By using an AAV vector that contains a liver-specific promoter, Margaritis and colleagues demonstrate that FVIIa gene delivery leads to long-term expression of FVIIa in treated hemophilic dogs. This results in a partial correction of hemostatic parameters and an absence of spontaneous bleeding episodes.
Hemophilia A and B are severe X-linked inherited bleeding disorders caused by deficiency of blood coagulation factors (F) VIII and FIX, respectively. Currently, hemophilia is treated with protein replacement therapy using either plasma-derived or recombinant coagulation factors. Although replacement therapy is extremely effective and has significantly enhanced the quality and life expectancy of patients with hemophilia, a number of challenges still remain. Significantly, some patients develop neutralizing (or inhibitor) antibodies after replacement therapy, resulting in the clinical failure of standard replacement treatment; 20% to 30% of patients with severe hemophilia A and about 5% with severe hemophilia B. Patients who have developed such antibodies may undergo immune tolerance therapy; however, this is very expensive and may not be effective. Inhibitor patients may be treated with “bypass” agents, such as activated prothrombin complex concentrate, but this has a low efficacy and has an associated thromboembolic risk. More recently, recombinant (r) FVIIa (NovoSeven) has been a highly successful treatment for hemophilia inhibitor patients. rFVIIa is believed to produce a “thrombin burst” on the surface of activated platelets, leading to activation of FIX and FX in a tissue factor–independent mechanism. rFVIIa can thus be used to treat both hemophilia A and B patients with inhibitors. However, although rFVIIa is effective, it is extremely expensive because of the large dose required for efficacy and the short half-life of 2.7 hours.
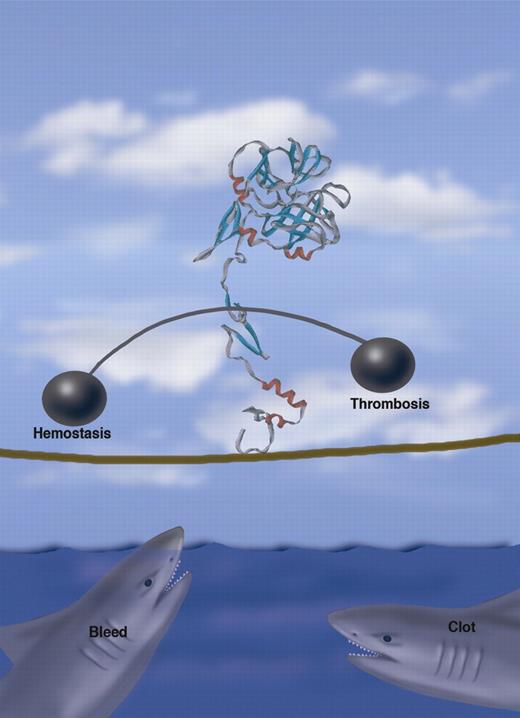
FVIIa walks a tightrope balancing hemostasis and thrombosis. Professional illustration by Marie Dauenheimer.
FVIIa walks a tightrope balancing hemostasis and thrombosis. Professional illustration by Marie Dauenheimer.
There have been major advances in gene therapy for hemophilia, particularly in vector design, that have resulted in sustained therapeutic expression in mice, hemophilic dogs, and nonhuman primates. However, as the Scottish poet Robert Burns noted in his poem, “To a Mouse,” “the best laid schemes of mouse and man often go askew.” So results from rodent species have not always translated to larger animals with quite the same efficacy. Thus, a safe, effective gene therapy treatment for hemophilia in humans has not yet been realized.
Treating hemophilia inhibitor patients with gene therapy that will lead to expression of a protein to which these patients have preexisting antibodies is a complex issue. Two alternative approaches to tackle this problem have been studied: in one, the expression of the replacement factor (FVIII) has been targeted to the α-granules of platelets. Thus, the FVIII is protected from inhibitory antibodies in the plasma and is released only at the site of vascular damage upon platelet activation.1 In the second approach, the utility of delivering an engineered secreted FVIIa by gene therapy has been investigated, thus avoiding the need for repeated pharmacologic dosing via in vivo expression. It is the second of these 2 approaches that Margaritis et al present in this issue of Blood,2 demonstrating the safety and efficacy of this approach in hemophilic dogs. In a systematic and thorough proof of concept, the authors previously showed that a novel human FVII transgene engineered to contain a cleavage site for the intracellular protease furin resulted in secretion of correctly processed fully active FVIIa and that treatment of hemophilia B mice with an adeno-associated viral (AAV) vector expressing the murine version of this FVII transgene led to sustained expression and phenotypic correction of the bleeding diathesis.3 As discussed above, some caution is warranted in translating results from mice to men; therefore, in the present paper they examine the safety and efficacy of this approach in a larger outbred animal model, namely canine hemophilia A and B. First, they generated a species-specific version of the transgene, canine FVIIa (cFVIIa) and demonstrated by in vitro expression of cFVIIa that it is biologically active. Similar to their previous studies in mice, they generate an AAV vector directing liver-specific expression of the transgene. This vector was used to treat 4 animals, 1 hemophilia B and 3 hemophilia A dogs, with 3 different vector doses. With the exception of the hemophilia B dog that received the lowest vector dose, all animals showed long-term stable expression of cFVIIa and reduction of the whole-blood clotting time and the prothrombin time. Importantly, this was associated with cessation of spontaneous bleeding episodes. Interestingly, the hemophilia B dog that demonstrated no appreciable change in cFVIIa levels, whole-blood clotting time, or prothrombin time also had no spontaneous bleeding episodes following treatment. The effective weight-normalized dose in the hemophilic dogs was found to be higher than the dose required in mice, highlighting the importance of using large animal models. This is an important study because, for the first time, it demonstrates proof of concept in a large animal model. However, it will be important to assess the efficacy of the treatment in a large animal subjected to a hemostatic challenge, because in the murine model expression of FVIIa reduced bleeding times following tail clip injury (though not to levels seen in wild-type littermates) and improved hemostasis in a microvascular injury model but had no effect on a carotid artery injury model.
The issue of rFVIIa's safety has been questioned, due to reports of thromboembolic events. Aljamali et al have investigated the potential thrombotic risk using mice transgenic for mFVIIa.4 They found that expression levels of 2 μg/mL or higher were associated with thrombosis and early mortality. In the present study, cFVIIa levels were generally below this level and, to date, no thrombotic complications have been reported and there was no evidence of a prothrombotic state. However, achieving the appropriate levels of FVIIa that provide adequate hemostasis without incurring the risk of thrombosis may be similar to walking a tightrope!
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal