Abstract
Wnt/Fzd signaling is known to play a key role in development, tissue-specific stem-cell maintenance, and tumorigenesis, particularly through the canonical pathway involving stabilization of β-catenin. We have previously shown that Fzd9−/− mice have a deficiency in pre-B cells at a stage when self-renewing division is occurring in preference to further differentiation, before light chain immunoglobulin recombination. To determine whether pathologic usurpation of this pathway plays a role in B-cell leukemogenesis, we examined the expression of Wnt/Fzd pathway genes in the Eμ-TCL1 mouse model of chronic lymphocytic leukemia. We find that, in the course of leukemogenesis, the expression of Wnt16, Wnt10α, Fzd1, and most dramatically, Fzd6, is progressively up-regulated in the transformed CD5+ B cells of these mice, as are β-catenin protein levels. Elimination of Fzd6 expression by crossing into Fzd6−/− mice significantly delays development of chronic lymphocytic leukemia in this model. Our findings suggest that the self-renewal signals mediated by Wnt/Fzd that are enlisted during B-cell development may be pathologically reactivated in the neoplastic transformation of mature B cells.
Introduction
The binding of soluble Wnt ligands to their Fzd receptors results in a complex array of downstream signals that play a central role in development,1 tissue-specific stem cell renewal,2 and tumorigenesis.3 Activation of the canonical Wnt/Fzd pathway through Wnt family members binding to the Fzd 7-pass membrane proteins and the LRP5/6 coreceptors results in the disruption of a protein complex containing GSK-3β, adenomatous polyposis coli (APC), and axin that is responsible for the constitutive phosphorylation and proteasome targeting of β-catenin. Inhibition of this complex increases intracellular levels of β-catenin and promotes its translocation to the nucleus, where it binds to the LEF/TCF family of transcription factors and up-regulates β-catenin target genes
There is accumulating evidence that the Wnt/Fzd signaling pathway is involved in lymphoid development and function. Elimination of downstream β-catenin partners LEF-1 and TCF-1 in mice generates lymphoid phenotypes,4,5 including deficits in B-cell development and a complete block of T-cell development in double LEF-1 plus TCF-1 knockouts. Elimination of β-catenin expression in hematopoietic cells, however, has given conflicting results.6-8 Overexpression of nondegradable β-catenin results in maturation abnormalities in numerous hematopoietic lineages, including B and T cells.8,9 Analysis of mice with gene ablation of upstream proteins, such as the family of Fzd receptors, revealed a variety of neural and other phenotypes,10-12 but no hematopoietic system defects until our analysis of the Fzd9−/− mice found abnormalities in B-cell development.13 Given the apparent role of the Wnt/Fzd pathway in regulating self-renewal and/or survival during lymphoid development and its known role in numerous epithelial-based cancers,3 we hypothesized that pathologic reactivation of the pathway in mature lymphocytes could contribute to neoplastic transformation. Indeed, there is evidence for this in human chronic lymphocytic leukemia (CLL).14-18
CLL is the most common leukemia in Western countries and may represent a malignant transformation of normal CD5+ (or B1a) B cells.19 CLL B cells are characterized by a low growth fraction, enhanced in vitro survival with up-regulation of antiapoptotic proteins, and production of immunosuppressive factors, such as transforming growth factor-β20 and soluble CD27.21 In many hematologic malignancies, a pathognomonic chromosomal translocation (eg, t(8;14) in Burkitt or t(14;18) in follicular lymphoma) can be directly linked to the pathobiology of the malignant B cells. In CLL, the area of 13q14 chromosomal deletion has recently been shown to encode a micro RNA that regulates BCL-2 expression.22,23 Other recurring abnormalities in CLL, such as trisomy 12, however, have not yet been linked with a specific biologic effect. Furthermore, no clear association between CLL and radiation or toxin exposure has been established, leaving the underlying pathophysiology of this disease unresolved.
Initial gene expression array analysis comparing human CLL B cells with “normal” B cells surprisingly has shown that 2 of the most up-regulated transcripts are Wnt3a14 and LEF-1.15 Dysregulation of this pathway has been partially confirmed by reverse-transcribed polymerase chain reaction (RT-PCR) analysis showing up-regulated Fzd3 and several different Wnt proteins in human CLL B cells.16 Finally, Liu et al noted that the genes encoding soluble inhibitors of the Wnt signaling pathway, including secreted frizzled-related protein 4, were silenced by hypermethylation in CLL B cells.17 Although these early findings are compelling and suggest a role for the Wnt/Fzd pathway in CLL, its potential function in these malignant B cells is not clear.
To further investigate a role for this critical signaling pathway in CLL, we used a currently accepted mouse model of the disease, the Eμ-TCL1 mouse,24,25 kindly provided by Carlo Croce (The Ohio State University, Columbus, OH). The Eμ-TCL1 mouse spontaneously develops a hyperplasia of CD5+ B cells in the peripheral blood and peritoneum, which progresses toward a monoclonal B-cell leukemia/lymphoma with infiltration of spleen, bone marrow, and other organs. This model has allowed us to evaluate changes in expression in the early and late stages of leukemogenesis, and we find that several Wnt/Fzd-related genes are up-regulated during this process. We demonstrate that the up-regulation of Fzd6, in particular, is critical in the malignant progression of CD5+ B cells in murine CLL.
Methods
Mice
Eμ-TCL1 transgenic mice (C3H and C57Bl/6 mixed background, provided by Carlo Croce, The Ohio State University, Columbus, OH) were crossed over 8 generations to C57Bl/6 mice (The Jackson Laboratory, Bar Harbor, ME), Fzd9−/− (129 background, provided by Uta Franke, Stanford University, Stanford, CA), and Fzd6−/− mice (C57Bl/6 and 129 mixed background, provided by Jeremy Nathans, Johns Hopkins University, Baltimore, MD) and housed, cared for, and used in accordance with the Guide for Care and Use of Laboratory Animals. The study protocol was approved by the Institutional Animal Care and Use Committee at the University of Wisconsin (Madison, WI). Eμ-TCL1 transgenic mice were crossed with Fzd6 KO mice to generate TCL1+/Fzd6−/− mice. A PCR protocol for genotyping of tail DNA was used and is available on request.
IgH gene rearrangement
Genomic DNA was isolated in Trizol (Invitrogen, Carlsbad, CA) and subjected to PCR using a Platinum DNA polymerase kit (Invitrogen) using V gene primers (Table S2, available on the Blood website; see the Supplemental Materials link at the top of the online article) for 35 cycles with a DNA Engine (MJ Research, Watertown, MA) thermal cycler at 60°C annealing temperature. Products were run on a 2% agarose gel and identified by ethidium bromide staining visualized under ultraviolet light. This protocol (courtesy of M. Teitell, University of California–Los Angeles, derived from Kawamoto et al26 ) amplifies 4 distinct immunoglobulin (Ig) heavy chain (HC)-derived products of different molecular weights (reflecting DJH1, DJH2, DJH3, and DJH4 rearrangements) in polyclonal B-cell populations (eg, mouse spleen or lymph nodes) with loss of these bands in oligoclonal or monoclonal populations.
Total RNA isolation, cDNA synthesis, and PCR primers
For real-time PCR and RT-PCR, Eμ-TCL1 mice CD19+CD5+Igλ+, CD19+CD5+Igλ− tumor B cells, and CD19+CD5− normal B cell subsets were flow sorted to 98% purity using the markers CD19, CD5, and Igλ (FACSVantage running FACSDiva software, BD Biosciences, San Jose, CA). For real-time PCR array, tumor-bearing Eμ-TCL1 mice (∼10 months old), CD19+CD5+ tumor cells, and CD19+CD5− normal B cells from young Eμ-TCL1 mice (6 weeks old) were positively selected to 90% to 95% purity by labeling with anti–B220-biotin and Streptavidin MicroBeads (Miltenyi Biotec, Auburn, CA). Total RNA was purified by RNeasy mini kit (QIAGEN, Valencia, CA), according to the manufacturer's instructions. The quality of mRNA was assessed by gel electrophoresis. Total RNA was reverse transcribed into first-strand cDNA by iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Gene-specific primers sets are listed in Table S2.
Real-time PCR
The mRNA levels were quantified in duplicate by real-time RT-PCR on the Bio-Rad iCycler or MyiQ Cycler system. cDNA derived from 1000 to 2000 cells was used for real-time PCR using iQ SYBR Green supermix (Bio-Rad), according to the manufacturer's instructions. PCR cycles consisted of an initial denaturation step at 95°C for 15 seconds and annealing at 58°C for 30 seconds. PCR amplification of β-actin was performed for each sample as a control of sample loading and to allow for normalization between samples. The data were analyzed using the comparative ΔΔCt method, where Ct is the cycle number at which fluorescence first exceeds a threshold set in the linear portion of the amplification curve. The ΔCt values from each gene were obtained by subtracting the values for the β-actin Ct from the gene-of-interest Ct. Differences of one Ct value represent a 2-fold difference in the level of mRNA. To ensure specific amplification, samples were checked for appropriate size by agarose gel electrophoresis.
Real-time PCR array
mRNA levels were quantified by real-time RT-PCR array on the RT2 Profiler TM PCR Array of mouse Wnt pathway (SABiosciences, Frederick, MD) according to the manufacturer's instructions. Briefly, total RNA (2 μg) was reverse-transcribed into first-strand cDNA and used as template to perform real-time PCR on the Bio-Rad iCycler system (Bio-Rad). PCR annealing step was at 60°C for 1 minute. PCR amplification of glyceraldehyde-3-phosphate dehydrogenase and hypoxanthine guanine phosphoribosyl transferase 1 was performed for each sample to control for sample loading and to allow for normalization between samples. The data were analyzed using the comparative ΔΔCt method, according to the PCR Array Data Analysis downloaded from the SABiosciences website.
Proliferation assay
Splenocytes from an Eμ-TCL1 tumor-bearing mouse (90% CD19+CD5+ tumor B cells) were cultured in Dulbecco modified Eagle medium plus 10% fetal calf serum (FCS) and 0.5 μM β-mercaptoethanol at 37°C in a humidified 5% CO2 atmosphere at a concentration of 106 cells/mL with or without 75 ng/mL recombinant mouse Wnt3a, 100 ng/mL Wnt5a, 100 ng/mL Dickkopf-1 (DKK-1) (R&D Systems, Minneapolis, MN), or 20 mM LiCl (KCl as the control) for 16, 24, 48, or 72 hours. For the last 6 hours, wells were pulsed with 1 μCi/well of 3H-TdR. 3H-TdR incorporation was quantified using a beta counter (MicroBeta Trilux; PerkinElmer Life and Analytical Sciences, Waltham, MA). Results are presented as counts per minute (plus or minus SE) for triplicate wells.
Flow cytometry
Anti–mouse CD19-allophycocyanin APC and CD5-phycoerythrin were purchased from eBioscience (San Diego, CA). Anti–mouse immunoglobulin λ-fluorescein isothiocyanate (FITC) was from Southern Biotechnology (Birmingham, AL). Propidium iodide was purchased from Invitrogen. Cells were harvested from spleen or blood into single-cell suspensions and stained with indicated antibodies in 106 cells/100 μL for 30 minutes at 4°C in phosphate-buffered saline (PBS) plus 2% FCS for surface proteins. Propidium iodide was added to stain dead cells that subsequently were excluded from the analysis. For intracellular β-catenin staining, after performing surface antigen staining, the cell pellet was resuspended in 4% paraformaldehyde (Fisher Scientific, Pittsburgh, PA) solution at 5 × 105 cells/100 μL and incubated for 20 minutes at room temperature and then washed once in flow buffer (PBS + 2% FCS). Before performing the intracellular staining, cell membranes were permeabilized with permeabilization buffer (PBS + 2% FCS + 0.1% saponin; MP Biomedicals, Irvine, CA) for 15 minutes at room temperature and washed in the same buffer once. The pellet was resuspended in 50 μL of permeabilization buffer, and cells were stained with FITC-conjugated mouse anti–β-catenin monoclonal antibody (BD Biosciences Transduction Laboratories, Lexington, KY) at 1 μg/106 cells at room temperature for 30 minutes. As a reference and isotype Ab control, FITC–mouse IgG1 (BD Biosciences Pharmingen, San Diego, CA) was used. Finally, cells were washed twice in permeabilization buffer and resuspended in 0.3 mL of flow buffer. Analysis was done using an LSRII or FACSCaliber (BD Biosciences Pharmingen). The data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Results
The Eμ-TCL1 transgenic mouse is a model of CLL that allows evaluation of Wnt/Fzd pathway expression throughout leukemogenesis
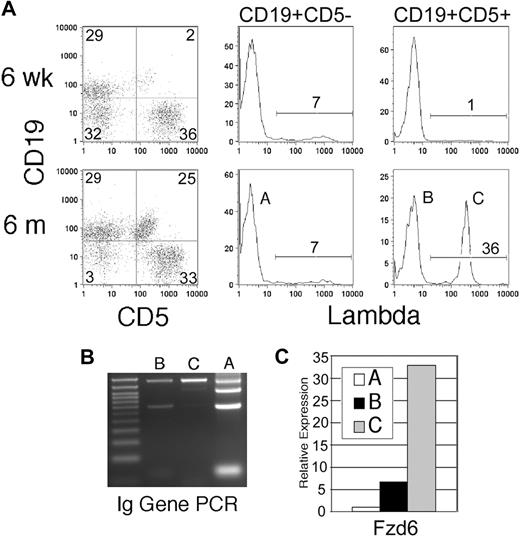
The Eμ-TCL1 transgenic mouse24 develops a malignancy of CD5+ B cells that accurately mimics human CLL.25 In this mouse, an oligoclonal expansion of CD5+CD19+ cells similar in morphologic appearance, cell cycle status, and in vitro behavior to human CLL 24 (Q.-L.W., E.A.R., unpublished observation, December 2005) is noted in the peripheral blood and peritoneal cavity (Figure 1A population B) that gradually evolves into a monoclonal, more frankly malignant disease involving the spleen and other organs (Figure 1A population C). Although the majority of Eμ-TCL1 mice possess κ light chain-expressing tumor cells, in some mice we are able to distinguish the oligoclonal background of expanded CD5+ B cells from an early arising, dominant clone by virtue of its expression of λ light chain. Normally, less than 5% of mouse B cells express λ; thus, a large expansion of CD5+λ+ B cells strongly suggests the emergence of a single dominant clone. This was confirmed by PCR amplification of variable regions in rearranged Ig HCs in flow-sorted populations as shown in Figure 1B, with a progressive loss of the heterogeneous products that are present in the CD5− “normal” B cells in population A, in the oligoclonal population B, and a monoclonal population C. These mice allowed us to examine the changes in the β-catenin signaling pathway by comparing B cells from young mice without CD5+ B-cell expansions (but expressing the TCL1 transgene) with those in the “preleukemic” expansion phase and frankly malignant, monoclonal B cells.
Fzd/Wnt expression can be monitored throughout leukemogenesis in Eμ-TCL1 transgenic mice. (A) Eμ-TCL1 transgenic mice show expansion of CD5+ B cells in blood at 6 months, but not at 6 weeks, of age. Gating on CD5+ and CD5− B cells reveals the usual 2% to 7% Igλ-expressing cells in CD5− B cells (population A), but a large clonal expansion of Igλ+ cells in the CD5+ compartment in this mouse (bottom right histogram, population C). (B) PCR of genomic DNA for Ig HC rearrangement confirms flow analysis, with the normal 4 bands in population A, oligoclonal (reduced) bands in population B, and a single intense band in population C. (C) Quantitative RT-PCR is performed on flow cytometrically sorted populations A, B, and C (> 95% purity) for Fzd6 expression, which is normalized within each sample to the β-actin RNA level. The data are representative of 3 separate analyses of λ+ clonal populations in different mice.
Fzd/Wnt expression can be monitored throughout leukemogenesis in Eμ-TCL1 transgenic mice. (A) Eμ-TCL1 transgenic mice show expansion of CD5+ B cells in blood at 6 months, but not at 6 weeks, of age. Gating on CD5+ and CD5− B cells reveals the usual 2% to 7% Igλ-expressing cells in CD5− B cells (population A), but a large clonal expansion of Igλ+ cells in the CD5+ compartment in this mouse (bottom right histogram, population C). (B) PCR of genomic DNA for Ig HC rearrangement confirms flow analysis, with the normal 4 bands in population A, oligoclonal (reduced) bands in population B, and a single intense band in population C. (C) Quantitative RT-PCR is performed on flow cytometrically sorted populations A, B, and C (> 95% purity) for Fzd6 expression, which is normalized within each sample to the β-actin RNA level. The data are representative of 3 separate analyses of λ+ clonal populations in different mice.
Wnt, Fzd, and other genes involved in the β-catenin pathway are up-regulated during B-cell leukemogenesis
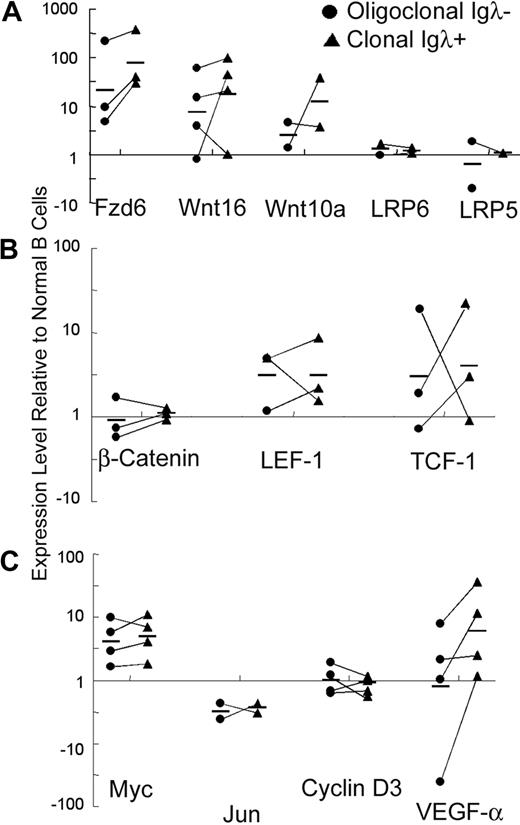
Real-time RT-PCR array analysis, specifically targeted to the Wnt signaling pathway, shows that the expression of several β-catenin signaling pathway-related genes was detectable in normal CD5− B cells from young Eμ-TCL1 mice (6 weeks) and tumor CD5+ B cells from aged Eμ-TCL1 (10 months or older) mice (Table S1). Wnt10a, Fzd5, and Fzd6 are the most abundantly expressed of the Wnt/Fzd family members in normal and malignant mouse B cells. Compared with normal cells, the tumor B cells had at least 4-fold higher expression of Fzd1, Fzd6, Wnt10a, and Wnt16 (Table S1a). The changes in Fzd6 expression were particularly striking. Fzd6 is mildly up-regulated in normal CD5+ B cells compared with CD5− B cells (data not shown) but increases dramatically on oligoclonal expansion and then again as a dominant clone arises, as can be seen in populations A, B, and C within single animals (Figures 1C, 2A). This jump in Fzd6 expression during leukemogenic progression was noted in 2 other mice with a λ-expressing monoclonal component and consistently found in κ-bearing tumor cells at late stage (Figure 2A; and data not shown), where monoclonality could be inferred from genomic PCR analysis as performed in Figure 1B. Notably, although B-cell tumors in individual mice arise spontaneously and vary somewhat in the degree of different Wnt/Fzd gene up-regulation, significant Fzd6 up-regulation is consistently found whether we compare CD5− B and CD5+ clonal B cells from the same animal or bulk B cells from young Eμ-TCL1 mice without CD5+ B-cell expansion versus those from tumor-bearing older mice. These results support the conclusion that Fzd6 up-regulation is not simply a result of TCL1 overexpression, as B cells in young Eμ-TCL1 mice as well as CD5− B cells in old and young animals also express TCL1. More interestingly, Fzd6 expression in monoclonal CD19+CD5+Igλ+ populations is increased over the oligoclonal CD19+CD5+Igλ− B cells in 3 separate mice, in which oligoclonal expansions of CD5+Igλ− (presumably κ+) B cells coexisted with a clonal expansion of CD5+Igλ+ cells (Figure 2A). These data show that progressively increased expression of Fzd6 is associated with malignant transformation in B-cell leukemogenesis in this CLL model.
Relative expression of Wnt/Fzd components during malignant transformation. The relative mRNA levels of Fzd6, Wnt16, Wnt10a, LRP5, and LRP6 (A), of transcription cofactors β-catenin, LEF-1, and TCF-1 (B), and of Wnt/β-catenin pathway target genes Myc, Jun, Cyclin D3, and VEGF-α (C) were determined using real-time PCR. Expression levels in flow-sorted oligoclonal CD19+CD5+Igλ− tumor B cells (●; same as population B in Figure 1) and monoclonal CD19+CD5+Igλ+ B tumor B cells (■; same as population C in Figure 1) are depicted, relative to levels in normal CD19+CD5− B cells (same as population A in Figure 1) from the same animals. The relative expression levels were determined by normalizing the ΔCt values of the tumor population against the ΔCt values for normal CD19+CD5− B cells for the specific genes from the specific mouse. The solid lines connect the oligoclonal and monoclonal tumor cell populations from the same mouse. All data are shown, but in some cases there was inadequate mRNA obtained to do all analyses. By 2-sample, equal variance t test, Fzd6 expression is significantly increased between CD5− B cells and CD5+ oligoclonal B cells (P = .027) and CD5+ monoclonal B cells (P = .006). Other consistently increased genes, such as Wnt10a and Wnt16, did not reach statistical significance.
Relative expression of Wnt/Fzd components during malignant transformation. The relative mRNA levels of Fzd6, Wnt16, Wnt10a, LRP5, and LRP6 (A), of transcription cofactors β-catenin, LEF-1, and TCF-1 (B), and of Wnt/β-catenin pathway target genes Myc, Jun, Cyclin D3, and VEGF-α (C) were determined using real-time PCR. Expression levels in flow-sorted oligoclonal CD19+CD5+Igλ− tumor B cells (●; same as population B in Figure 1) and monoclonal CD19+CD5+Igλ+ B tumor B cells (■; same as population C in Figure 1) are depicted, relative to levels in normal CD19+CD5− B cells (same as population A in Figure 1) from the same animals. The relative expression levels were determined by normalizing the ΔCt values of the tumor population against the ΔCt values for normal CD19+CD5− B cells for the specific genes from the specific mouse. The solid lines connect the oligoclonal and monoclonal tumor cell populations from the same mouse. All data are shown, but in some cases there was inadequate mRNA obtained to do all analyses. By 2-sample, equal variance t test, Fzd6 expression is significantly increased between CD5− B cells and CD5+ oligoclonal B cells (P = .027) and CD5+ monoclonal B cells (P = .006). Other consistently increased genes, such as Wnt10a and Wnt16, did not reach statistical significance.
Other β-catenin signaling pathway genes that consistently show up-regulation on initiation of leukemogenesis include Fzd1, Wnt10a, and Wnt16 (Table S1a; Figure 2). In some but not all cases, these showed further up-regulation comparing preleukemic cells to the dominant clonal population in the same animal (Figure 2). In some cases, such as LEF-1, changes noted comparing populations between 2 different animals were not consistently noted comparing different populations from the same mouse as in Figure 2. The mRNA expression levels of the Wnt coreceptors LRP5 and LRP6 and β-catenin itself do not change in the tumor B cells (Figure 2A,B). In addition to the up-regulation of presumably “pro–β-catenin” factors, the expression of Tle 1 and 2 (Transducin-Like Enhancer of Split, the vertebrate homologs of Drosophila Groucho), which encode transcriptional corepressors that act with LEF/TCF at β-catenin responsive elements, are down-regulated more than 4-fold in tumor B cells versus nonneoplastic B cells (Table S1b). Decreased levels of these inhibitors could effect net increases in the expression of β-catenin/LEF-responsive genes.
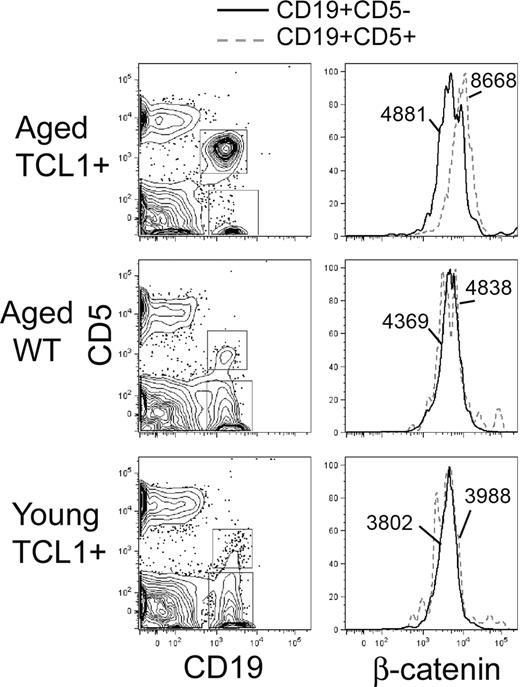
Fzd engagement by Wnt can result in both stabilization of β-catenin (canonical pathway) as well as induce noncanonical signals, including Ca++ flux and activation of JNK, although these are less well characterized in mammalian cells. To determine whether up-regulation of Wnt/Fzd pathway members is associated with evidence of canonical signaling, we assessed the levels of intracellular β-catenin in normal and malignant B cells in this model by FACS analysis. We consistently note an increase in β-catenin levels in the CD5+ tumor cells with a nearly 2-fold increase of the net geometric mean of the fluorescence intensity (Geo Mean β-catenin − Geo Mean Isotypecontrol), compared with CD5− normal B cells, in older, tumor-bearing Eμ-TCL1 mice (Figure 3). There is no difference in β-catenin levels between CD5+ and CD5− B cells in either WT or young TCL1 mice before tumor emergence (Figure 3), demonstrating that overexpression of Eμ-TCL1 alone cannot increase β-catenin protein levels. Western blotting shows the β-catenin to be primarily in the active, dephosphorylated state but is not sensitive enough to detect measurable differences between CD5+ and CD5− B cells (data not shown). Fluorescent immunostaining on purified, cytospun B cells shows nuclear and cytoplasmic localization of β-catenin, again with little discernable difference between normal and leukemic B cells (data not shown).
Intracellular β-catenin protein levels are increased in Eμ-TCL1–induced leukemia cells. Flow staining was performed on lymphocytes isolated from the peripheral blood of tumor bearing Eμ-TCL1 mice (> 10 months old), aged WT mice (> 10 months old), and young Eμ-TCL1 mice (6 weeks old). As shown in the left panels, anti–mouse CD19-APC and CD5-phycoerythrin are used to gate CD19+CD5+ and CD19+CD5− B cells. The right panels show the fluorescence intensity of FITC-conjugated anti–β-catenin in CD19+CD5+ (dotted line) and CD19+CD5− (solid line) B-cell populations. The numbers state the net geometric mean of the fluorescence intensity (Geo Meanβ-catenin − Geo Meanisotype control). Representative data of 3 independent analyses are shown.
Intracellular β-catenin protein levels are increased in Eμ-TCL1–induced leukemia cells. Flow staining was performed on lymphocytes isolated from the peripheral blood of tumor bearing Eμ-TCL1 mice (> 10 months old), aged WT mice (> 10 months old), and young Eμ-TCL1 mice (6 weeks old). As shown in the left panels, anti–mouse CD19-APC and CD5-phycoerythrin are used to gate CD19+CD5+ and CD19+CD5− B cells. The right panels show the fluorescence intensity of FITC-conjugated anti–β-catenin in CD19+CD5+ (dotted line) and CD19+CD5− (solid line) B-cell populations. The numbers state the net geometric mean of the fluorescence intensity (Geo Meanβ-catenin − Geo Meanisotype control). Representative data of 3 independent analyses are shown.
The canonical Wnt/β-catenin signaling target genes, Myc and VEGF-α, are up-regulated in the tumor B cells, whereas Cyclin D3 and Jun are not (Figure 2C). Up-regulation of Myc appears to be an early event, whereas VEGF-α expression increases primarily on monoclonal transformation.
LiCl Increases the proliferation of Eμ-TCL1–induced CD5+ tumor B cells
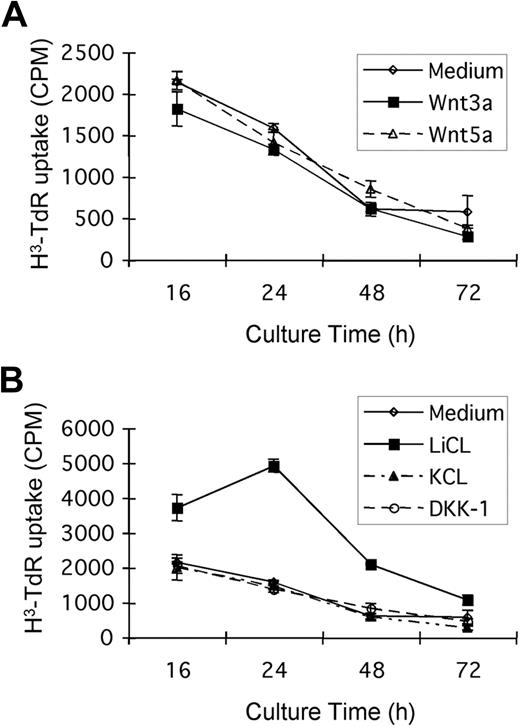
To assess the functional importance of the canonical β-catenin pathway in tumor cell biology, we examined the effects of currently available recombinant Wnt proteins on tumor B-cell proliferation and survival in vitro. The presence of Wnt3a, Wnt5, or the canonical inhibitor DKK-1 results in inconsistent, but in any case modest, effects on tumor cell survival (as measured by viability or apoptosis induction by flow cytometry; data not shown) or proliferation (Figure 4). The data shown are from experiments using spontaneous tumor B-cell proliferation, although similar results are noted in the presence of mitogenic stimuli, such as lipopolysaccharide (data not shown). These negative results may be the result of the relative dilution of tumor cells in culture compared with the in vivo situation, diminishing necessary paracrine interactions, or the absence of a critical second signal or second cell population that is missing in vitro. A more direct and potent inhibitor of GSK-3β, LiCl, does induce significant proliferation of the Eμ-TCL1 mouse CD5+ tumor cells (Figure 4). Increased cellular levels of β-catenin can be detected by Western blot in LiCl-treated cells, but these changes are too small to be detected by flow cytometry (data not shown).
The effects of Wnt, LiCL, and DKK-1 on the proliferation of Eμ-TCL1–induced tumor B cells. CD5+ tumor B cells were incubated with or without 75 ng/mL recombinant mouse Wnt3a, 100 ng/mL Wnt 5a, 100 ng/mL DKK-1, or 20 mM LiCl (KCl as the control) for 16, 24, 48, or 72 hours. (A) Wnt3a and Wnt5a do not significantly affect the proliferation of CD5+ tumor B cells from Eμ-TCL1 mice. (B) Tumor B cells proliferate in response to the GSK-3β inhibitor, LiCl, but not to identical concentrations of control KCl. DKK-1, a canonical pathway inhibitor that blocks Wnt binding to LRP5/6, had only minimal effects at a range of concentrations tested.
The effects of Wnt, LiCL, and DKK-1 on the proliferation of Eμ-TCL1–induced tumor B cells. CD5+ tumor B cells were incubated with or without 75 ng/mL recombinant mouse Wnt3a, 100 ng/mL Wnt 5a, 100 ng/mL DKK-1, or 20 mM LiCl (KCl as the control) for 16, 24, 48, or 72 hours. (A) Wnt3a and Wnt5a do not significantly affect the proliferation of CD5+ tumor B cells from Eμ-TCL1 mice. (B) Tumor B cells proliferate in response to the GSK-3β inhibitor, LiCl, but not to identical concentrations of control KCl. DKK-1, a canonical pathway inhibitor that blocks Wnt binding to LRP5/6, had only minimal effects at a range of concentrations tested.
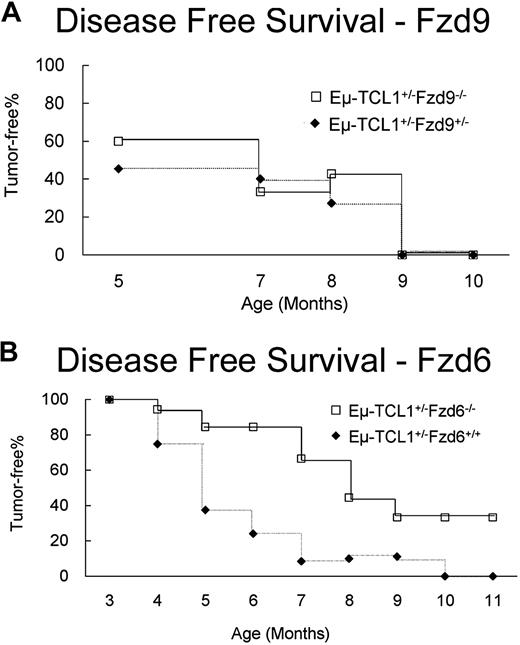
Absence of Fzd6 but not Fzd9 significantly impairs TCL1-induced leukemogenesis
To assess more directly the role of the Wnt/Fzd pathway in CLL leukemogenesis in the mouse, we crossed Eμ-TCL1 mice with strains lacking Fzd9 or Fzd6 and monitored tumor appearance and growth kinetics. We have found that the tumor cell (CD19+CD5+) percentage in peripheral blood closely approximates their percentage in spleen and peritoneal cavity in more than 95% of the Eμ-TCL1 mice we analyzed. Thus, we use tumor cell percentage in peripheral blood as a marker of tumor growth. As we knew that the absence of Fzd9 impacted pre-B development13 and therefore might restrict the number of putative lymphoma-precursor cells in the periphery that suffered initial genetic hits during Ig gene rearrangement, we first examined the effect of the absence of Fzd9 on TCL1-induced leukemogenesis. We found no changes in incidence or delay in the kinetics of CLL emergence in the Eμ-TCL1+/− × Fzd9−/− mice (Figure 5A). These findings agree with the low to absent expression of Fzd9 in tumor cells in the Eμ-TCL1 model (Table S1).
Fzd6, but not Fzd9, gene ablation delays Eμ-TCL1–induced tumor growth. Eμ-TCL1 mice were crossed with Fzd9−/− (A, n = 40) and Fzd6−/− mice (B, n = 18) to obtain Eμ-TCL1+/− animals either heterozygous for Fzd6+/− or homozygous Fzd6−/−. The fraction of CD5+CD19+ cells in peripheral blood was determined by flow cytometry at the indicated times. Disease-free survival was defined as having less than 4% peripheral blood CD5+CD19+ cells. Disease-free survival was significantly longer in mice lacking Fzd6 as determined by log-rank test (P < .001).
Fzd6, but not Fzd9, gene ablation delays Eμ-TCL1–induced tumor growth. Eμ-TCL1 mice were crossed with Fzd9−/− (A, n = 40) and Fzd6−/− mice (B, n = 18) to obtain Eμ-TCL1+/− animals either heterozygous for Fzd6+/− or homozygous Fzd6−/−. The fraction of CD5+CD19+ cells in peripheral blood was determined by flow cytometry at the indicated times. Disease-free survival was defined as having less than 4% peripheral blood CD5+CD19+ cells. Disease-free survival was significantly longer in mice lacking Fzd6 as determined by log-rank test (P < .001).
Fzd6 expression is mildly elevated in normal CD5+ B cells (data not shown) and shows dramatic up-regulation throughout leukemogenesis. We evaluated the hematopoietic compartment of Fzd6−/− mice (kind gift of Jeremy Nathans, Johns Hopkins University)10 and found no abnormalities in B- or T-cell numbers or distribution of immature, follicular, or marginal zone compartments as quantified by flow cytometric analysis of B-cell expression of CD21 and CD23 (data not shown). We find that Fzd6−/− mice also possess normal numbers of B1 (CD5+) B cells, demonstrating that Fzd6 expression is not necessary for the development of this lineage (data not shown). Analysis of Eμ-TCL1 × Fzd6−/− crosses confirms a role for Fzd6 in leukemogenesis in this model. In comparison with Eμ-TCL1+/−Fzd6+/− and Eμ-TCL1+/−Fzd6+/+ animals, mice lacking Fzd6 showed significantly delayed emergence of CLL-like disease (Figure 5B). Heterozygosity for Fzd6 did not alter tumor emergence compared with that in Eμ-TCL1+/−/Fzd6+/+ mice (data not shown). More than 30% of Eμ-TCL1+/−/Fzd6−/− mice do not show peripheral blood evidence of leukemia, even at 10 to 12 months of age, whereas all Eμ-TCL1+/−Fzd6+/− and Eμ-TCL1+/−/Fzd6+/+ mice exhibit CD5+ B-cell expansions by this time. Although most Fzd6−/− mice eventually die of leukemia by 15 months of age, in 2 animals fatal T-cell and myeloid tumors arose before any evidence of B-cell leukemia in this age range. B-cell leukemias that do arise in the absence of Fzd6 appear to have similar surface phenotype, morphology, growth kinetics, and lethality (data not shown) to Fzd6+/+ leukemias.
We do not think that “strain effects” impact the leukemia resistance or tumor biology in mice lacking Fzd6. The Fzd9−/− mice are kept on a pure 129 background, whereas the Fzd6−/− mice are mixed C57Bl/6 and 129. Because there is no significant difference in disease kinetics between Eμ-TCL1+/−Fzd9+/− mice, which are 75% 129 background, Eμ-TCL1+/−Fzd6+/− mice, which are approximately 25% 129 background, and our normal Eμ-TCL1+/−, which are C57Bl/6 backcrossed at least 7 generations from the original C3H × C57Bl/6 F1 animals, the 129 strain background itself does not appear to impact leukemogenesis either positively or negatively (Figure 5).
Wnt/Fzd/β-catenin expression in B-cell leukemias differs in the presence or absence of Fzd6
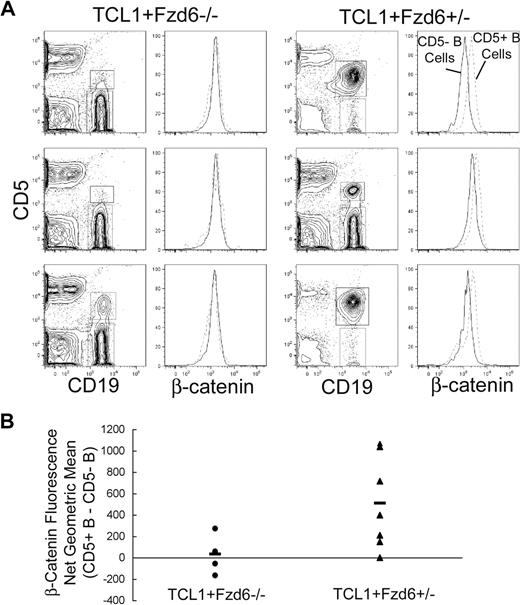
Flow cytometric analysis of the total intracellular β-catenin protein level in normal and malignant B cells from 8-month-old Eμ-TCL1+/−Fzd6+/− confirms an increase in β-catenin levels in the CD5+ tumor cells, compared with CD5− normal B cells, as seen in Figure 3 with Eμ-TCL1+/−/Fzd6+/+ mice. Tumor B cells that do arise in Eμ-TCL1+/−/Fzd6−/− littermates, however, do not show increases in β-catenin levels (Figure 6), strongly suggesting a direct link between Fzd6 up-regulation and the increased cellular β-catenin in Eμ-TCL1 leukemias. These differences in β-catenin expression cannot be explained by differences in cell size, as the average forward scatter (FSC) intensity and distribution were identical in tumor cells of either strain (data not shown). Because of the delayed appearance of CD5+ B-cell expansions in Eμ-TCL1+/−/Fzd6−/− mice, the prominence of this population is lower in mice lacking Fzd6 at this time point, as seen in Figure 6A. However, in Fzd6+/− mice, the level of net β-catenin increase, as measured by flow cytometry (Figure 6B), did not correlate with tumor bulk. Mice with only 1% to 5% CD19+CD5+ B cells exhibited some of the highest β-catenin levels in this group. Further, even Eμ-TCL1+/−/Fzd6−/− mice with advanced disease do not show increased β-catenin levels (Figure 5B and data not shown).
Beta-catenin levels are increased in Eμ-TCL1–induced tumor cells of Fzd6+/−, but not Fzd6−/−, mice. Flow staining is performed on lymphocytes isolated from peripheral blood of 8-month-old Eμ-TCL1+/−Fzd6−/− and Eμ-TCL1+/−Fzd6+/− littermates as in Figure 3. (A) CD19+CD5+ and CD19+CD5− B cells are gated as shown in the left panels. The right panels show histograms of β-catenin–FITC staining in these 2 B-cell populations (CD19+CD5+ cells are within the dashed lines). Results for 3 representative mice from each group are shown in panel A. (B) The difference in β-catenin geometric mean fluorescence intensity between CD19+CD5+ and CD19+CD5− populations for every individual mouse and the mean value for each group of mice are shown. The geometric mean difference in Eμ-TCL1+/−Fzd6+/− (▲) mice is significantly higher than that of Eμ-TCL1+/−Fzd6−/− (●) mice at P < .05 (Student t test).
Beta-catenin levels are increased in Eμ-TCL1–induced tumor cells of Fzd6+/−, but not Fzd6−/−, mice. Flow staining is performed on lymphocytes isolated from peripheral blood of 8-month-old Eμ-TCL1+/−Fzd6−/− and Eμ-TCL1+/−Fzd6+/− littermates as in Figure 3. (A) CD19+CD5+ and CD19+CD5− B cells are gated as shown in the left panels. The right panels show histograms of β-catenin–FITC staining in these 2 B-cell populations (CD19+CD5+ cells are within the dashed lines). Results for 3 representative mice from each group are shown in panel A. (B) The difference in β-catenin geometric mean fluorescence intensity between CD19+CD5+ and CD19+CD5− populations for every individual mouse and the mean value for each group of mice are shown. The geometric mean difference in Eμ-TCL1+/−Fzd6+/− (▲) mice is significantly higher than that of Eμ-TCL1+/−Fzd6−/− (●) mice at P < .05 (Student t test).
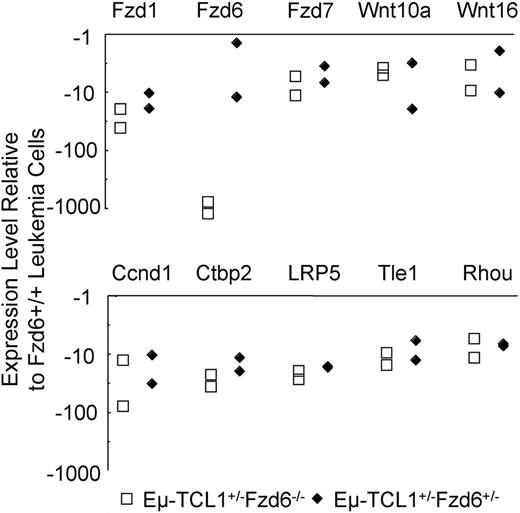
Whereas the elimination of the modest β-catenin increases in tumor cells lacking Fzd6 suggests that unrelated pathways are responsible for oncogenesis in these leukemias, we wished to assess whether alternative Fzd pathway genes were up-regulated in these cells. Comparison of gene expression in tumor cells lacking one or both copies of the Fzd6 gene with those from Eμ-TCL1+/−Fzd6+/+ mice revealed that, rather than up-regulation of alternative Fzd genes in the absence of Fzd6, these tumors have lower levels of Fzd1 and Fzd7 mRNA, as well as Wnt10a and Wnt16 mRNA in comparison with Fzd6+/+ leukemic B cells (Figure 7). In contrast to the transformed B cells in Eμ-TCL1+/−Fzd6−/− mice, comparison of “normal” B-cell gene signatures in Eμ-TCL1 Fzd6+/+ versus Fzd6−/− mice reveals that normal, CD19+CD5− B cells lacking Fzd6 overexpress Fzd1, Fzd3, and Fzd4 mRNA from 3.7- to 7.7-fold compared with their Fzd6+/+ counterparts (data not shown). Thus, in nonmalignant B cells, the absence of Fzd6 appears to trigger an increase in some other Fzd mRNA species. Although Fzd3 and Fzd4 expression did not differ between Fzd6+/+ and Fzd6−/− leukemic cells, this finding makes the relative decrease in Fzd1 mRNA in Fzd6−/− tumor cells even more significant. Finally, unlike all tested cases of Fzd6+/+ leukemias, levels of c-MYC mRNA were not elevated in Fzd6−/− tumor B cells in comparison with residual normal B cells (data not shown). This suggests the possibility that β-catenin elevations do play a role in transcriptional up-regulation of MYC in these tumors.
Impact of Fzd6 KO on the expression of Wnt, Fzd, and other Wnt pathway components on Eμ-TCL1–induced tumor B cells. Total RNA was isolated from flow cytometry–sorted CD19+CD5+ tumor B cells of age and tumor burden matched Eμ-TCL1+/−Fzd6−/−, Eμ-TCL1+/−Fzd6+/−, and Eμ-TCL1+/−Fzd6+/+ tumor-bearing mice (n = 2). Gene expression of Wnt, Fzd families, and other components related with the Wnt pathway was determined by real-time PCR array of mouse Wnt signaling as described in “Real-time PCR array.” Data are shown as relative expression levels determined by normalizing the ΔCt values of the Eμ-TCL1+/−Fzd6−/− or Eμ-TCL1+/−Fzd6+/− against the ΔCt values for Eμ-TCL1+/−Fzd6+/+ mice for the specific genes. The genes, which on average show no less than 4-fold changes in both Eμ-TCL1+/−Fzd6−/− and Eμ-TCL1+/−Fzd6+/− mice, are indicated in the plot.
Impact of Fzd6 KO on the expression of Wnt, Fzd, and other Wnt pathway components on Eμ-TCL1–induced tumor B cells. Total RNA was isolated from flow cytometry–sorted CD19+CD5+ tumor B cells of age and tumor burden matched Eμ-TCL1+/−Fzd6−/−, Eμ-TCL1+/−Fzd6+/−, and Eμ-TCL1+/−Fzd6+/+ tumor-bearing mice (n = 2). Gene expression of Wnt, Fzd families, and other components related with the Wnt pathway was determined by real-time PCR array of mouse Wnt signaling as described in “Real-time PCR array.” Data are shown as relative expression levels determined by normalizing the ΔCt values of the Eμ-TCL1+/−Fzd6−/− or Eμ-TCL1+/−Fzd6+/− against the ΔCt values for Eμ-TCL1+/−Fzd6+/+ mice for the specific genes. The genes, which on average show no less than 4-fold changes in both Eμ-TCL1+/−Fzd6−/− and Eμ-TCL1+/−Fzd6+/− mice, are indicated in the plot.
Discussion
Previous gene expression studies in human CLL suggested that Wnt/Fzd signaling components, including Fzd3, Wnts 10a and 16, and LEF-1, are significantly up-regulated compared with their expression in normal B cells14-16 but were unable to determine whether these changes were secondary to other genetic aberrations or critical for the neoplastic phenotype. In this study, we describe a strikingly similar pattern of Wnt/Fzd pathway up-regulation both in early and late stages of neoplastic B-cell development in a mouse model of CLL and show that the up-regulation of Fzd6 is an important, although not essential, component of leukemogenesis induced by the TCL1 oncogene.
Although it is only with caution that findings in mouse models can be directly applied to human disease, our findings do partially mirror the published human CLL data, with the same 2 Wnt genes (10a and 16) up-regulated in the mouse leukemia model. In contrast to human CLL where Fzd3 was the most up-regulated Fzd family member in a quantitative PCR-based study,16 we noted dramatic up-regulation of Fzd6, both during a preleukemic oligoclonal expansion and again on transformation to a monoclonal leukemia. Fzd6 and Fzd3 are the most closely phylogenetically related Fzd proteins27 and have been shown to function redundantly to control neural tube closure and the orientation of a subset of auditory and vestibular sensory cells in mice.28 Interestingly, human Fzd6 has been shown to inhibit the canonical signaling pathway via phosphorylation of LEF/TCF targets,29 but mouse Fzd6 differs more substantially from its human homolog (89% similar) compared with the near 99% identity between mouse and human Fzd3. Indeed, it is difficult to predict how a given pair of Wnt and Fzd proteins will interact in a given species and in a specific, nonfibroblast cell lineage such as B cells.
Up-regulation of Fzd6 expression has been detected in other human malignancies, including squamous cell and prostate carcinomas,30,31 although its function in these tumors remains unexplored. Fzd6 also is one of the genes showing the highest expression level in the slow-dividing fraction of human cord blood hematopoietic progenitor cells, suggesting a potential role in self-renewal.32 The role of β-catenin and Wnt/Fzd signaling in tissue-specific stem cell maintenance2,33 and its role in self-renewing proliferation versus differentiation in developing B cells13 were what originally induced us to examine the expression of these signaling components during leukemogenesis. A primary role for Fzd6 up-regulation in this mouse model of CLL is confirmed by the substantial delay and decreased incidence of B-cell leukemogenesis in Eμ-TCL1 animals lacking the Fzd6 gene. The up-regulation of intracellular β-catenin levels in Fzd6+/+ leukemic B cells and the presence of normal β-catenin levels in leukemic cells developing in the absence of Fzd6 further suggest that up-regulated Fzd6 protein is providing canonical pathway signals. This is of particular importance in this model, as TCL1 is thought to function primarily by augmentation of the Akt pathway.34 Akt is capable of phosphorylating and thus inhibiting GSK-3β,35 and phosphorylating β-catenin itself at an activating locus,36 both of which could stabilize β-catenin in a Wnt/Fzd-independent fashion. However, in mouse B cells, overexpression of TCL1 alone does not increase β-catenin levels, as nontransformed CD5+ B cells from young Eμ-TCL1 mice, CD5− B cells from aged mice, and tumor B cells in Eμ-TCL1+/−Fzd6−/− mice show identical β-catenin levels compared with WT B or T cells. In addition, Fzd6 expression is not increased by the TCL1 transgene alone in CD5− or CD5+ B cells compared with nontransgenic cells (data not shown). Although TCL1-induced malignant transformation can occur in the absence of up-regulated Fzd6, this is not associated with β-catenin up-regulation. The mechanism(s) of Fzd6 up-regulation in Eμ-TCL1 tumor cells is unknown and currently under investigation.
The precise function of β-catenin and Wnt/Fzd signaling in mouse or human CLL B cells is not clear. In human CLL cells, there is an indirect indication that this pathway protects from apoptosis in vitro.16 In the Eμ-TCL1 mouse, direct in vitro effects on proliferation and apoptosis of tumor B cells by Wnt3a, Wnt5a, or the canonical inhibitor DKK-1 have been inconsistent thus far. The effects of different Wnt proteins, particularly in vivo in the presence of other hematopoietic and stromal elements, are probably complex and difficult to reproduce in vitro.
Contrary to our expectations, B-cell tumors that arose, in a delayed fashion, in Fzd6−/− mice did not appear to compensate for the absence of Fzd6 by up-regulating an alternative Fzd protein. Indeed, compared with Fzd6+/+ tumor B cells, there was substantial down-modulation of Fzd1, Fzd7, LRP5, Wnt10a, and Wnt16. This suggests the possibility that Wnt/Fzd-mediated signals both positively and negatively impact the growth and survival of the malignant clone in vivo. Wnt10a and/or Wnt16 binding Fzd6 may mediate pro-leukemia signals that are partially balanced by negative signals mediated through Fzd1 and/or Fzd7. Thus, in the absence of the Fzd6 gene, making up-regulation impossible (or diminished, in the case of Fzd6+/− animals), potential negative signals may need to be down-modulated for late leukemia development, thereby explaining the decrease in Fzd1, Fzd7, LRP5 coreceptor, and Wnt expression in the malignant cells that emerge in Fzd6−/− mice. The decrease in expression of these genes is even more striking given that they are up-regulated in the normal B cells of Eμ-TCL1+/−Fzd6−/− mice compared with Eμ-TCL1+/−Fzd6+/+ B cells. As we have been unable to find obvious defects in B-cell development or proliferation in Fzd6−/− mice, it is as yet unclear how these pathways may interact in the nontransformed state.
This study provides further support for a direct role of the Wnt/Fzd pathway in B-cell leukemogenesis in CLL. We are currently assessing the role of Fzd6 and β-catenin in the initiation and progression of CLL in the mouse model by directly transducing B cells with inhibitors and promoters of this pathway. The emergence of more specific small molecule inhibitors of Wnt/Fzd signaling37 will allow for the testing of the effectiveness of therapeutic disruption of this pathway in vitro and in vivo in animal models, such as the Eμ-TCL1 mouse.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Songwon Seo (University of Wisconsin) for expert assistance with statistical analysis.
This work was supported by National Institutes of Health (grant 5KO8CA090450-03), the University of Wisconsin Medical Education and Research Committee, and the V Foundation for Cancer Research (Cary, NC).
National Institutes of Health
Authorship
Contribution: Q.-L.W. designed and performed research, collected, analyzed, and interpreted data, performed statistical analyses, and assisted in writing the manuscript; C.Z. designed and performed research, analyzed and interpreted data, and edited the manuscript; and E.A.R. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Erik A. Ranheim, 600 Highland Avenue, K4/850 CSC, Madison, WI 53792-8550; e-mail: earanheim@wisc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal