient characteristics
| Clinical characteristic . | CHOP treated, no. (%), n = 82 . | R-CHOP treated, no. (%), n = 190 . |
|---|---|---|
| Age > 60 y | 41 (50) | 107 (56) |
| Male sex | 51 (63) | 128 (67) |
| PS > 1 | 30 (37) | 66 (35) |
| LDH > normal | 46 (56) | 90 (47) |
| Extranodal sites > 1 | 14 (17) | 39 (21) |
| Stage III/I | 45 (55) | 97 (51) |
| IPI score at diagnosis | ||
| 0 | 12 (15) | 26 (14) |
| 1, 2 | 32 (40) | 91 (48) |
| 3-5 | 37 (45) | 73 (38) |
| Pathology of biopsy | ||
| DLBCL | 82 (100) | 181 (95) |
| PMBCL | 9 (5) | |
| Site | ||
| Nodal | 70 (85) | 145 (76) |
| Extranodal | 12 (15) | 45 (24) |
| Relapse or progression | 38 (46) | 55 (29) |
| Clinical characteristic . | CHOP treated, no. (%), n = 82 . | R-CHOP treated, no. (%), n = 190 . |
|---|---|---|
| Age > 60 y | 41 (50) | 107 (56) |
| Male sex | 51 (63) | 128 (67) |
| PS > 1 | 30 (37) | 66 (35) |
| LDH > normal | 46 (56) | 90 (47) |
| Extranodal sites > 1 | 14 (17) | 39 (21) |
| Stage III/I | 45 (55) | 97 (51) |
| IPI score at diagnosis | ||
| 0 | 12 (15) | 26 (14) |
| 1, 2 | 32 (40) | 91 (48) |
| 3-5 | 37 (45) | 73 (38) |
| Pathology of biopsy | ||
| DLBCL | 82 (100) | 181 (95) |
| PMBCL | 9 (5) | |
| Site | ||
| Nodal | 70 (85) | 145 (76) |
| Extranodal | 12 (15) | 45 (24) |
| Relapse or progression | 38 (46) | 55 (29) |
PS indicates Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; IPI, International Prognostic Index; DLBCL, diffuse large B-cell lymphoma; and PMBCL, primary mediastinal B-cell lymphoma.
CD20 expression by FCM is heterogeneous
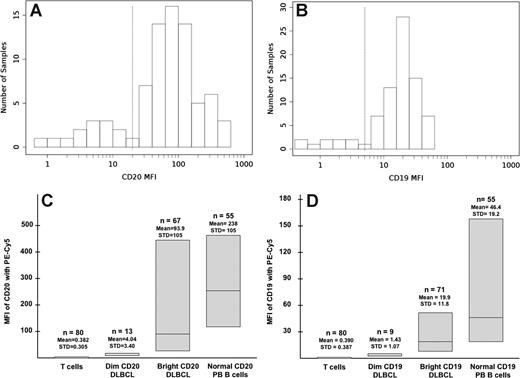
The CD20 MFI varied considerably within each of the 3 time windows during which instrument settings and laboratory protocols remained constant. This heterogeneity was very similar to that observed by Olejniczak et al who used a more sensitive quantitative assay for determining CD20 density on DLBCL.6 Figure 1A demonstrates the distribution of MFI in the DLBCL samples analyzed from 2004 to 2007. Two distinct groups could be identified based on CD20 expression. Thirteen samples (16% of the group) had a very low MFI (range, 0.85-11.57) and 67 samples had higher MFIs (range, 23.9-450). The first group was defined as having “dim” or reduced CD20 expression, whereas the remaining samples were considered “bright” because their CD20 expression was closer to that of normal PB lymphocytes, as seen in Figure 1C. The mean MFI of CD20 of these 67 samples was 93.9 compared with 238 for normal PB lymphocytes. T cells, which were present in all of the samples, served as an internal negative control and had a mean MFI of 0.38. Because of the staining and acquisition protocols used in the first 2 time windows (1997-2002 and 2002-2004), the dynamic range of CD20 expression was compressed relative to the 2004 to 2007 period. As such, a definitive “trough” could not be identified to demarcate “dim” from “bright” cases, despite the obvious presence of a “dim” subset. To define a cutoff MFI to segregate “dim” from “bright” in this situation, we made the assumption that the fraction of “dim” versus “bright” cases should be similar between the 3 time frames and arbitrarily defined the dimmest 16% of cases (ranked by MFI) to be “dim” and the rest as “bright.”
CD20 and CD19 expression in DLBCL by flow cytometry. (A) Distribution of MFI in CD20 expression in the DLBCL samples from 2004 to 2007. (B) Distribution of MFI in CD19 expression in the DLBCL samples from 2004 to 2007. (C) Heterogeneity in CD20 expression in DLBCL and normal peripheral blood lymphocytes from 2004 to 2007. From 2002 to 2004: dim CD20, mean MFI: 2.1; STD: 0.8; range: 0.55-3.91; bright CD20 mean, MFI: 17.6; STD: 19.4; range: 4.13-103. From 1997 to 2002: dim CD20, mean MFI: 1.1; STD: 0.4; range: 0.69-1.92; bright CD20, mean MFI: 14.1; STD: 18.5; range: 2.4-85.1. (D) Heterogeneity in CD19 expression in DLBCL and normal peripheral blood lymphocytes from 2004 to 2007. From 2002 to 2004: dim CD19, mean MFI: 0.44; STD: 0.11; range: 0.27-0.57; bright CD19, mean MFI: 4.79; STD: 4.28; range: 0.6-17.5. From 1997 to 2002: dim CD19, mean MFI: 0.52; STD: 0.22; range: 0.15-0.85; bright CD19, mean MFI: 6.71; STD: 6.70; range: 1.79-36.5. MFI indicates mean fluorescence intensity; DLBCL, diffuse large B-cell lymphoma; STD, standard deviation; PB, peripheral blood.
CD20 and CD19 expression in DLBCL by flow cytometry. (A) Distribution of MFI in CD20 expression in the DLBCL samples from 2004 to 2007. (B) Distribution of MFI in CD19 expression in the DLBCL samples from 2004 to 2007. (C) Heterogeneity in CD20 expression in DLBCL and normal peripheral blood lymphocytes from 2004 to 2007. From 2002 to 2004: dim CD20, mean MFI: 2.1; STD: 0.8; range: 0.55-3.91; bright CD20 mean, MFI: 17.6; STD: 19.4; range: 4.13-103. From 1997 to 2002: dim CD20, mean MFI: 1.1; STD: 0.4; range: 0.69-1.92; bright CD20, mean MFI: 14.1; STD: 18.5; range: 2.4-85.1. (D) Heterogeneity in CD19 expression in DLBCL and normal peripheral blood lymphocytes from 2004 to 2007. From 2002 to 2004: dim CD19, mean MFI: 0.44; STD: 0.11; range: 0.27-0.57; bright CD19, mean MFI: 4.79; STD: 4.28; range: 0.6-17.5. From 1997 to 2002: dim CD19, mean MFI: 0.52; STD: 0.22; range: 0.15-0.85; bright CD19, mean MFI: 6.71; STD: 6.70; range: 1.79-36.5. MFI indicates mean fluorescence intensity; DLBCL, diffuse large B-cell lymphoma; STD, standard deviation; PB, peripheral blood.
CD19 expression by FCM is heterogeneous
CD19 expression was also very heterogeneous and showed similar distribution patterns to CD20 (Figure 1B,D). This was also true for FMC7, an epitope of CD20 (data not shown).16,17 Interestingly, one sample showed at least 3 populations of CD19+ cells displaying different intensities of FMC7, suggesting that clonal populations with different CD20 expression can exist within the same tumor (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). All but one sample with “dim” CD20 expression also had “dim” expression for FMC7. However, 12% of the “dim” FMC7 samples were “bright” for CD20. Overall, 4 major groups were defined based on the pattern of their CD19 and CD20 expression (Table 2). These groups will be referred to as concordant bright, concordant dim, discordant CD19 (“dim” CD19 and “bright” CD20), and discordant CD20 (“dim” CD20 and “bright” CD19).
Incidence of DLBCL samples stratified according to CD19 and CD20 expression
| . | Bright CD20 expression, no. (%) . | Dim CD20 expression, no. (%) . |
|---|---|---|
| Bright CD19 expression | 203 (75) | 35 (13) |
| Dim CD19 expression | 26 (10) | 8 (3) |
| . | Bright CD20 expression, no. (%) . | Dim CD20 expression, no. (%) . |
|---|---|---|
| Bright CD19 expression | 203 (75) | 35 (13) |
| Dim CD19 expression | 26 (10) | 8 (3) |
CD20 expression by FCM is more sensitive than IHC
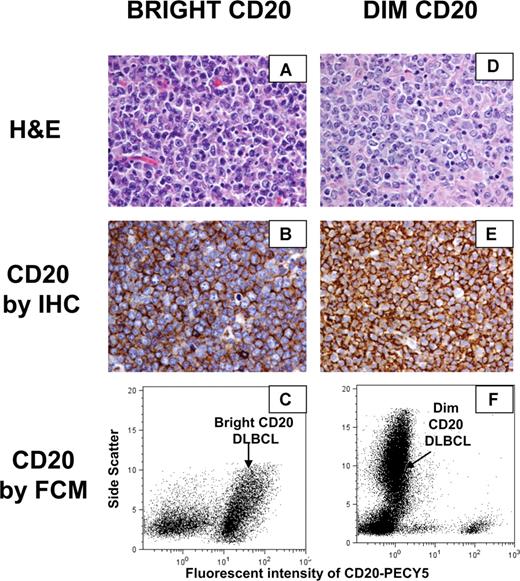
We then compared CD20 protein expression determined by FCM to that obtained by IHC. The B1 antibody used in most clinical FCM laboratories targets the same critical amino acid sequence on the extracellular CD20 epitope as rituximab, whereas the L26 antibody used routinely on FFPET targets the cytoplasmic portion of CD20.8,18 In total, 16% (n = 43) of the DLBCL samples were “dim” CD20 (including both discordant CD20 and concordant dim) by FCM, but only 3 cases were CD20− by IHC. This relative low frequency of CD20− biopsies reflects that CD20 expression by IHC was a requirement to be treated with rituximab at our institution. Thus, these 3 negative biopsies were in CHOP-treated patients only. Dots plots and histology sections of representative “dim” CD20 and “bright” CD20 samples in Figure 2 demonstrate that the one log intensity difference in CD20 expression detected by FCM could not be detected using routine IHC. Side scatter, representing internal cellular complexity, was the best parameter to distinguish the CD20− T cells from the “dim” CD20 malignant B cells.
CD20 expression by IHC and FCM of representative “dim” CD20 and “bright” CD20 DLBCL samples. (A) Representative hematoxylin and eosin stain of a “bright” CD20 DLBCL. (B) CD20 protein expression by IHC of a “bright” CD20 DLBCL. (C) CD20 expression by flow cytometry of a “bright” CD20 DLBCL. (D) Representative hematoxylin and eosin stain of a “dim” CD20 DLBCL. (E) CD20 protein expression by IHC of a “dim” CD20 DLBCL. (F) CD20 expression by flow cytometry of a “dim” CD20 DLBCL. (A,B,D,E) Slides were viewed with a Nikon Eclipse E600 microscope (Nikon Canada, Mississauga, ON) at a magnification of 400×. Images were acquired using a Nikon Digital Camera DXM1200 (Nikon Canada) and were processed with Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA).
CD20 expression by IHC and FCM of representative “dim” CD20 and “bright” CD20 DLBCL samples. (A) Representative hematoxylin and eosin stain of a “bright” CD20 DLBCL. (B) CD20 protein expression by IHC of a “bright” CD20 DLBCL. (C) CD20 expression by flow cytometry of a “bright” CD20 DLBCL. (D) Representative hematoxylin and eosin stain of a “dim” CD20 DLBCL. (E) CD20 protein expression by IHC of a “dim” CD20 DLBCL. (F) CD20 expression by flow cytometry of a “dim” CD20 DLBCL. (A,B,D,E) Slides were viewed with a Nikon Eclipse E600 microscope (Nikon Canada, Mississauga, ON) at a magnification of 400×. Images were acquired using a Nikon Digital Camera DXM1200 (Nikon Canada) and were processed with Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA).
Reduced CD20 expression is associated with an inferior survival
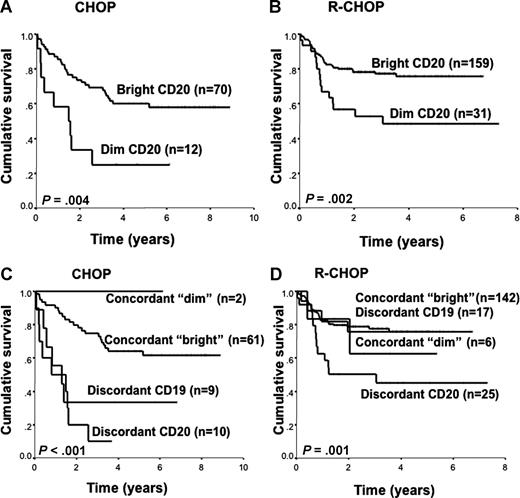
Reduced CD20 expression (“dim” CD20) in primary DLBCL was associated with a median OS of 1.2 years and 3 years for the “dim” CD20 versus median survival not reached in the “bright” CD20 group in CHOP- and R-CHOP–treated patients, respectively (Figure 3A,B). Dichotomizing the data according to CD20 and CD19 expression, we found that patients whose biopsies were discordant CD20 (ie, “dim” CD20 but “bright” CD19) had the worst OS compared with patients whose biopsies were concordant dim or concordant bright, irrespective of treatment regimen (Figure 3C,D). Interestingly, the poor prognostic effect of discordant CD20 was also seen in the CHOP-treated patients, suggesting that CD20 expression correlates with the cellular biology of the malignant lymphocytes and that the CD20 antigen is important beyond merely serving as a rituximab target. Indeed, 8 of 10 (87%) and 22 of 35 (63%) of patients with discordant CD20 eventually relapsed after CHOP and R-CHOP, suggesting that these were very high-risk patients even when rituximab was introduced into the treatment regimen. The discordant CD19 group had a slightly inferior survival compared with the concordant bright group in CHOP-treated patients, but this nonsignificant negative prognostic effect disappeared when rituximab was included in the treatment regimen. Although all the discrepant CD20 samples were also “dim” or negative for FMC7, FMC7 expression alone was not correlated with survival. Thus, in DLBCL, a reduced CD20 expression was associated with an inferior survival if CD19 expression was “bright” (discordant CD20).
Overall survival. (A,B) Patients with DLBCL according to CD20 expression. (A) CHOP treated. (B) R-CHOP treated. (C,D) Patients with DLBCL according to CD20 and CD19 expression. (C) CHOP treated. (D) R-CHOP treated.
Overall survival. (A,B) Patients with DLBCL according to CD20 expression. (A) CHOP treated. (B) R-CHOP treated. (C,D) Patients with DLBCL according to CD20 and CD19 expression. (C) CHOP treated. (D) R-CHOP treated.
Discordant CD20 expression is associated with CD5 expression and Bcl-2 expression
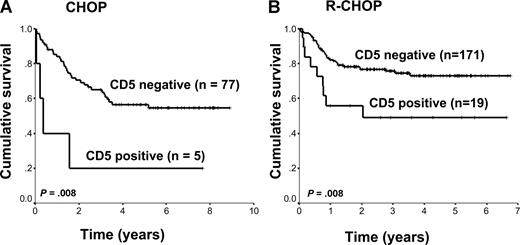
The clinical and pathologic characteristics of patients whose biopsies had discordant CD20 expression were slightly different from the other groups. These patients tended to present more often with advanced-stage disease and higher IPI scores. In addition, 11 (31%) of these patients had biopsies that showed coexpression of CD19 and CD5 (CD5+). Importantly, these were not patients with “Richter transformation” because their staging bone marrow biopsies did not contain CLL, nor were they cases of misdiagnosed mantle cell lymphomas because the biopsies were all negative for cyclin D1.19 Given that CD5+ DLBCL has been previously shown to be associated with an inferior survival in CHOP- and more recently in R-CHOP–treated patients, we determined the association of CD5 coexpression on B cells with clinical outcome.20,21 Indeed, CD5+ DLBCL was associated with an inferior survival in both CHOP- and R-CHOP–treated patients (P = .008 and P = .008, respectively; Figure 4). Similar to the discordant CD20 group, these patients also presented with advanced-stage disease and higher IPI scores, but unlike previous reports, this was predominantly seen in older men. However, 13 of 24 (54%) biopsies that were CD5+ were also discordant for CD20. Thus, CD5+ DLBCL is highly associated with reduced CD20 expression (P < .001).
Overall survival of patients with DLBCL according to CD5 expression. (A) CHOP treated. (B) R-CHOP treated.
Overall survival of patients with DLBCL according to CD5 expression. (A) CHOP treated. (B) R-CHOP treated.
Eighty-one percent and 83% of biopsies in the discordant CD20 and CD5+ groups were also positive for Bcl-2 protein, respectively, which, as expected, correlated with a significantly inferior survival in CHOP- but not R-CHOP–treated patients (P = .01 and P = .9, respectively). Other parameters, such as CD10 expression and CD4/CD8 ratio, were not associated with OS or discordant CD20 status.
Discordant CD20 expression remains a predictor of outcome on multivariate analysis
When CD5 status, discordant CD20, and IPI were included as covariates in a Cox regression analysis in R-CHOP–treated patients, only IPI and discordant CD20 remained as statistically significant predictors of OS (IPI, P = .007; discordant CD20, P = .002). Thus, the negative prognostic effect of CD5+ appears to result from its association with reduced CD20 expression and high-risk clinical features.
Reduced CD20 expression is not caused by mutations in exon 5 of the MS4A1 gene
To explain the discrepancy between dim CD20 by FCM and bright CD20 by IHC observed in 94% of the discordant CD20 samples, we sequenced exon 5 of the MS4A1 gene, which codes for the extracellular loop of the CD20 protein. Mutations at the critical ANPS and YCYSI motifs at amino acids 170 to 173 and 182 to 185 have, in previous in vitro studies, sufficiently altered the quaternary structure of CD20 to affect the binding affinity of B1 and other CD20 antibodies.22 In this study, 15 of the discordant CD20 cases were successfully sequenced, and no mutations were detected. DLBCL subtype defined by gene expression profiling has been shown to be associated with OS in CHOP and more recently R-CHOP–treated patients.23-25 Thus, we determined whether there was an association between discordant CD20 and cell of origin in 18 discordant CD20 biopsies and 13 CD5+ biopsies. We found a similar proportion of GCB and ABC subtypes in the CD5+ group but a relatively high proportion of the ABC subtype in the discordant CD20 group (12 of 18; Table 3). Thus, cell of origin may be a confounding factor in the prognostic effect of discordant CD20 expression.
Clinical and pathologic characteristics of patients with DLBCL biopsies having discordant CD20 or CD5 expression
| Variable . | Discordant CD20, no. (%), n = 35 . | CD5 expression, no. (%), n = 24 . |
|---|---|---|
| Age > 60 y | 22 (63) | 14 (58) |
| Male sex | 26 (74) | 16 (64) |
| PS > 1 | 16 (45) | 11 (46) |
| LDH > normal | 24 (69) | 15 (62) |
| Extranodal sites > 1 | 10 (29) | 6 (25) |
| Stage III/IV | 32 (91) | 14 (60) |
| IPI score at diagnosis | ||
| 0 | 3 (9) | 2 (8) |
| 1, 2 | 9 (26) | 9 (38) |
| 3-5 | 23 (65) | 13 (54) |
| CD5 expression | 11 (31) | 24 (100) |
| Discordant CD20 by FCM | 35 (100) | 13 (54) |
| Bright CD20 expression by IHC | 33 (94) | 24 (100) |
| BCL2 protein expression | 28 (81) | 20 (83) |
| DLBCL subtype (cell of origin) | ||
| GCB | 6 (17) | 5 (21) |
| Non-GCB, ABC, or unclassifiable | 12 (34) | 8 (33) |
| Not available | 17 (49) | 11 (46) |
| Relapse or progression | 22 (63) | 12 (50) |
| Variable . | Discordant CD20, no. (%), n = 35 . | CD5 expression, no. (%), n = 24 . |
|---|---|---|
| Age > 60 y | 22 (63) | 14 (58) |
| Male sex | 26 (74) | 16 (64) |
| PS > 1 | 16 (45) | 11 (46) |
| LDH > normal | 24 (69) | 15 (62) |
| Extranodal sites > 1 | 10 (29) | 6 (25) |
| Stage III/IV | 32 (91) | 14 (60) |
| IPI score at diagnosis | ||
| 0 | 3 (9) | 2 (8) |
| 1, 2 | 9 (26) | 9 (38) |
| 3-5 | 23 (65) | 13 (54) |
| CD5 expression | 11 (31) | 24 (100) |
| Discordant CD20 by FCM | 35 (100) | 13 (54) |
| Bright CD20 expression by IHC | 33 (94) | 24 (100) |
| BCL2 protein expression | 28 (81) | 20 (83) |
| DLBCL subtype (cell of origin) | ||
| GCB | 6 (17) | 5 (21) |
| Non-GCB, ABC, or unclassifiable | 12 (34) | 8 (33) |
| Not available | 17 (49) | 11 (46) |
| Relapse or progression | 22 (63) | 12 (50) |
PS indicates Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; IPI, International Prognostic Index; FCM, flow cytometry; IHC, immunohistochemistry; DLBCL, diffuse large B-cell lymphoma; GCB, germinal B-cell type; ABC, activated B-cell type; and Discordant CD20, reduced CD20 but bright CD19 expression by flow cytometry.
Discussion
We show that CD20 expression in DLBCL is heterogeneous and that at least 16% of cases (3% concordant dim and 13% discordant CD20) have reduced levels of CD20 similar to what is observed in a sizable proportion of cases of SLL/CLL. The prognostic significance of CD20 expression is contentious in other lymphoma subtypes and, to our knowledge, has never been specifically examined in DLBCL.6,26-29 We demonstrate that patients who have reduced CD20 expression but bright CD19 expression (discordant CD20) on their biopsies taken at diagnosis have a markedly inferior OS after treatment with CHOP with or without rituximab, independent of the IPI.
Quantitative measurements of fluorescence intensity using microbead standards would be considered the “gold standard” in determining the number of antigens on specific cell populations of interest.30 Recently, 3 such assays were tested in CLL and they were found to be very reproducible at assessing quantitative CD20 antigen expression.31 We did not use such methods to assess antigen expression in our study, possibly accounting for some of the variability we encountered. However, our FCM data were accrued on a single instrument with constant configuration for the entire cohort of samples included in this study and with only 2 significant alterations in voltage settings over a 10-year period. All other instrument parameters were held constant for the entire decade, thus allowing the analysis of hundreds of samples on a consistent instrument platform. The results of this study provide sufficient evidence that the use of FCM with proper calibration standards should be used to study more B-cell neoplasms, including SLL/CLL, whenever patients are candidates for anti-CD20 immunotherapy.
Importantly, the immunofluorescence assay by FCM used in our study was more sensitive at detecting differences in CD20 antigen expression than IHC. Indeed, IHC missed 41 of 43 of the dim CD20 cases. L26 staining is not usually graded by pathologists. The original report by Mason et al recommended that hematopathologists report all lymphomas that react with antibody L26 as “CD20 positive” regardless of intensity.8 However, the intensity of CD20 staining by IHC in most of our “dim” cases could not be distinguished from our “bright” cases. The dynamic resolution of IHC is too low to detect this difference. Other alternative explanations for the discordance between FCM and IHC could be a conformational change in the extracellular domain prohibiting proper binding of the B1 antibody to its epitope. For example, interleukin-4 may induce a conformational change in CD20 to prevent one but not other antibodies from binding to their extracellular epitopes.32 Importantly, we have shown that mutations in exon 5 of the MS4A1 gene that codes for the extracellular domain of the CD20 protein do not explain discrepancy between FCM and IHC. PCR-based direct sequencing would not, however, detect complete loss of one copy of the gene, nor would it detect loss of exon 5. Methylation of the promoter of the gene causing a decrease in its transcription would also not be detected by this technique.
Discordant CD20 is not synonymous with “dim” or negative FMC7. Negative or “dim” FMC7 was more common and, unlike discordant CD20, was not predictive of OS. In a study by Hübl et al investigating CD20 and FMC7 intensity in various lymphomas, 2 of 11 (19%) of their “aggressive” lymphoma samples (mainly DLBCL) were FMC7− and CD20+, which is in agreement with our results.33 In addition, they and others found that the correlation between CD20 and FMC7 is the lowest in CLL and that little additional information is gained using FMC7 if the intensity of CD20 expression is considered.33,34 Interestingly, Polyak et al found that FMC7 may be an indicator of membrane cholesterol content as cholesterol depletion markedly diminishes the expression of FMC7.17,35 Thus, samples with discordant CD20 may represent a form of DLBCL that has an altered membrane cholesterol metabolism.
Reduced levels of membrane CD20 could be associated with other confounding factors that were not measured in this study. In CLL, CD20 expression was recently shown to be associated with specific cytogenetic alterations and clinical outcome.36 For instance, trisomy 12 was associated with a high CD20 expression and the best response to rituximab, whereas 11q deletions were associated with the lowest CD20 expression and the worst responses to rituximab.36 Although we cannot exclude 11q deletions as a potential cause of discordant CD20, these genetic events are too infrequent in DLBCL to be the sole explanation for the relatively high incidence of discordant CD20 observed in our study.37 Interestingly, the majority of our discordant CD20 and CD5+ samples had positive staining for Bcl-2 protein, suggesting that inhibition of apoptosis may be involved in these cases. These results are in agreement with the recent study looking at the outcome of CD5+ DLBCL where 90% of CD5+ biopsies were also Bcl-2 protein positive.21 Although CD5+ is associated with clinical outcome, our Cox regression analysis suggests that discordant CD20 or high-risk clinical features, not CD5+, is the main contributor of the negative prognostic effect of CD5+ DLBCL. Another possibility is that discordant CD20 may be surrogate marker for cells that are “frozen” at a different stage of differentiation reflected by a slightly higher proportion of ABC subtypes in the discordant CD20 group.
Discordant CD20 appears to be a marker for a more aggressive DLBCL biology given its association with poor survival in CHOP only-treated patients. The B-cell receptor (BCR) is crucial to B-cell survival and signaling, and it is modulated by coreceptors, such as CD19, CD20, and CD5.38-41 CD19 and CD20 both function as calcium (Ca2+) channels, and through their interaction with the BCR, direct B-cell fate through various pathways, including activating nuclear factor-κB.41 These receptors aggregate together on lipid rafts that act to compartmentalize and stabilize BCR signaling.42,43 Recently, it was shown that lymphoma cells are dependent on Ca2+ entry into the cell to be killed by rituximab and that the Ca2+ influx by CD20 is dependent on BCR.44,45 Finally, as with FMC7, reduced surface CD20 may reflect an imbalance in cholesterol and lipid metabolism in the tumor cells. For instance, the levels of ganglioside GM1 by FCM, used frequently as a marker for lipid rafts, has recently been shown to be highly correlated with rituximab response in cell lines and primary lymphoma samples.46 Cross-linking of CD20 antigen by rituximab onto lipid rafts appears to be important in mediating rituximab induced apoptosis and complement-dependent cytotoxicity. Thus, in vitro evidence confirms an important role for CD20 and CD19 in lymphoma biology.
The results of this study appear to identify a group of high-risk patients who may be good candidates for novel targeted therapies. Indeed, 13% of patients with DLBCL had discordant CD20 on their diagnostic biopsies and the majority (63%) developed a lymphoma relapse after R-CHOP. The high proportion of discordant CD20 cases with strong Bcl-2 protein expression suggests that these tumors may be “Bcl-2 dependent” and may benefit from targeted therapy with novel BH3 mimetics that bind to and inhibit Bcl-2 family proteins.47 Another approach may be to use newer generations of anti-CD20 monoclonal antibodies that may be more active in lymphomas with a low CD20 density. These fully humanized antibodies appear to be more effective than rituximab at mobilizing CD20 onto lipid rafts and activating complement-dependent cytotoxicity. These agents have already been shown to be active and safe in phase 1/2 clinical trials that have included patients with relapsed CLL.48-50 Thus, identifying patients with discordant CD20 at the time of diagnosis could be crucial as they may derive the most benefit from these novel agents. Furthermore, FCM is considered routine in many clinical laboratories; thus, the recognition of these patients is already possible with currently available data.
In conclusion, we have demonstrated that discordant CD20 expression by FCM using diagnostic DLBCL biopsies may be a novel biomarker that could identify a subgroup of high-risk patients treated with R-CHOP. Moreover, this biomarker could be identified using flow cytometry, a technique that is already used in most clinical laboratories. More sensitive methods of quantifying CD19 and CD20 expression should be studied further to determine their association with outcome in different lymphoma subtypes. Studies to explore the basis of the interpatient heterogeneity in expression, for example, by assessing the methylation status of the gene, are also warranted. Currently, CD20+ staining by IHC, not FCM, is one of the criteria for inclusion into clinical trials investigating the activity of novel anti-CD20 agents. Determination of CD20 and CD19 expression by FCM may be very helpful in these patients because it would allow more efficient investigation of novel anti-CD20 agents that may be able to overcome the negative prognostic effect of CD20 discordance. If so, we may reduce lymphoma relapses because of discordant CD20 by identifying high-risk patients early and treating them with more effective first line therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Diponkar Banerjee for his helpful discussions, which prompted us to investigate “dim” CD20 as a possible cause of R-CHOP resistance, as well as the dedicated clinical flow cytometry laboratory technologists at the British Columbia Cancer Agency for the hard work.
This work was in part supported by a research grant from Roche Canada. N.A.J. is a research fellow of the Terry Fox Foundation through an award from the National Cancer Institute of Canada (019005) and the Michael Smith Foundation for Health Research (ST-PDF-01793). She was previously supported by a training award from the Canadian Institute of Health Research (STP-53912) during which part of this work was performed. J.M.C. and R.D.G. received support through the National Cancer Institute of Canada, Terry Fox Foundation Program Project (grant 016003). A.B., R.R.B., and A.P.W. are supported by the Michael Smith Foundation for Health Research. A.B. is also supported by Natural Sciences and Engineering Research Council of Canada. A.P.W. is also supported by the US National Cancer Institute (National Institutes of Health). A.B.-W. is a Senior Scholar of the Michael Smith Foundation for Health Research.
National Institutes of Health
Authorship
Contribution: N.A.J. designed and performed the research, analyzed the data, and wrote the manuscript; M.B., A.B., R.R.B., S.L., and A.B.-W. contributed to data analysis and reviewed the manuscript; M.C. contributed to pathologic review; L.H.S. and J.M.C. contributed clinical data collection and helped with editing and revising the manuscript; A.P.W. contributed with the design of the research and helped with editing and revising of the manuscript; and R.D.G. contributed with the design of the research, pathologic review of the tissue, editing and revision and final review of the manuscript.
Conflict-of-interest disclosure: R.D.G., L.H.S., and J.M.C. have received research funding from Roche Canada. The remaining authors declare no competing financial interests.
Correspondence: Randy D. Gascoyne, Department of Pathology, British Columbia Cancer Agency, 600 W 10th Ave, Vancouver, BC V5Z 4E6, Canada; e-mail: rgascoyn@bccancer.bc.ca.