Abstract
Localization of primed T cells to antigenic tissue is essential for the development of effective immunity. Together with tissue-selective homing molecules, T-cell receptor (TCR)– and CD28-mediated signals have been shown to promote transendothelial migration of specific T cells into nonlymphoid antigen-rich tissue. However, the cellular and molecular requirements for T-cell accumulation to target tissue following their recruitment are largely undefined. The guanine nucleotide exchange factor (GEF) Vav1 has an integral role in coupling TCR and CD28 to signaling pathways that regulate T-cell activation and migration. Here, we have investigated the contribution of TCR- and CD28-induced Vav1 activity to the trafficking and localization of primed HY-specific CD4+ T cells to antigenic sites. Severe migratory defects displayed by Vav1−/− T cells in vitro were fully compensated by a combination of shear flow and chemokines, leading to normal recruitment of Vav1−/− T cells in vivo. In contrast, Vav1−/− T-cell retention into antigen-rich tissue was severely impaired, reflecting T cells' inability to engage in sustained TCR- and CD28-mediated interactions with tissue-resident antigen-presenting cells (APCs). This novel function of APC-induced, and TCR- and CD28-mediated Vav1 activity in the regulation of effector T-cell immunity highlights its potential as a therapeutic target in T cell–mediated tissue damage.
Introduction
Following priming, specific T cells need to migrate and reside into antigenic sites where they are further reactivated and carry out their effector functions. Primed T-cell migration to nonlymphoid antigenic tissues is orchestrated by the expression of tissue-selective homing receptors by T cells, which engage tissue-specific endothelial cell (EC) ligands.1 T-cell recruitment to target tissue is also induced by cognate recognition of antigen presented by EC surface major histocompatibility complex (MHC)2-5 and by CD28 triggering6 both in vitro and in vivo. Cognate recognition of resident conventional antigen-presenting cells (APCs) has been suggested to promote the selective accumulation of specific T cells into target tissue by delivering stop signals and preventing them from leaving the tissue.7,8 The molecular mechanisms underlying the effects of T-cell receptor (TCR) and CD28 triggering on T-cell migration and retention are at present only partially characterized,5 but they probably involve pathways conveying TCR and costimulatory receptor signaling to the molecules that regulate adhesion and/or cytoskeletal rearrangements
Vav1 is a 95-kDa guanine nucleotide exchange factor (GEF) for Rho GTPases, which is present in cells of all hematopoietic lineages, including T cells. Vav1 has been found to have an important role in T-cell development9-13 and proliferation, interleukin-2 (IL-2) production, and Ca2+ flux induction.12,14
In addition, Vav1 regulates the cytoskeletal rearrangements that are necessary for T-cell migration. For example, Vav1 controls integrin-mediated adhesion of thymocytes to extracellular-matrix proteins.15,16 Vav1 has also been implicated in CXC-chemokine ligand 12 (CXCL12)–driven chemotaxis of T cells.17,18 The possibility that Vav1 activity mediates the TCR- and CD28-induced signaling pathways that mediate T-cell motility has been explored only partially.15,16
The involvement of Vav1-mediated signals in the regulation of T-cell localization to target tissue could explain recent findings showing that in experimental autoimmune encephalomyelitis,19 T cells from Vav1−/− mice were significantly less able to infiltrate the brain compared with their wild-type (WT) counterparts despite being activated; this led to decreased disease penetrance. Similarly, Vav1−/− recipients of heart allografts displayed diminished graft infiltration by T cells, and this was associated with reduced rejection of the transplant.20
Based on this evidence, we have examined the contribution by Vav1-mediated signals to the constitutive, inflammation-induced and TCR/CD28-dependent primed T-cell recruitment and accumulation into antigenic tissue.
Methods
Mice
Male and female 129sv mice aged 7 to 9 weeks were purchased from Olac (Bicester, United Kingdom). Vav1−/− mice were previously described.11 Procedures were carried out in accordance with the Home Office authority Act (1986) and were approved by the Imperial College London institutional review board.
Reagents, monoclonal antibodies, and intravital dyes
The HY Dby peptide21 was a gift from D. Scott (Imperial College London). Mouse IFNγ was purchased from PeproTech (London, United Kingdom). Golgi-plug was purchased from BD Pharmingen (Oxford, United Kingdom).
Antimouse CD4 was obtained from Caltag Laboratories (Burlingame, CA). Anti–mouse CD69, CD25, and CD62L were purchased from Cambridge Biosciences (Cambridge, United Kingdom). All the other antibodies were purchased from BD Biosciences (Oxford, United Kingdom). The cell linker PKH26 and CFSE were purchased from Sigma-Aldrich (Gillingham, United Kingdom). For labeling, the PKH26 and CFSE were added at a final concentration of 5 μM and 1 μM, respectively.
Cells
Mouse microvascular ECs were purified and cultured from mouse lung tissue as previously described.22 For functional assays the ECs were used between passages 4 to 6 and treated with 300 U/mL mouse IFNγ (PeproTech) for 72 hours to induce MHC class II expression (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) prior to use in experiments.
CD4+ WT and Vav1−/− T cells specific for the male-specific minor histocompatibility antigen HY epitope Dby in the context of H2-Ab were obtained by 2 fortnightly intraperitoneal immunizations of female Vav1−/− mice or WT littermates with splenocytes (5 × 108/mouse) from WT male littermates. Cells were maintained in vitro by fortnightly restimulation with irradiated (60 Gy) male splenocytes (50 × 106 splenocytes per 5 × 106 T cells) and 20 U/mL rIL2 (Roche, Welwyn Garden City, United Kingdom) in T-cell medium (RPMI 1640 medium supplemented with 10% FCS [fetal calf serum], 2 mM glutamine, 50 IU/mL penicillin, 50 μg/mL streptomycin, 10 mM HEPES, and 50 mM 2-mercaptoethanol). T-cell specificity was determined by 3HTdR incorporation and IFNγ production following recognition of Dby peptide–pulsed female-derived splenocytes and ECs (Figure S2). T cells were used 2 weeks after stimulation, following isolation on a Ficoll-Paque gradient and incubation in medium alone overnight. The phenotype of T cells at the time of injection and following activation is shown in Figures S2 through S4.
BM-derived dendritic cells (DCs) were obtained by flushing femurs from 7- to 10-week-old syngeneic female mice. BM cells (5 × 106/well) were seeded in a 6-well plate (Helena Bioscience, Gateshead, United Kingdom) in RPMI 1640 medium supplemented with 10% FCS, 2 mM glutamine, 50 IU/mL penicillin, 50 μg/mL streptomycin, 50 mM 2-ME, and 8% to 16% murine GM-CSF obtained from the supernatant of the GM-CSF hybridoma (gift from A. George, Imperial College London). On days 3 and 5, fresh culture medium was added to the plates. For functional assays, DCs were matured overnight with 100 ng/mL LPS (Sigma-Aldrich) and used between 7 and 10 days after isolation.
In vitro T-cell migration assays
In adhesion assays, 96-well plates were coated with rICAM-1 (2 μg/mL; R&D Systems, Abingdon, United Kingdom) in Tris, pH 8.5, for 2 hours at 37°C. Control wells were incubated with PBS alone. The plate was subsequently blocked with 2.5% BSA (Sigma-Aldrich) PBS at 37°C for 1 hour, and washed with 0.5% BSA. PKH26-labeled T cells were plated at 103/well and incubated for 10 to 60 minutes. T cells were washed once and the number of adherent cells was analyzed with wide-field fluorescence microscopy. Control wells were not washed. The percentage adhesion was calculated using the following formula: (Experimental adhesion-min adhesion/Control adhesion-min adhesion) ×100.
The transwell assays were carried out using either plastic-bound rICAM-1 or EC monolayers (2 × 104 cells/well) on Transwell tissue-culture well inserts (diameter, 6.5 mm) mounted with polycarbonate membranes with a 3-μm pore size (Costar, High Wycombe, United Kingdom), as previously described.23 T cells (5 × 105/well) were added in each insert and left to migrate. The number of migrated T cells was determined by hemocytometric counting of the cells present in the well media at different time points over a 24-hour period. Results are expressed as percentage of transmigrated cells.
In time-lapse microscopy migration assays, 35-mm dishes were coated with rICAM-1 in PBS and incubated at 4°C overnight. The plate was subsequently blocked with PBS containing 2.5% BSA at 37°C for 1 hour and washed with 0.5% BSA/PBS. T cells were serum-starved in RPMI 2% FCS medium for 2 hours, seeded on the rICAM-1–coated dishes at a concentration of 1 × 106/mL per dish, and incubated at 37°C for 5 to 30 minutes. Plates were washed once to remove nonadherent cells. T-cell migration was observed by time-lapse microscopy using Tempus software (Kinetic Imaging, Nottingham, United Kingdom). Images were acquired with a KPM1E/K-S10 CCD camera (Hitachi Denshi, Japan) using Kinetic Imaging software (Andor Technology, Belfast, United Kingdom) every 15 to 30 seconds for 25 to 50 minutes. The path of each cell was tracked for the whole of the time-lapse sequence using Tempus Meteor software (Andor Technology). Analysis of migration speed was then carried out using Mathematica 6.0 (Wolfram Research Institute, Long Hanborough, United Kingdom) notebooks.
In chemotaxis assays, T cells were seeded (5-10 × 105/well) in the upper chamber of a 5-μm pore polycarbonate Transwell. A 0.5-mL volume of chemotaxis medium (RPMI 2% FCS) containing either CXCL10 (300 ng/mL; PeproTech) or CXCL12 (50 ng/mL; PeproTech) was added to the bottom chamber, while 0.2 mL cell suspension was added to the top chamber. Transwells were incubated for 6 hours at 37°C with 5% CO2. The number of migrated cells was evaluated by hemocytometric counting.
Flow chamber assays
Male pulmonary ECs were grown to confluence in Nunc Slide Flaskettes (9 cm2; Nalge Nunc International, Roskilde, Denmark) that were precoated with fibronectin (10 μg/mL; Sigma-Aldrich); some cultures were stimulated for 48 hours with IFNγ (300 U/mL) and in some experiments ECs were coated with CXCL10 (300 ng/mL) for 2 hours prior to the assay. The flasks were then disassembled to use the slide for rolling assays. Slides were washed twice with PBS, 0.05% Tween 20 and mounted in a parallel-plate flow chamber (channel height, 0.15 cm). The outer housing of the slide flaskette was removed and the remaining slide was mounted onto the flow chamber, which was maintained at a constant temperature of 37°C. WT and Vav1−/− T cells were perfused onto the flow chamber using a Harvard 2000 pump (Harvard Apparatus, Holliston, MA) at a fixed shear stress of 2.5 dyn/cm2. For a single experiment, a 20-mL mixture containing WT (green) and Vav1−/− (red) T cells was perfused on the flow chamber at a final density of 2.5 × 105 cells/mL. Perfusion of T cells was stopped at 17 minutes and the remaining nonadherent T cells were removed by perfusion of the T-cell medium through the flow chamber for 2 minutes. The remaining 3 mL of the WT and Vav1−/− T-cell mixture was retained to determine the exact “starting ratio” of the T-cell perfusion mixture using flow cytometry. Bound/transmigrated T cells were harvested by removing the slide from the flow chamber and treating it with trypsin/EDTA. Ratios of red and green cells were calculated using flow cytometry, which was subsequently corrected against the value of the starting ratio in the perfusion mixture.
DC/lymphocyte conjugate formation assays
These experiments were carried out following a previously established protocol.15,16 Dby peptide–pulsed (50 nM), CFSE-labeled, female-derived DCs were used at 105 DCs/condition. PKH26-labeled WT and Vav1−/− T cells were used at 2.5 × 105 cells/condition. DCs and T cells were spun at 150g for 5 minutes to increase the possibility of conjugate formation and coincubated for 2 hours at 37°C. Conjugate formation was analyzed by flow cytometry. T cells and DCs show a distinct pattern when analyzed by FSC and SSC. Larger-sized cells (DCs) were gated and analyzed for the presence of anti-CD4 APC-positive T cells. Conjugate formation was analyzed with flow cytometry and FlowJo software version 7.1.2 (TreeStar, Ashland, OR).
Activation experiments
To induce the stop signal, T cells were activated for 45 minutes with plate-bound 1 μg/mL anti-CD3 and 5 μg/mL anti-CD28, or 1 μg/mL rat IgG and 5 μg/mL hamster IgG as a control (Sigma-Aldrich), and subsequently stained for analysis. To induce CD28 signaling, the T cells were treated with a mixture of hamster antimouse CD28 (5 μg/5 × 106 cells) and rabbit anti–hamster Ig (2.5 μg/5 × 106 cells) for 30 to 45 minutes at 37°C. As a control, T cells were treated with hamster Ig (5 μg/5 × 106 cells) and rabbit anti–hamster Ig (2.5 μg/5 × 106 cells).
Wide-field fluorescence microscopy and flow cytometry
Tissues were sampled and embedded in Optimal Cutting Temperature compound (OCT; Agar Scientific, Stansted, United Kingdom), snap-frozen, and stored until analysis. Peritoneal membranes or frozen tissue sections were laid onto Polysine Microscope slides (VWR International, Lutterworth, United Kingdom), left to dry overnight, and then mounted in Vectorshield mounting medium for fluorescence with DAPI (Vector Laboratories, Peterborough, United Kingdom), to visualize the nuclei (blue fluorescence). Slides were visualized with a Coolview 12-cooled CCD camera (Photonic Science, Newbury, United Kingdom) mounted over a Zeiss Axiovert S100 microscope equipped with Metamorph software (Zeiss, Welwyn Garden City, United Kingdom). Standard epi-illuminating fluoresceine and rhodamine fluorescence filter cube and 10×/0.6 NA and 40×/0.6 NA objectives were used and 12-bit image data sets were generated. Tissue infiltration was quantified by randomly selecting 6 to 10 10×-magnified fields and assessing the number of fluorescent cells in each field, as previously described.23 Quantification of T-cell infiltrates observed by wide-field fluorescence microscopy was performed using a specifically designed software to run in the LabView (V7.1; National Instruments, Austin, TX) environment. This automatic cell-counting algorithm is based on a combination of background subtraction, multiple thresholding, and morphologic processing approaches,6 which allows for the identification of single fluorescent cells in the tissue. The number of infiltrating cells obtained were then averaged and assessed statistically. Infiltration is expressed as the mean of fluorescent cells per ×10 fields in a given experimental condition plus or minus standard error.
DC/T-cell interactions in tissues were also assessed by 3-color analysis. Cell-cell contact (yellow fluorescence) is revealed by areas of overlapping membrane between CFSE-labeled DCs (green) and PKH26-labeled T cells (red). The mean number of DC/T-cell conjugates in 6 to 10 10×-magnified tissue samples was assessed by automatic cell counting. The data shown are based on the number of DCs engaged.
The presence of labeled cells in the peritoneal lavage was analyzed by flow cytometry using a FACSCalibur (Becton Dickinson, Mountain View, CA) and FlowJo software.
Experimental models of T-cell trafficking
Details on the recirculation patterns of adoptively transferred T cells and DCs in the experimental models can be found in Figure S5.
Statistical analysis
In the in vitro experiments, comparisons between groups were made using the Student t test. All reported P values are 2-sided.
Results
Constitutive and chemokine-induced Vav1−/− T-cell migration is severely impaired in vitro, but preserved in vivo
HY(Dby)-specific Ab-restricted CD4+ memory T cells were generated by intraperitoneal immunization of Vav1−/− female mice and WT littermates with male splenocytes, as previously described.5 WT and Vav1−/− T cells displayed similar specificity, and phenotypic and functional characteristics (Figures S2Figure S3. Phenotype of HY-specific T cells at the time of injection (JPG, 71.5 KB)–S4), although functional responses, such as proliferation and interferon-γ (IFNγ) secretion by Vav1−/− T cells were reduced compared with their WT counterpart.
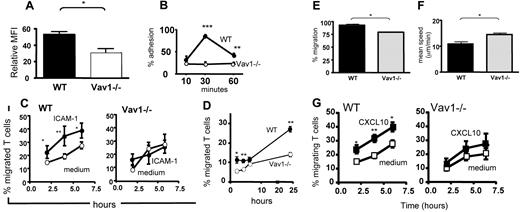
Numerous phenotypic and functional defects were revealed by in vitro experiments that analyzed the migratory ability of Vav1−/− T cells, including decreased LFA-1 expression (Figure 1A), adhesion, and migration to intracellular adhesion molecule 1 (ICAM1; Figure 1B,C,E,F) and through endothelial cells (Figure 1D), impaired de-adhesion and retraction of the uropode (Videos S1,S2), and significantly decreased response to chemokines (Figure 1G).
Motility of Vav1−/− T cells in vitro. (A) Mean expression (from at least 3 independent experiments) of LFA-1 by HY-specific CD4+ WT and Vav1−/− T cells 7 days following antigen stimulation. (B) Mean adhesion (from at least 3 independent experiments) by WT and Vav1−/− T cells to ICAM1 (2 μg/mL)–coated 96-well plates at the indicated time points. (C) Mean migration (from 4 independent experiments) by WT and Vav1−/− T cells 6 hours after plating onto ICAM1-coated transwells. (D) Mean migration by WT and Vav1−/− T cells through syngeneic female EC monolayers. Migration was measured at 2, 4, 6, and 24 hours. The percentage of migrated cells was calculated by dividing the number of cells in the bottom chamber by the total input of T cells from the mean of 3 experiments. (E,F) Migration by WT and Vav1−/− T cells plated on ICAM1-coated dishes was analyzed by time-lapse microscopy. The number of cells migrating was quantified by counting motile T cells (E). T cells were tracked using Kinetiq tracking software (Kinetiq Media, Chichester, United Kingdom) and their migratory speed (F, μm/min) was quantified using Mathematica spreadsheets. The mean percentage of motile cells and mean speed was calculated from data of 3 independent experiments. (G) WT and Vav1−/− T-cell migration in response to CXCL10 through a transwell was assessed by counting the cells in the bottom chamber at 2, 4, and 6 hours. Percentage migration was calculated by dividing the number of cells in the bottom chamber by the original number of cells plated on the transwell. The mean percentage migration from 4 independent experiments is shown. Error bars indicate standard error (*P < .05, ** P < .01, ***P < .001).
Motility of Vav1−/− T cells in vitro. (A) Mean expression (from at least 3 independent experiments) of LFA-1 by HY-specific CD4+ WT and Vav1−/− T cells 7 days following antigen stimulation. (B) Mean adhesion (from at least 3 independent experiments) by WT and Vav1−/− T cells to ICAM1 (2 μg/mL)–coated 96-well plates at the indicated time points. (C) Mean migration (from 4 independent experiments) by WT and Vav1−/− T cells 6 hours after plating onto ICAM1-coated transwells. (D) Mean migration by WT and Vav1−/− T cells through syngeneic female EC monolayers. Migration was measured at 2, 4, 6, and 24 hours. The percentage of migrated cells was calculated by dividing the number of cells in the bottom chamber by the total input of T cells from the mean of 3 experiments. (E,F) Migration by WT and Vav1−/− T cells plated on ICAM1-coated dishes was analyzed by time-lapse microscopy. The number of cells migrating was quantified by counting motile T cells (E). T cells were tracked using Kinetiq tracking software (Kinetiq Media, Chichester, United Kingdom) and their migratory speed (F, μm/min) was quantified using Mathematica spreadsheets. The mean percentage of motile cells and mean speed was calculated from data of 3 independent experiments. (G) WT and Vav1−/− T-cell migration in response to CXCL10 through a transwell was assessed by counting the cells in the bottom chamber at 2, 4, and 6 hours. Percentage migration was calculated by dividing the number of cells in the bottom chamber by the original number of cells plated on the transwell. The mean percentage migration from 4 independent experiments is shown. Error bars indicate standard error (*P < .05, ** P < .01, ***P < .001).
To establish whether the migratory defects that were observed in Vav1−/− T cells in vitro were reflected in altered constitutive trafficking in vivo, labeled HY-specific H2-Db–restricted CD4+ WT and Vav1−/− T cells (107) were injected intravenously into female WT mice and their localization to the liver, kidney, lung, and spleen was quantified 2, 6, and 24 hours after injection by wide-field fluorescence microscopy.
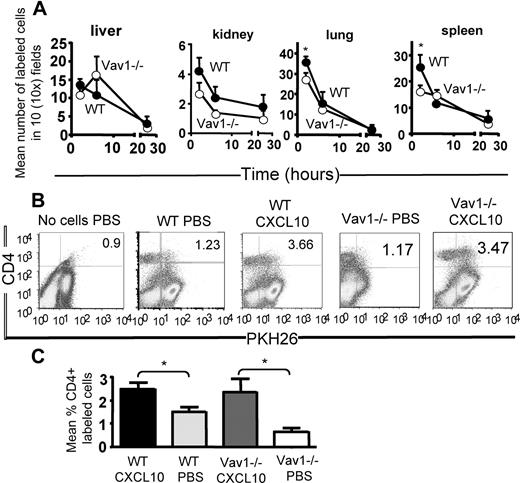
Although higher numbers of WT T cells were found in the spleen and lung of recipients 2 hours after injection, there was no significant difference in the number of WT and Vav1−/− T cells that localized in all the tissues at all the other time points, suggesting that constitutive trafficking of primed Vav1−/− T cells is not severely impaired in vivo (Figure 2A).
Constitutive and chemokine Vav1−/− effector T-cell trafficking in vivo. (A) HY-specific CD4+ WT and Vav1−/− T cells were labeled with PKH26 (red) or CFSE (green), respectively, and injected intravenously in syngeneic female mice. Trafficking into kidney, liver, lung, and spleen was monitored at 2, 6, and 24 hours after injection by harvesting, snap-freezing the tissue, and taking 5- to 10-μm sections. The mean number of cells from at least 6 tissue sections from at least 3 experiments was quantified by wide-field fluorescence microscopy, as described in “Wide-field fluorescence microscopy and flow cytometry.” (B,C) WT and Vav1−/− T cells were labeled with PKH26 and injected intravenously into syngeneic female mice that had received an intraperitoneal injection of 1.2 μg CXCL10. Some mice were also injected with PBS alone (ie, no T cells) as an autofluorescence control. Mice were killed 16 hours later, and the presence of PKH26-labeled, CD4+ T cells was analyzed by flow cytometry. Representative dot plots are shown in panel B. The mean percentage of cells present in the peritoneal lavage (calculated by subtracting the average background migration) from the percentage of migrated cells in the presence of CXCL10 is shown in panel C. Owing to the presence of an autofluorescent population of non-T cells often detected in FL-2 (also in control mice that received saline solution), cells were double-stained with an APC-conjugated anti-CD4 antibody following harvesting, and the percentage of PKH26 (FL-2)–labeled T cells gated in the CD4+ T-cell population is shown in the histogram and the graph, representing cumulative data from at least 3 animals. The mean plus or minus SEM observed in samples from at least 3 animals are shown. Error bars indicate standard error (*P < .05).
Constitutive and chemokine Vav1−/− effector T-cell trafficking in vivo. (A) HY-specific CD4+ WT and Vav1−/− T cells were labeled with PKH26 (red) or CFSE (green), respectively, and injected intravenously in syngeneic female mice. Trafficking into kidney, liver, lung, and spleen was monitored at 2, 6, and 24 hours after injection by harvesting, snap-freezing the tissue, and taking 5- to 10-μm sections. The mean number of cells from at least 6 tissue sections from at least 3 experiments was quantified by wide-field fluorescence microscopy, as described in “Wide-field fluorescence microscopy and flow cytometry.” (B,C) WT and Vav1−/− T cells were labeled with PKH26 and injected intravenously into syngeneic female mice that had received an intraperitoneal injection of 1.2 μg CXCL10. Some mice were also injected with PBS alone (ie, no T cells) as an autofluorescence control. Mice were killed 16 hours later, and the presence of PKH26-labeled, CD4+ T cells was analyzed by flow cytometry. Representative dot plots are shown in panel B. The mean percentage of cells present in the peritoneal lavage (calculated by subtracting the average background migration) from the percentage of migrated cells in the presence of CXCL10 is shown in panel C. Owing to the presence of an autofluorescent population of non-T cells often detected in FL-2 (also in control mice that received saline solution), cells were double-stained with an APC-conjugated anti-CD4 antibody following harvesting, and the percentage of PKH26 (FL-2)–labeled T cells gated in the CD4+ T-cell population is shown in the histogram and the graph, representing cumulative data from at least 3 animals. The mean plus or minus SEM observed in samples from at least 3 animals are shown. Error bars indicate standard error (*P < .05).
In parallel, the recruitment of intravenously injected labeled HY-specific CD4+ WT and Vav1−/− T cells to the peritoneal cavity in response to the inflammatory chemokine CXC-chemokine ligand 10 (CXCL10; also known as IP-10) injected intraperitoneally into syngeneic female mice was compared. In contrast to what we had observed in vitro, CXCL10 induced equal migration of WT and Vav1−/− T cells to the peritoneal cavity (Figure 2B,C). Owing to the presence of an autofluorescent population of non-T cells that is often detected in FL-2, cells were double-stained with an APC-conjugated anti-CD4 antibody following harvesting; the percentage of PKH26 (FL-2)–labeled T cells gated in the CD4+ T-cell population is shown. These results suggest that T cells lacking Vav1 activity undergo normal trafficking in response to constitutive or nonspecific inflammatory stimuli in vivo despite displaying defective migration in vitro.
Vav1−/− T cells are recruited to but not retained into antigenic tissue
Previous reports (also by our group) have shown that the TCR engagement by the antigen-presenting endothelium enhances antigen-specific T-cell migration and contributes to their recruitment to target tissue.2-5 Given that Vav1 activity is induced by TCR triggering and is involved in the regulation of cytoskeletal reorganization, we examined the possibility that Vav1−/− T cells have a defect in TCR-driven migration.
Antigen-induced migration of HY-specific CD4+ H2-Ab–restricted WT and Vav1−/− T cells (3-5 × 105) through IFNγ-treated antigenic (male) and nonantigenic (female) syngeneic EC monolayers was first compared. As expected, WT T cells showed increased migration through male-derived ECs (Figure 3A). In contrast, Vav1−/− T cells showed reduced and comparable levels of migration through both male and female ECs. Similar observations were made when the TCR-driven migration of HY-specific CD4+ H2-Ab–restricted WT and Vav1−/− T cells was examined by time-lapse microscopy (Figure 3B).
Antigen-driven Vav1−/− T-cell migration. HY-specific CD4+ WT and Vav1−/− T cells (3 × 105) were seeded onto IFNγ-treated antigenic (male) or nonantigenic (female) syngeneic EC monolayers grown on transwells. The mean percentage migration at the indicated time points from 3 experiments of similar design is shown. (B) T cells (1 × 106) were plated on 35-mm dishes coated with IFNγ-treated antigenic (male) or nonantigenic (female) ECs and allowed to migrate for 50 minutes. Pictures were taken every 30 seconds and analyzed as described in “Methods.” Transmigrating T cells were defined as changing phase (ie, turning from bright to dark once under the endothelial monolayer). Mean percentage of cells transmigrating was calculated from 3 independent experiments from a sample of 100 cells per movie. (C,D) HY-specific CD4+ WT and Vav1−/− T cells labeled with PKH26 (red) and CFSE (green), respectively, were coinjected intravenously into syngeneic male or female recipients that had previously received an intraperitoneal injection of IFNγ. Labeled T-cell enrichment of the peritoneal lavage was analyzed by flow cytometry 24 hours later (C). The mean percentage of CD4+-labeled (PKH26 or CFSE) T cells in the peritoneal lavage from at least 3 animals is shown (C right panel). Nuclei are stained by DAPI (blue). Retention of T cells into the peritoneal membrane (D) was analyzed by wide-field fluorescence microscopy as described in the legend to Figure 2. The mean number of cells in 6 tissue samples from at least 3 mice is shown (D right panel). Error bars indicate standard error (*P < .05, **P < .01).
Antigen-driven Vav1−/− T-cell migration. HY-specific CD4+ WT and Vav1−/− T cells (3 × 105) were seeded onto IFNγ-treated antigenic (male) or nonantigenic (female) syngeneic EC monolayers grown on transwells. The mean percentage migration at the indicated time points from 3 experiments of similar design is shown. (B) T cells (1 × 106) were plated on 35-mm dishes coated with IFNγ-treated antigenic (male) or nonantigenic (female) ECs and allowed to migrate for 50 minutes. Pictures were taken every 30 seconds and analyzed as described in “Methods.” Transmigrating T cells were defined as changing phase (ie, turning from bright to dark once under the endothelial monolayer). Mean percentage of cells transmigrating was calculated from 3 independent experiments from a sample of 100 cells per movie. (C,D) HY-specific CD4+ WT and Vav1−/− T cells labeled with PKH26 (red) and CFSE (green), respectively, were coinjected intravenously into syngeneic male or female recipients that had previously received an intraperitoneal injection of IFNγ. Labeled T-cell enrichment of the peritoneal lavage was analyzed by flow cytometry 24 hours later (C). The mean percentage of CD4+-labeled (PKH26 or CFSE) T cells in the peritoneal lavage from at least 3 animals is shown (C right panel). Nuclei are stained by DAPI (blue). Retention of T cells into the peritoneal membrane (D) was analyzed by wide-field fluorescence microscopy as described in the legend to Figure 2. The mean number of cells in 6 tissue samples from at least 3 mice is shown (D right panel). Error bars indicate standard error (*P < .05, **P < .01).
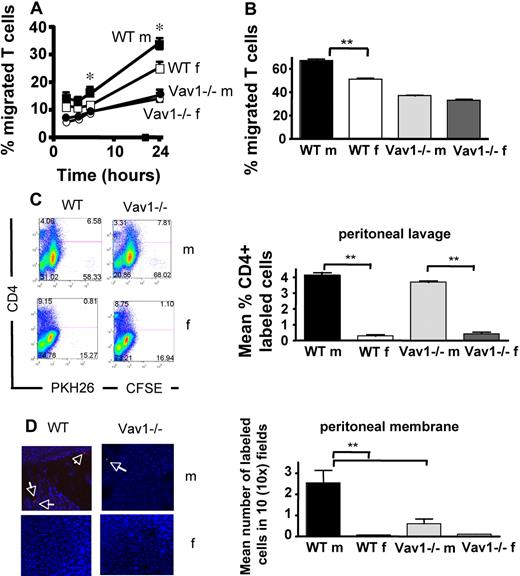
To assess whether this defect was reflected in impaired antigen-dependent T-cell recruitment in vivo, HY-specific WT (PKH26-labeled) and Vav1−/− (CFSE-labeled) T cells were coinjected intravenously in male and female mice (107/mouse) that had previously received an optimal dose of IFNγ intraperitoneally to induce local up-regulation of MHC molecules, and consequently HY antigen presentation, by the microvessels as we have previously described4,5 (Figure S6). WT T cells were recruited and retained in the membrane and some migrated to the peritoneal cavity of male but not female mice (Figure 3C,D; also Figure S5). In contrast, Vav1−/− T cells were recruited but not retained into the antigenic peritoneal tissue (Figure 3E,F), although they were readily detectable in the peritoneal cavity of male mice (Figure 3C). As expected, neither WT nor Vav1−/− T cells reached the peritoneal membrane or cavity of female mice (Figure 3C,D). These data suggest that, although HY-specific CD4+ Vav1−/− T cells can be efficiently recruited to the site of antigen presentation, they are not retained in the antigenic tissue (the peritoneal membrane).
A combination of flow and chemokines compensates for the lack of Vav1 activity during T-cell recruitment in vivo
The discrepancy between defective migration by Vav1−/− T cells in vitro and their normal recruitment in vivo suggests that additional Vav1-independent mechanisms are in place to compensate for the loss of Vav1 activity in vivo. Shear flow and chemokine-induced signals have been shown to provide essential stimuli for the recruitment of T cells in physiological settings,24 including following cognate recognition of the endothelium.2,25
To address this possibility, we used a previously described model of antigen-dependent tissue infiltration in vivo under static conditions.23 In this model, HY-specific CD4+ H2-Ab–restricted WT and Vav1−/− T cells (2 × 106) are injected intraperitoneally into syngeneic male and female mice that have previously received an optimal dose of IFNγ intraperitoneally (to induce local antigen presentation); therefore, T-cell recruitment in the peritoneal membrane occurs in the absence of shear flow (also Figure S5). HY-specific WT T cells were promptly recruited to the peritoneal membrane and depleted from the peritoneal cavity of male, but not female, mice (Figure 4A,B). In contrast, Vav1−/− T cells failed to migrate to the peritoneal membrane of antigen-expressing male mice and remained localized in the peritoneal cavity. These data suggest that the antigen-dependent cell-cell interactions leading to Vav1−/− T-cell migration are impaired in static condition in vivo.
A combination of shear flow and chemokines sustains Vav1−/− T-cell migration. (A,B) PKH26-labeled HY-specific CD4+ WT or Vav1 T cells (3 × 10b) were injected intraperitoneally in syngeneic male mice that had previously received an intraperitoneal injection of IFNγ to induce MHC class II expression and antigen presentation. The percentage of T cells remaining in the peritoneal cavity and T-cell infiltration of the peritoneal membrane were evaluated 24 hours later by flow cytometry and wide-field fluorescence microscopy, respectively, as described in the legend to Figure 2. Representative examples of peritoneal lavage dot plots and peritoneal membrane sections (×10 magnification) are shown. The mean number of cells in the peritoneal membrane and lavage from at least 4 mice are shown. Error bars indicate standard error (*P < .05, **P < .01). (C) HY-specific WT and Vav1−/− CD4+ T cells were perfused at 37°C over male-derived EC-coated slides at a fixed shear stress of 2.5 dyn/cm2 for 10 minutes. Some ECs were pretreated with IFNγ for 48 hours to induce antigen presentation (indicated as IFNγ, C). In some experiments, ECs were overlaid with CXCL10 (300 ng/mL) for 2 hours prior to use in the flow assay. Slides were then removed, gently washed with warm PBS, and exposed to 0.05% trypsin-EDTA solution to obtain a cell suspension. The number of labeled T cells in this suspension was evaluated by flow cytometry (by gating on the small lymphocyte population and comparing the number of green and red fluorescent cells). The graphs summarize data obtained from at least 3 experiments. Error bars indicate standard error (*P < .05, **P < .01).
A combination of shear flow and chemokines sustains Vav1−/− T-cell migration. (A,B) PKH26-labeled HY-specific CD4+ WT or Vav1 T cells (3 × 10b) were injected intraperitoneally in syngeneic male mice that had previously received an intraperitoneal injection of IFNγ to induce MHC class II expression and antigen presentation. The percentage of T cells remaining in the peritoneal cavity and T-cell infiltration of the peritoneal membrane were evaluated 24 hours later by flow cytometry and wide-field fluorescence microscopy, respectively, as described in the legend to Figure 2. Representative examples of peritoneal lavage dot plots and peritoneal membrane sections (×10 magnification) are shown. The mean number of cells in the peritoneal membrane and lavage from at least 4 mice are shown. Error bars indicate standard error (*P < .05, **P < .01). (C) HY-specific WT and Vav1−/− CD4+ T cells were perfused at 37°C over male-derived EC-coated slides at a fixed shear stress of 2.5 dyn/cm2 for 10 minutes. Some ECs were pretreated with IFNγ for 48 hours to induce antigen presentation (indicated as IFNγ, C). In some experiments, ECs were overlaid with CXCL10 (300 ng/mL) for 2 hours prior to use in the flow assay. Slides were then removed, gently washed with warm PBS, and exposed to 0.05% trypsin-EDTA solution to obtain a cell suspension. The number of labeled T cells in this suspension was evaluated by flow cytometry (by gating on the small lymphocyte population and comparing the number of green and red fluorescent cells). The graphs summarize data obtained from at least 3 experiments. Error bars indicate standard error (*P < .05, **P < .01).
Prompted by these observations, we then sought to investigate whether exposure to shear flow and/or other stimuli could compensate for the migratory defects that were displayed by the Vav1−/− T cells in vitro. HY-specific CD4+ WT (PKH26-labeled) and Vav1−/− T cells were perfused over untreated or IFNγ-treated (antigen-presenting) male-derived EC monolayers.24 As shown in Figure 4C, WT T cells displayed a 5-fold increase in their recruitment through IFNγ-treated male-derived ECs, whereas Vav1−/− T cells still displayed defective migration that was not increased in the presence of shear flow. As chemokine-mediated signals have been shown to cooperate with flow in the recruitment of T cells,24 we carried out further experiments in which untreated and IFNγ-treated male-derived EC monolayers were exposed to CXCL10 (300 ng/mL) for 2 hours prior to use in the flow chamber assays. This led to increased and quantitatively similar recruitment of both WT and Vav1−/− T cells irrespectively of antigen presentation. As experiments of similar design did not rescue T-cell migration in static transwell-based assays in vitro (data not shown), this suggests that a combination of shear flow and chemokine stimulation can rescue Vav1−/− T-cell recruitment independently of antigen presentation by the endothelium.
Vav1−/− T cells are not susceptible to stop signals in vitro and in vivo
Retention of specific T cells into nonlymphoid antigenic tissue has been thought to require their interaction with resident conventional (ie, B7-expressing) APCs.7,8 The observation that memory Vav1−/− T cells were efficiently recruited to antigenic sites but were not retained in the antigenic tissue prompted us to investigate the ability of Vav1−/− T cells to establish sustained interactions with conventional tissue-resident APCs. To address this issue, the following approaches were taken.
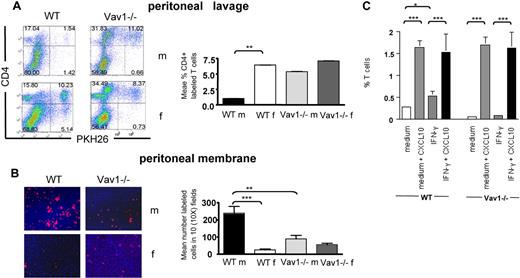
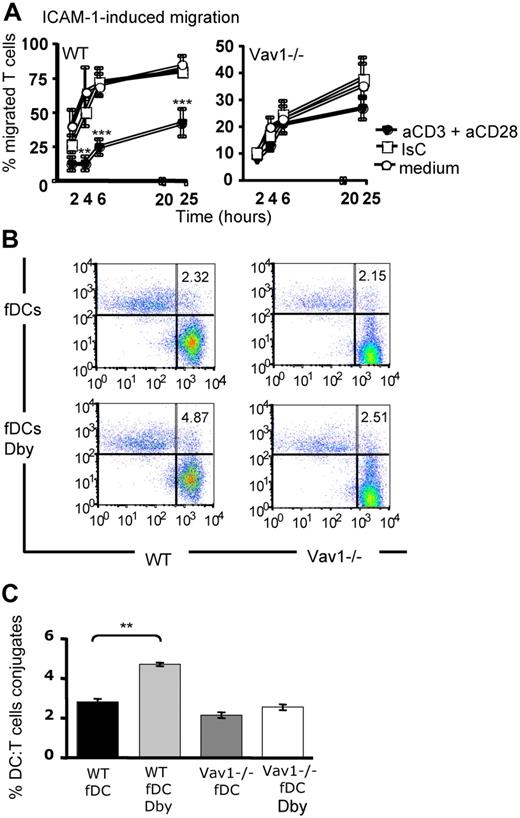
First, we compared the ability of primed HY-specific CD4+ H2-Ab–restricted WT and Vav1−/− T cells (3-5 × 105) that had previously been treated with antibodies specific for CD3 and CD28 (45 minutes at 37°C) to migrate through ICAM-1–coated transwells for 24 hours. The doses of CD3 and CD28 elicited similar levels of proliferation by WT and Vav1−/− T cells (data not shown). HY-specific activated WT T cells migrated significantly less than those exposed to isotype control antibodies or medium alone (Figure 5A). As previously observed (Figure 1), the baseline migration of Vav1−/− T cells was less efficient than that of their WT counterparts. In addition, migration of CD3/CD28-activated Vav1−/− T cells was unchanged, suggesting that these cells are not susceptible to stop signals in vitro.
Vav1−/− T cells are not susceptible to antigen-induced stop signals. (A) HY-specific Ab-restricted WT and Vav1−/− T cells were incubated with plastic bound anti-CD3 and anti-CD28 for 45 minutes and plated on rICAM-1–coated transwells. As a control, T cells were exposed to hamster Ig isotype control or medium alone. Migration was measured as indicated in the legend to Figure 1. The percentage of migrated cells was calculated by dividing the number of cells in the lower chamber with the number of cells plated on the transwells. Error bars indicate standard error (** P < .01, *** P < .001). (B) DCs were obtained from bone marrow of syngeneic female mice (fDCs) and cultured for 7 days in GM-CSF, followed by overnight LPS-induced maturation. HY-specific Ab-restricted CD4+ PKH26-labeled WT or Vav1−/− T cells were incubated with CFSE-labeled female-derived DCs pulsed with 50 nM Dby peptide for 2 hours (fDCs Dby). Nonantigenic DCs (fDCs) were used as a control. Conjugate formation was analyzed by flow cytometry as described in “DC/lymphocyte conjugate formation assays.” The mean percentage of conjugate formation from 4 experiments is shown in panel C. Error bars indicate standard error (**P < .01, ***P < .001).
Vav1−/− T cells are not susceptible to antigen-induced stop signals. (A) HY-specific Ab-restricted WT and Vav1−/− T cells were incubated with plastic bound anti-CD3 and anti-CD28 for 45 minutes and plated on rICAM-1–coated transwells. As a control, T cells were exposed to hamster Ig isotype control or medium alone. Migration was measured as indicated in the legend to Figure 1. The percentage of migrated cells was calculated by dividing the number of cells in the lower chamber with the number of cells plated on the transwells. Error bars indicate standard error (** P < .01, *** P < .001). (B) DCs were obtained from bone marrow of syngeneic female mice (fDCs) and cultured for 7 days in GM-CSF, followed by overnight LPS-induced maturation. HY-specific Ab-restricted CD4+ PKH26-labeled WT or Vav1−/− T cells were incubated with CFSE-labeled female-derived DCs pulsed with 50 nM Dby peptide for 2 hours (fDCs Dby). Nonantigenic DCs (fDCs) were used as a control. Conjugate formation was analyzed by flow cytometry as described in “DC/lymphocyte conjugate formation assays.” The mean percentage of conjugate formation from 4 experiments is shown in panel C. Error bars indicate standard error (**P < .01, ***P < .001).
We then compared the ability of WT and Vav1−/− HY-specific memory T cells to form conjugates with syngeneic dendritic cells (DCs). HY-specific CD4+ H2-Ab–restricted WT and Vav1−/− T cells (2.5 × 105) were labeled with PKH26 and incubated with lipopolysaccharide (LPS)–matured, carboxyfluorescein succinimidyl ester (CFSE)–labeled female DCs (105) that were either untreated or preloaded with the cognate HY Dby peptide (50 nM). The dose of peptide was chosen based on its ability to induce similar levels of proliferation by both WT and Vav1−/− T cells (Figure S2). As expected, a higher percentage of HY-specific WT T cells engaged Dby peptide–pulsed female DCs compared with nonantigenic syngeneic DCs (Figure 5B,C). In contrast, antigen presentation did not enhance conjugate formation by Vav1−/− T cells, in line with previous findings in immature and naive T cells.15,16
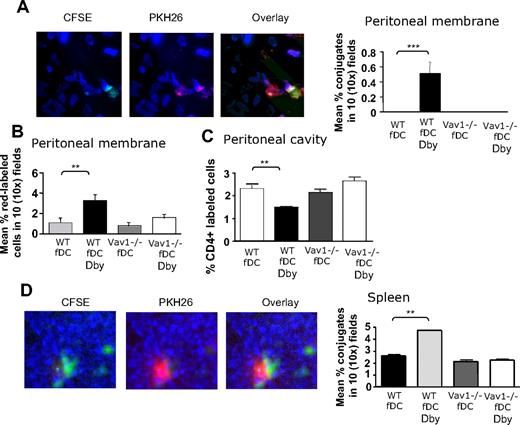
Finally, we investigated the interactions between HY-specific CD4+ H2-Ab–restricted WT or Vav1−/− T cells and tissue-resident conventional APCs in vivo. LPS-matured CFSE-labeled Dby peptide–pulsed (50 nM) female DCs were injected intraperitoneally in female mice (2 × 106/mouse), which then received an intravenous injection of PKH26 labeled HY-specific CD4+ WT or Vav1−/− T cells (107/mouse). As a control, nonantigenic DCs were also injected in some T-cell recipients. In addition, some mice received T cells or Dby-pulsed or nonantigenic DCs alone. A schematic representation of the trafficking patterns of intravenous-injected T cells and intraperitoneal-injected DCs is provided for clarity in Figure S5. The presence of interacting labeled T cells and DCs in the peritoneal tissue and the spleen of recipient mice was analyzed 24 hours later. Yellow fluorescence was observed in the areas of cell-cell contact as a result of the interacting red-labeled T cells and green-labeled DCs. As expected, a significantly higher number of DC/T-cell conjugates was observed in the peritoneal membrane of mice that had been injected with female DCs prepulsed with Dby peptide (Figure 6A) than those injected with nonantigenic female DCs. This effect was not seen when Vav1−/− T cells were injected. Furthermore, WT T cells, but not Vav1−/− T cells, that were not engaged by DCs were detected in the peritoneal membrane of mice that had been injected with peptide-pulsed DCs (Figure 6B), suggesting that WT-specific T cells are temporarily retained in the target tissue following a previous encounter with antigen-presenting DCs; this has previously been described in lymphoid tissue.26 In addition, a lower number of labeled T cells was retrieved from the peritoneal cavity of recipients of HY-specific WT T cells and peptide-loaded DCs than recipients of WT T cells and nonantigenic DCs, or Vav1−/− T cells and either antigenic DCs or non–peptide-loaded DCs (Figure 6C). This suggests that T cells that had not engaged with antigenic DCs did not remain in the peritoneal tissue and migrated to the peritoneal cavity.
Vav1−/− T cells display defective T-cell/antigen-presenting DC interactions in vivo. (A) PKH26-labeled T cells (107) were injected intravenously in syngeneic female mice that simultaneously received an intraperitoneal injection of Dby peptide–pulsed female-derived matured DCs labeled with CFSE, or DCs alone as a control. HY-specific CD4+ WT and Vav1−/− T cells and DCs were injected alone as a control. In this model, T cells travel into the bloodstream and reach the peritoneal membrane and the spleen, whereas DCs travel out of the peritoneal cavity, through the peritoneal membrane, enter the bloodstream, and reach the spleen. The presence of T-cell/DC conjugates in the peritoneal membrane and spleen was quantified 24 hours later by wide-field fluorescence microscopy as described in “Methods.” The occurrence of cell-cell interactions was apparent as yellow fluorescence. Representative 40× images from the peritoneal membrane (A) and the spleen (D) are shown. The number of conjugates in the peritoneal membrane (A) and spleen (D) were averaged and quantified with the algorithm described in “Wide-field fluorescence microscopy and flow cytometry” in 10 × 10 images obtained from samples from at least 6 animals. In addition, the mean number of labeled T cells (not engaged by DCs) in the peritoneal membrane and cavity was measured as described in the legend to Figure 3, and is shown in panels B and C, respectively. No T-cell/DC conjugates were detected in the peritoneal lavage (data not shown). Error bars indicate standard error (**P < .01, ***P < .001).
Vav1−/− T cells display defective T-cell/antigen-presenting DC interactions in vivo. (A) PKH26-labeled T cells (107) were injected intravenously in syngeneic female mice that simultaneously received an intraperitoneal injection of Dby peptide–pulsed female-derived matured DCs labeled with CFSE, or DCs alone as a control. HY-specific CD4+ WT and Vav1−/− T cells and DCs were injected alone as a control. In this model, T cells travel into the bloodstream and reach the peritoneal membrane and the spleen, whereas DCs travel out of the peritoneal cavity, through the peritoneal membrane, enter the bloodstream, and reach the spleen. The presence of T-cell/DC conjugates in the peritoneal membrane and spleen was quantified 24 hours later by wide-field fluorescence microscopy as described in “Methods.” The occurrence of cell-cell interactions was apparent as yellow fluorescence. Representative 40× images from the peritoneal membrane (A) and the spleen (D) are shown. The number of conjugates in the peritoneal membrane (A) and spleen (D) were averaged and quantified with the algorithm described in “Wide-field fluorescence microscopy and flow cytometry” in 10 × 10 images obtained from samples from at least 6 animals. In addition, the mean number of labeled T cells (not engaged by DCs) in the peritoneal membrane and cavity was measured as described in the legend to Figure 3, and is shown in panels B and C, respectively. No T-cell/DC conjugates were detected in the peritoneal lavage (data not shown). Error bars indicate standard error (**P < .01, ***P < .001).
In line with these observations, a significantly higher percentage of T cell/DC conjugates was detected in the spleen of recipient mice that had received Dby peptide–loaded DCs and WT T cells than Vav1−/− T cells (Figure 6D), confirming that Vav1 activity is required for the delivery of stop signals to trafficking T cells following cognate interactions with resident APCs, which led to their accumulation into antigen-rich tissue.
Vav1−/− T-cell motility is not susceptible to CD28-mediated regulation
Our results suggest that Vav1 activity is dispensable for T-cell recruitment that is mediated by antigen-presenting ECs, but is instrumental for their retention into target tissue, possibly following interactions with resident conventional APCs. A prominent feature of fully mature conventional APCs is the high expression of CD28 ligands, which is not observed in other parenchymal cells. We have recently reported that CD28 triggering promotes interactions with APCs by inducing integrin clustering and is required for the localization of primed T cells to nonlymphoid antigen-rich tissues.6 As Vav1 can be activated by CD28 signals,27,28 we sought to assess the effect of CD28-induced Vav1 activity on T-cell trafficking in the absence of—or concomitant to—TCR triggering.
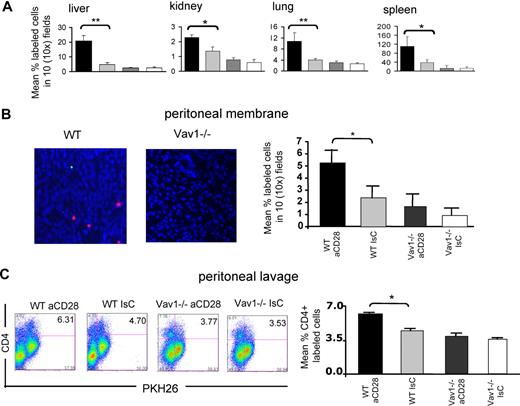
HY-specific CD4+ H2-Ab–restricted WT and Vav1−/− T cells were pretreated with a mixture of hamster antimouse CD28 and rabbit anti–hamster Ig and labeled with PKH26 prior to intravenous injection into female mice. As a control, T cells that had been treated with hamster Ig and rabbit anti–hamster Ig, and labeled with CFSE, were coinjected. Localization to nonlymphoid tissue by T cells was assessed 24 hours after injection. As previously described, CD28-stimulated WT T cells showed increased trafficking to kidney, lung, liver, and spleen compared with the controls (Figure 7A). In contrast, HY-specific Vav1−/− T-cell infiltration of the same tissues was unaffected by CD28 triggering. This was surprising, as TCR-dependent recruitment was not affected by Vav1 activity in vivo.
Vav1−/− T-cell motility is not susceptible to CD28-mediated regulation. (A) HY-specific CD4+ WT and Vav1−/− T cells that had either undergone antibody-mediated CD28 ligation (30 minutes at 37°C, PKH26-labeled) or had been pretreated with an antibody isotype control (CFSE-labeled) were injected intravenously (107/mouse) into syngeneic female recipients. The presence of fluorescently labeled cells in the indicated organs was assessed 24 hours later as described in the legend to Figure 2. The mean T-cell number plus or minus SEM observed in samples from at least 6 animals are shown (*P < .05, **P < .01). (B-C) HY-specific CD4+ WT and Vav1−/− T cells that had undergone either antibody-mediated CD28 ligation (PKH26-labeled) or had been pretreated with an antibody isotype control (CFSE-labeled) were injected intravenously (107/mouse) into male mice that had received an intraperitoneal injection of IFNγ 48 hours earlier. The presence of fluorescently labeled cells in the peritoneal membrane (B) and cavity (C) was assessed 24 hours later as described in the legend to Figure 3. The mean T-cell number plus or minus SEM observed in samples from at least 3 animals is shown in the right-hand side panels (*P < .05, **P < .01).
Vav1−/− T-cell motility is not susceptible to CD28-mediated regulation. (A) HY-specific CD4+ WT and Vav1−/− T cells that had either undergone antibody-mediated CD28 ligation (30 minutes at 37°C, PKH26-labeled) or had been pretreated with an antibody isotype control (CFSE-labeled) were injected intravenously (107/mouse) into syngeneic female recipients. The presence of fluorescently labeled cells in the indicated organs was assessed 24 hours later as described in the legend to Figure 2. The mean T-cell number plus or minus SEM observed in samples from at least 6 animals are shown (*P < .05, **P < .01). (B-C) HY-specific CD4+ WT and Vav1−/− T cells that had undergone either antibody-mediated CD28 ligation (PKH26-labeled) or had been pretreated with an antibody isotype control (CFSE-labeled) were injected intravenously (107/mouse) into male mice that had received an intraperitoneal injection of IFNγ 48 hours earlier. The presence of fluorescently labeled cells in the peritoneal membrane (B) and cavity (C) was assessed 24 hours later as described in the legend to Figure 3. The mean T-cell number plus or minus SEM observed in samples from at least 3 animals is shown in the right-hand side panels (*P < .05, **P < .01).
As in physiologic settings, CD28 triggering is delivered in conjunction with TCR engagement; the ability of CD28-mediated Vav1−/− activity to enhance T-cell localization to target tissue in conjunction with TCR-mediated signals was also analyzed. HY-specific CD4+ H2-Ab–restricted WT and Vav1−/− T cells were pretreated with a mixture of hamster antimouse CD28 and rabbit anti–hamster Ig, and labeled with PKH26 prior to intravenous injection into male mice that had previously received an optimal dose of IFNγ to induce local antigen presentation in the peritoneal tissue (Figure 7B-C). CD28-activated WT T cells showed increased localization to the peritoneal membrane, but this effect was not observed with the Vav1−/− T cells (Figure 5B). Similarly, CD28 triggering did not increase Vav1−/− T-cell accumulation into the peritoneal cavity (Figure 5C). These data suggest that in the absence of Vav1, CD28-mediated regulation of T-cell trafficking and tissue localization is impaired.
Discussion
It is becoming increasingly clear that TCR and CD28 coengagement not only sustains the differentiation, expansion, and development of effector function of T cells, but also optimizes the efficiency of the immune response by coordinating its anatomy.29 Recognition of antigen displayed by ECs directs T-cell extravasation to antigenic sites by facilitating the access of antigen-specific T cells.2-5 The cellular and molecular mechanisms that subsequently sustain T-cell retention into antigenic nonlymphoid tissue are less well understood. As a prominent role in mediating T-cell arrest has been ascribed to antigen-receptor engagement, it is likely that parenchymal APCs have a fundamental role in this effect.7,8,30
Several reports indicate that the GEF Vav1 is a key mediator in the transduction of TCR- and CD28-mediated signals to the cytoskeleton owing to its ability to activate Rho GTPases.15,16,27,28
Despite the observation that adhesion, chemotactic responses, and antigen-induced migration by Vav1−/− T cells were severely compromised in vitro, constitutive and antigen-induced recruitment of primed Vav1−/− T cells were unaffected in vivo owing to compensatory mechanisms that are mediated by shear flow in conjunction with endothelium-displayed chemokines. We have recently reported that loss of TCR-induced phosphoinositide 3 kinase (PI3K) p110δ subunit activity completely abrogated the recruitment of antigen-specific T cells to target tissue.5 Although both molecules are activated by TCR triggering and can influence each other's activity, it is possible that p110δ activity may be coengaged by signaling pathways that cooperate with the TCR in the regulation of T-cell migration. In this context, it has recently been shown that that a ζ-chain–associated protein kinase 70 (ZAP70)–mediated chemokine and TCR cross-talk is required to induce T-cell migration.17
Despite being dispensable for primed T-cell recruitment to antigenic nonlymphoid tissue in vivo, Vav1 activity was required for T-cell infiltration and retention in a shear-free microenvironment, as recruited Vav1−/− T cells failed to accumulate in target tissue and continued to migrate. Similarly to what has been described in lymph nodes,26 retention of antigen-specific T cells to nonlymphoid antigenic tissue is presumably mediated by cognate interactions with conventional resident APCs7,8 and can be independent of chemokine-induced integrin activation,31 while requiring distinct additional signals. The observation that CD28 triggering of Vav1−/− T cells does not promote their localization to nonlymphoid tissue is consistent with a cooperation between TCR- and CD28-mediated signals in the regulation of T-cell motility. The contribution of CD28-mediated signals to the establishment of T cell/APC interactions that sustain T-cell localization to target tissue has been suggested by several studies.7,8 Reduced T-cell infiltrates are also commonly observed in B7-deficient target tissue despite efficient T-cell activation.32,33 Importantly, specific inhibition of the Vav1/Rac1 interaction following CD28 signaling in human CD4+ T cells resulted in reduced lamellipodium formation and inhibition of T-cell/APC conjugate formation.34,35
We propose that 2 mechanisms are likely to contribute to this effect. First, Vav1 activity is needed for T-cell/APC cognate interactions in static conditions, such as those that occur in parenchymal tissue. Second, retention of T cells in the tissue may require additional signals that are delivered concomitantly to TCR triggering and that also require Vav1 activity. Based on the evidence presented here, TCR triggering by B7−/− parenchymal cells, such as the those of the mesothelium or stromal cells (in which MHC-molecule up-regulation is induced by IFNγ4 ), is not enough to sustain antigen-specific Vav1−/− T-cell retention. The observation that Vav1−/− T cells are not susceptible to CD28-mediated regulation of T-cell migration suggests that additional CD28-induced, Vav1-mediated signals may be necessary to allow this effect.
From a clinical perspective, given the recent reports of the effectiveness of pharmacological inhibition of Vav1 activity in autoimmunity and transplantation,34,35 our observations provide a molecular platform for pharmacologic targeting of Vav1 in the control of T cell–mediated inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to F. Vianello and K. Okkenhaug for critical review of the paper.
R.D. was supported by a BHF (London, United Kingdom) scholarship (BHF FS/04/065).
Authorship
Contribution: R.D. performed research and wrote the paper; L.M. and A.T. performed research; A.I. and J.-G.C. designed and performed research; A.J.R. designed research; V.L.T. designed research and contributed reagents; and F.M.M.-B. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Federica M. Marelli-Berg, Department of Immunology, Imperial College London, Hammersmith Hospital Campus, Du Cane Rd, London W12 0NN, United Kingdom; e-mail: f.marelli@imperial.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal