Abstract
Twenty-two adult acute lymphoblastic leukemia (ALL) patients (21 of 22 in complete remission [CR]) received reduced-intensity conditioning followed by allogeneic transplantation. All patients were high risk. After a uniform preparative regimen (fludarabine 40 mg/m2 × 5, cyclophosphamide 50 mg/kg, 200 cGy total body irradiation), patients received either matched related (n = 4) or umbilical cord (n = 18) donor grafts. All patients reached neutrophil engraftment and 100% donor chimerism (median, days 10 and 23, respectively). Overall survival, treatment-related mortality (TRM) and relapse were 50% (95% confidence interval [CI], 27%-73%), 27% (95% CI, 9%-45%), and 36% (95% CI, 14%-58%) at 3 years, respectively. There were no relapses beyond 2 years. The cumulative incidence of acute and chronic graft-versus-host disease was 55% and 45%. Hematopoietic cell transplantation in CR1 (n = 14) led to significantly less TRM (8%, P < .04) and improved overall survival (81%, P < .01). For adults with ALL in CR, reduced intensity conditioning allografting results in modest TRM, limited risk of relapse, and promising leukemia-free survival. Clinical trial numbers are NCT00365287, NCT00305682, and NCT00303719.
Introduction
In contrast to 80% disease-free survival for children with acute lymphoblastic leukemia (ALL), adults fare much worse; only one-third survive beyond 5 years.1,2 Older adults with ALL are frequently high risk (Philadelphia chromosome–positive [Ph+] or high white blood count), yielding a poor prognosis.3 The more than 85% relapse rate in patients with Ph+ ALL was reduced by donor hematopoietic cell transplantation (HCT), implying that a graft-versus-leukemia effect may cure ALL.3-5 Recently, a landmark publication from Medical Research Council/Eastern Cooperative Oncology Group reported the outcomes of more than 1900 adult ALL patients in first complete remission (CR1) and showed improved survival (∼53%) for patients allocated to sibling HCT versus either consolidation/maintenance chemotherapy or autologous HCT.6 The best survival was observed in standard risk ALL patients younger than 35 years. The benefit for older patients was abrogated by unacceptably high treatment-related mortality (TRM).6,7 The optimal therapy for older patients or those unable to tolerate myeloablative conditioning is uncertain. Allogeneic HCT using reduced-intensity conditioning (RIC) has promise for limiting TRM, whereas its antileukemia potency is uncertain.8-10 We present results of 22 patients with high-risk ALL in CR undergoing RIC allotransplantation and show modest TRM and promising leukemia-free survival.
Methods
We analyzed data from 22 consecutive patients with ALL enrolled on allogeneic RIC HCT studies, which were prospectively collected in the University of Minnesota Blood and Marrow Transplantation Database between 2001 and 2008 (Table 1).
Patient and graft characteristics
| Characteristic . | n (%) . |
|---|---|
| Sex | |
| Male | 8 (36) |
| Female | 14 (64) |
| Diagnosis | |
| ALL Ph+ [CR1/ ≥ CR2] | 14 [10/4] (64) |
| ALL B-lineage | 6 (27) |
| ALL T-lineage | 2 (9) |
| Disease status at transplantation | |
| CR1 | 12 (55) |
| CR2 or greater | 9 (40) |
| PIF-sensitive | 1 (5) |
| CNS disease at diagnosis | |
| No | 16 (73) |
| Yes [cranial irradiation Y/N] | 6 [1/5] (27) |
| Prior autotransplantation/allotransplantation | |
| None | 19 (86) |
| Yes, autotransplantation | 2 (9) |
| Yes, allotransplantation | 1 (5) |
| Induction/intensification therapy (CR1 patients) | 12 (55) |
| Hyper-CVAD /M, Ara-C, P | 2 (9) |
| Da, V, C, L-asp, P/C, Ara-C, 6MP, V, L-asp | 4 (18) |
| Da, V, L-asp, P/C, Ara-C, 6MP | 5 (21) |
| Unknown | 1 (5) |
| Recipient CMV | |
| Negative | 9 (41) |
| Positive | 13 (59) |
| Donor CMV | |
| Negative | 14 (77) |
| Positive | 5 (23) |
| Year of transplantation | |
| 2001-2004 | 10 (47) |
| 2005-2008 | 12 (53) |
| Donor type (HLA locus matching) | |
| PBSC sibling (6 of 6) | 4 (18) |
| UCB (4 of 6) | 12 (55) |
| UCB (5 of 6) | 5 (23) |
| UCB (6 of 6) | 1 (5) |
| Comorbidity index | |
| 0 | 6 (36) |
| 1-2 | 4 (18) |
| 3 or higher | 11 (55) |
| Median (range) | 3 (0–8) |
| Median WBC at diagnosis | 20 (range, 1.4-248) |
| Weight, kg | 74.8 (range, 41.5-120) |
| Follow-up, mo | 33 (range, 5-76) |
| Characteristic . | n (%) . |
|---|---|
| Sex | |
| Male | 8 (36) |
| Female | 14 (64) |
| Diagnosis | |
| ALL Ph+ [CR1/ ≥ CR2] | 14 [10/4] (64) |
| ALL B-lineage | 6 (27) |
| ALL T-lineage | 2 (9) |
| Disease status at transplantation | |
| CR1 | 12 (55) |
| CR2 or greater | 9 (40) |
| PIF-sensitive | 1 (5) |
| CNS disease at diagnosis | |
| No | 16 (73) |
| Yes [cranial irradiation Y/N] | 6 [1/5] (27) |
| Prior autotransplantation/allotransplantation | |
| None | 19 (86) |
| Yes, autotransplantation | 2 (9) |
| Yes, allotransplantation | 1 (5) |
| Induction/intensification therapy (CR1 patients) | 12 (55) |
| Hyper-CVAD /M, Ara-C, P | 2 (9) |
| Da, V, C, L-asp, P/C, Ara-C, 6MP, V, L-asp | 4 (18) |
| Da, V, L-asp, P/C, Ara-C, 6MP | 5 (21) |
| Unknown | 1 (5) |
| Recipient CMV | |
| Negative | 9 (41) |
| Positive | 13 (59) |
| Donor CMV | |
| Negative | 14 (77) |
| Positive | 5 (23) |
| Year of transplantation | |
| 2001-2004 | 10 (47) |
| 2005-2008 | 12 (53) |
| Donor type (HLA locus matching) | |
| PBSC sibling (6 of 6) | 4 (18) |
| UCB (4 of 6) | 12 (55) |
| UCB (5 of 6) | 5 (23) |
| UCB (6 of 6) | 1 (5) |
| Comorbidity index | |
| 0 | 6 (36) |
| 1-2 | 4 (18) |
| 3 or higher | 11 (55) |
| Median (range) | 3 (0–8) |
| Median WBC at diagnosis | 20 (range, 1.4-248) |
| Weight, kg | 74.8 (range, 41.5-120) |
| Follow-up, mo | 33 (range, 5-76) |
ALL indicates acute lymphoblastic leukemia; Ph+, Philadelphia chromosome–positive; CR, complete remission; PIF, primary induction failure; C, cyclophosphamide; V, vincristine; A, doxorubicin; Ara-C, cytarabine; 6MP, 6-mercaptopurine; Da, daunorubicin; D, dexamethasone; P, prednisone; L-asp, L-asparaginase; M, methotrexate; CMV, cytomegalovirus; WBC, white blood cell count; HLA, human leukocyte antigen; PBSC, peripheral blood stem cell; and UCB, umbilical cord blood.
Median age was 49 years (range, 24-68 years); 3 were younger than 35 years. Indications for RIC included age (> 55 years for sibling HCT, n = 3; > 45 years for unrelated umbilical cord blood [UCB] HCT; n = 12), Karnofsky performance status less than 80% (n = 4), and prior HCT (n = 3). Patients had high-risk ALL defined as Ph+ (n = 14) and more than or equal to CR2 (n = 10). Twenty-one patients were in CR; 19 were in cytogenetic remission at HCT. Five patients with Ph+ ALL received tyrosine kinase inhibitors (TKIs; imatinib 600-800 mg, n = 3; dasatinib 140 mg, n = 2) before HCT. After transplantation, TKIs were used for molecular or morphologic relapse. Patients received either matched related (n = 4) or UCB (n = 18) donor grafts at a median 222 days (range, 91-3589 days) after diagnosis. Median follow-up among survivors is 33 months (range, 5-76 months).
Treatment plan
The transplantation protocol was approved by the University of Minnesota Institutional Review Board. All patients gave written informed consent in accordance with the Declaration of Helsinki. Twenty patients received 200 cGy total body irradiation (day −1) plus fludarabine 40 mg/m2 per day intravenously daily (days −6 through −2) and cyclophosphamide 50 mg/kg per day intravenously day −6. In one patient, busulfan 2 mg/kg orally every 12 hours for 4 doses (days −8, −7) replaced cyclophosphamide. All patients received cyclosporine (days −3 to 180) and mycophenolate mofetil (1 g intravenously or orally twice a day) from day −3 to day 30. Institutional practices for drug monitoring and supportive care have been reported.11
Statistical analysis
Kaplan-Meier estimates of overall survival (OS) and cumulative incidence estimates of TRM, engraftment, graft-versus-host disease (GVHD), and relapse were calculated as of June 2008.12,13 Log-rank statistics were used to compare time to event curves. Event times were measured from the date of transplantation to the date of death or last contact.
Results and discussion
All patients had sustained neutrophil engraftment (> 0.5 × 109/L) at median 10 days (range, 0-28 days), and 77% (95% CI, 54%-100%) had an unsupported platelet count more than 20 × 109/L at a median of 38 days after transplantation (range, 0-167 days). All patients achieved 100% donor chimerism at a median 23 days after transplantation (range, 14-99 days).
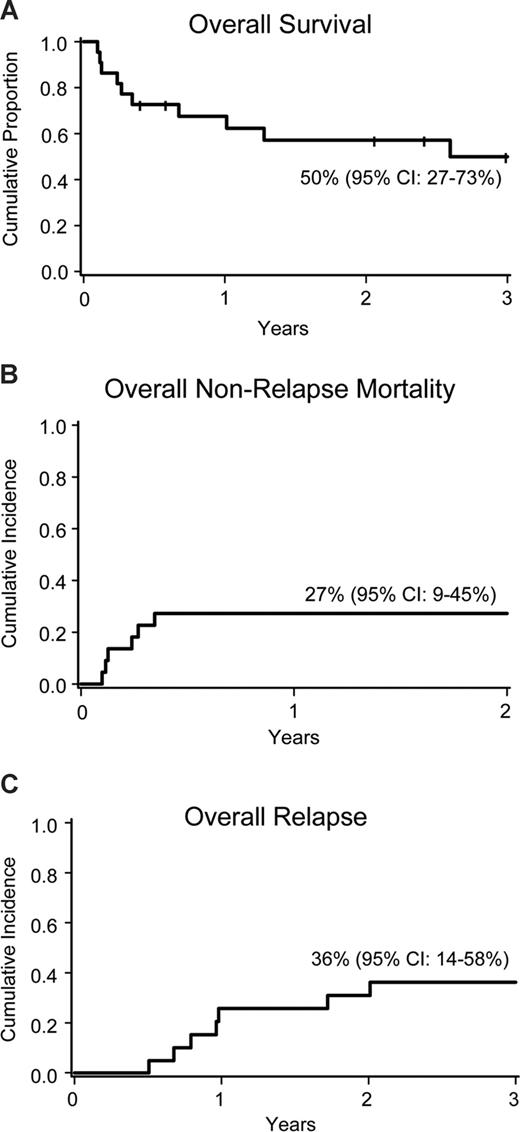
At 3 years, OS was 50% (95% CI, 27%-73%; Figure 1A). For 12 patients in CR1, 3-year OS was 81% (95% CI, 57%-100%) compared with 15% (95% CI, 0%-40%) for patients who received transplants in CR2 or more (P < .01). TRM at 3 years was 27% (95% CI, 9%-45%; Figure 1B) with no events after 6 months. Promisingly low TRM in CR1 patients (8%, 95% CI, 0%-23%) compared with CR2 or more patients (50%, 95% CI, 20%-80%, P = .04) was the main factor leading to improved OS. TRM for patients with less than 1 year (n = 14) between diagnosis and HCT was 14% (95% CI, 0%-32%) versus 50% (95% CI, 16%-84%) for those 1 year or more (P = .09). Cumulative incidence of relapse was 36% (95% CI, 14%-58%) with most events occurring before 1 year (Figure 1C). Relapse risk was similar in CR1 (n = 4) and CR2 or more (n = 3) and did not correlate with time from diagnosis to transplant (CR1 median 107 days; range, 91-231 days; patients with relapse 101, 109, 129, 214 days). OS, TRM, and relapse after UCB HCT (n = 18) were 49% (95% CI, 23%-75%), 28% (95% CI, 8%-48%), and 33% (95% CI, 9%-57%). Outcomes were similar for patients with Ph+ and Ph− ALL. No effect of patient age, weight, performance status, comorbidity index,14 cytomegalovirus serostatus, white blood cell count at diagnosis, total nucleated cell dose on TRM, or survival was observed, although the analysis is limited by small sample size. Patients with central nervous system leukemia (CR1, n = 1; ≥ CR2, n = 5) had increased risk of TRM (n = 3) and systemic relapse (n = 3).
Transplantation outcomes. (A) Overall survival. (B) Treatment-related mortality. (C) Relapse rate.
Transplantation outcomes. (A) Overall survival. (B) Treatment-related mortality. (C) Relapse rate.
In this high-risk cohort, 9 patients have durable and prolonged leukemia-free survival (6-77 months), suggesting potent antileukemia reactivity of both UCB and sibling grafts. Interestingly, 3 of 7 relapsed Ph+ ALL patients attained a subsequent CR with dasatinib. One survives in CR at 25 months after a second RIC HCT, 44 months after the initial HCT.
At day 100, the cumulative incidences of grade II-IV and III-IV acute GVHD were 55% (95% CI, 32%-88%) and 20% (95% CI, 3%-37%), respectively. One-year survival of patients with grade II-IV acute GVHD was significantly improved (relative risk = 0.2; 95% CI, 0.05-0.8; P = .02). One-year cumulative incidence of chronic GVHD was 45% (95% CI, 21%-69%); 7 patients had extensive chronic GVHD.
Whereas the Medical Research Council/Eastern Cooperative Oncology Group and Center for International Blood and Marrow Transplant Research reports of myeloablative allografts for adult ALL in CR1 demonstrate OS of 40% to 53%, patients older than 35 years had substantially worse TRM and survival.3,6,15 Our data suggest that survival can be improved using RIC HCT. RIC allograft for adult ALL may limit TRM, particularly if performed in CR1. Rapid, complete donor engraftment was achieved without donor lymphocyte infusion. Most of our patients received UCB grafts, and their outcomes suggest that UCB is a valuable option for older patients with ALL who lack a matched or sufficiently healthy sibling donor. Considerably higher TRM in patients with recurrent ALL may reflect the consequences of protracted prior chemotherapy, rendering older patients vulnerable to HCT complications. The otherwise dismal prognosis (disease-free survival ∼7%-12%) of advanced ALL in adults2,16 suggests that the prevention of relapse with allografting in CR1 is the best opportunity to achieve long-term survival. RIC HCT may be particularly relevant for Ph+ ALL. The widespread use of TKIs during induction chemotherapy can limit minimal residual leukemia17,18 and favorably influence subsequent outcome if RIC regimens are used. Whereas earlier reports suggest feasibility and reduced TRM of RIC allotransplantation for adult ALL, many enrolled patients had advanced persistent or refractory ALL (40% in European Group for Bone and Marrow Transplantation registry analysis),10 leading to poor leukemia control and relapse rates of 49% to 60%. Our data show that OS for ALL patients after RIC HCT is comparable with that achieved with conventional allografting.3,7,19-22 Long-term follow-up is warranted to confirm the rates of sustained and durable remissions. The results from this relatively small cohort are encouraging and justify enrolling patients in future trials testing RIC HCT for ALL patients in CR1 or later CR who are unsuited for more intensive, myeloablative conditioning.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.B. designed the study, collected and verified patient information, analyzed and interpreted data, and wrote the manuscript; C.G.B. assisted with data interpretation and critically reviewed the manuscript; M.R.V. contributed to the data analyses and assisted in writing the manuscript; T.D. collected and analyzed data and performed statistical analysis; and D.J.W. designed research, interpreted data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Veronika Bachanova, Blood and Marrow Transplant Program, University of Minnesota, Mayo Mail Code 480; 420 Delaware St SE, Minneapolis, MN 55455; e-mail: bach0173@umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal