In this issue of Blood, Di Nicola and colleagues report that vaccination of relapsed indolent non-Hodgkin lymphoma patients with DCs loaded with killed autologous tumor cells induced a multifaceted immunologic response that correlated with objective clinical responses including 3 continuous complete responses lasting beyond 45 months.
Despite the promise of harnessing the immune system to target cancer cells, most phase III trials of cancer vaccines have been disappointing. Potential reasons for these failures, despite the high immunogenicity of vaccines,1 may be categorized into factors affecting the afferent or priming phase of the immune response and factors influencing the efferent or effector phase of the immune response. For instance, during the afferent phase of the immune response, it is possible that the magnitude of the T-cell response or the avidity of the induced T cells was not sufficient to kill tumor cells effectively after vaccination. During the efferent phase of the immune response, it is possible that the antitumor T cells may not have trafficked to the tumor site or if they trafficked, they may not have been able to overcome newly recognized immunosuppressive mechanisms present in the tumor microenvironment.
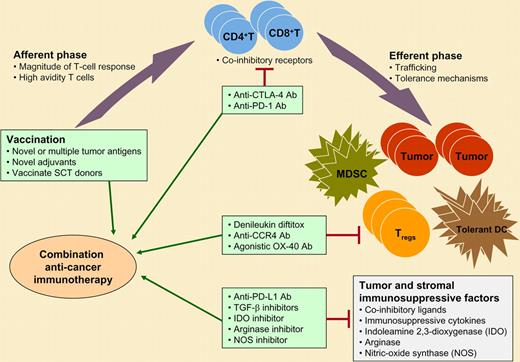
Combination immunotherapeutic strategies for cancer. The afferent or priming phase of the immune response may be enhanced by vaccines using novel tumor antigens or adjuvants or by vaccinating stem cell transplant (SCT) donors with healthy immune systems with the goal of adoptive transfer of the antitumor immunity to the patient. The efferent or effector phase of the immune response may be augmented by inhibiting various immunosuppressive and tolerance mechanisms in the tumor microenvironment. Potential strategies that are in various stages of preclinical and clinical development are shown. These agents may be used in combination with therapeutic cancer vaccines for optimal induction of antitumor immunity that, in turn, may lead to improved clinical outcome. MDSC, myeloid-derived suppressor cells; TGF-β, transforming growth factor beta.
Combination immunotherapeutic strategies for cancer. The afferent or priming phase of the immune response may be enhanced by vaccines using novel tumor antigens or adjuvants or by vaccinating stem cell transplant (SCT) donors with healthy immune systems with the goal of adoptive transfer of the antitumor immunity to the patient. The efferent or effector phase of the immune response may be augmented by inhibiting various immunosuppressive and tolerance mechanisms in the tumor microenvironment. Potential strategies that are in various stages of preclinical and clinical development are shown. These agents may be used in combination with therapeutic cancer vaccines for optimal induction of antitumor immunity that, in turn, may lead to improved clinical outcome. MDSC, myeloid-derived suppressor cells; TGF-β, transforming growth factor beta.
However, indolent B-cell non-Hodgkin lymphomas may be different from many other solid tumors, as they are considered highly immune responsive. In the current study, vaccination with dendritic cells (DCs) loaded with autologous heat-shocked, irradiated, and ultraviolet-C–treated lymphoma cells induced both T-cell and B-cell tumor-specific immune responses. As opposed to other vaccination strategies targeting a single tumor antigen, this vaccine formulation can potentially induce immune responses against multiple tumor-associated antigens and thus may minimize immune escape. Moreover, vaccination skewed the maturation of the T cells to effector memory and/or terminally differentiated phenotype, activated natural killer cells, and intriguingly, reduced the number of CD4+CD25+Foxp3+ regulatory T cells in the peripheral blood and/or tumor microenvironment. Aside from minor quibbles with specificity controls, the assays used by Di Nicola et al to measure immune responses were state-of-the-art and appeared to correlate with clinical responses, as has been described for other hematologic cancer vaccines.2,3 Collectively, the enhancement of multiple immunostimulatory signals and down-modulation of the immunoregulatory elements of the immune system may explain the higher objective clinical response rate (33.3%) observed with this and another previously reported DC vaccine formulation4 compared with non-DC vaccine formulations.5 However, the clinical responses were induced primarily in patients with low tumor burden, suggesting there may be opportunity for further optimization of vaccine strategies to eradicate disease in patients with large tumor masses prior to vaccination. Recent discoveries in basic immunology have also now provided us several novel agents to augment both the afferent and efferent phases of the immune response following therapeutic vaccination. The afferent phase of the immune response could be enhanced by using more potent vaccines with novel adjuvants, such as Toll-like receptor ligands, or by vaccinating donors of transplant recipients who have a healthy immune system as opposed to patients who may be immunocompromised either from the cancer or from therapy.3 Alternatively, vaccines could be used in combination with agents that inhibit the immunosuppressive mechanisms such as coinhibitory receptors/ligands6 and/or deplete regulatory T cells7 to augment the effector phase of the immune response.
The natural history of development of a novel therapeutic modality is often marked by initial enthusiasm (up) followed by periods of discouragement (down), as obstacles are encountered. The promising results reported by Di Nicola et al, the recent approval of a heat shock protein vaccine (vitespen) in Russia for the adjuvant treatment of patients with renal cell carcinoma at intermediate risk of disease recurrence,8 and advances in understanding of immune tolerance suggest that we may be very close to overcoming these barriers to success for therapeutic cancer vaccines.
Conflict-of-interest disclosure: S.S.N. declares no competing financial interests. L.W.K. is a consultant for Antigenics Inc. ■