The vitamin A metabolite retinoic acid (RA) plays a fundamental role in cellular functions by activating nuclear receptors. Retinaldehyde dehydrogenase-II (RALDH2) creates localized RA gradients needed for proper embryonic development, but very little is known regarding its regulated expression in adults. Using a human ex vivo model of aller-gic inflammation by coincubating IgE receptor–activated mast cells (MCs) with blood basophils, we observed prominent induction of a protein that was identified as RALDH2 by mass spectroscopy. RALDH2 was selectively induced in basophils by MC-derived interleukin-3 (IL-3) involving PI3-kinase and NF-κB pathways. Importantly, neither constitutive nor inducible RALDH2 expression was detectable in any other human myeloid or lymphoid leukocyte, including dendritic cells. RA generated by RALDH2 in basophils modulates IL-3–induced gene expression in an autocrine manner, providing positive (CD25) as well as negative (granzyme B) regulation. It also acts in a paracrine fashion on T-helper cells promoting the expression of CD38 and α4/β7 integrins. Furthermore, RA derived from IL-3–activated basophils provides a novel mechanism of Th2 polarization. Thus, RA must be viewed as a tightly controlled basophil-derived mediator with a high potential for regulating diverse functions of immune and resident cells in allergic diseases and other Th2-type immune responses.
Introduction
Vitamin A (retinol) is essential in vertebrate physiology, and vitamin A deficiency represents an important health problem in many third world countries.1 The retinol derivative RA is a major pleiotropic mediator regulating diverse developmental processes and cellular functions. It activates the nuclear receptors RA receptor RAR/RXR-heterodimers to regulate the transcription of target genes containing RA-response elements (RAREs). RA is synthesized from retinol via a retinaldehyde intermediate and the enzymes that determine the tissue specificity of RA synthesis are retinaldehyde dehydrogenases (RALDHs) that catalyze the last oxidative step. ALDH1A1 (also known as ALDH1, RALDH1), an important aldehyde-detoxifying enzyme that also accepts retinal as substrate, is widely expressed in adult tissues and is thus likely responsible for low RA levels present in tissues throughout the body.2,3 The 2 most efficient and selective RA-synthesizing enzymes are RALDH2 (gene name ALDH1A2) and RALDH3 (ALDH1A3).2,–4 Expression of RALDH2 is required for the spatial and temporal RA gradients needed for proper embryonic development, as evidenced by the lethal phenotype of RALDH2-KO mice and transgenic RARE reporter mice.5,,–8 In sharp contrast to the extensive research on RALDH2 in the embryo, very little is known regarding the function of this enzyme in the adult: it is unknown whether the enzyme continues to generate local foci of RA synthesis or whether it is involved in any pathophysiological processes, in particular with regard to immune-mediated diseases
It is well established that RA can affect gene expression and function of almost any cell, including cells of the immune system; however, the physiological implications of such findings are uncertain, particularly when pharmacologic RA concentrations are used. Several studies indicate that vitamin A deficiency compromises the function of the immune system, in particular humoral Th2-mediated immune responses.9 A role of RA in immune regulation is also supported by the observation that RA added as an adjuvant promotes a Th2 bias and inhibits experimental autoimmune disease in rodents.10,11 Furthermore, very recent findings indicate that RA regulates homeostatic lymphocyte function and trafficking to the gut.12,13 This large body of data on RA effects in vitro and in vivo contrasts greatly with the lack of knowledge about the regulation of expression of the major RA-synthesizing enzyme RALDH2 in different cell types of the immune system.
Immediate-type allergic diseases, such as atopic dermatitis, rhinoconjunctivitis, and asthma, have reached epidemic proportions in industrialized countries. Why the “Western lifestyle” has led to this strong increase in the prevalence of atopic diseases is unclear, although it is believed that better hygiene standards are a cause (hygiene hypothesis).14 Current concepts of the pathophysiology of allergic diseases emphasize the importance of Th2 lymphocytes and eosinophils,15 but at least in human asthma there is also increasing evidence for a key role of high-affinity IgE receptor (FcϵRI)–expressing tissue-resident mast cells (MCs)16 and basophils.17,18 Human basophils can produce major mediators of asthma, such as histamine, leukotriene C4, interleukin (IL)-4, and IL-13, implying an important contribution of this cell type to the pathology of allergic inflammation.17,,,–21 There is also increasing evidence for the importance of “basophil-like” FcϵRI+ non-B–non-T cells in several experimental mouse models of Th2-type immune responses.18,21,,–24 However, there are major differences between the phenotype and functions of mouse basophil-like cells and human basophils and between mouse models of asthma and the natural human disease. Thus, a better definition of the cooperation and interactions occurring between primary human cells involved in asthma is badly needed to understand the complex molecular and cellular events operating in allergic disease in humans.
Upon allergen challenge of allergic patients, blood basophils are attracted to sites of inflammation where they can interact with activated tissue MCs.25 To mimic such cellular interactions occurring in allergic disease, we established a human ex vivo system by exposing blood basophils to tissue-derived MCs activated by FcϵRI cross-linking. We show here that a MC-derived factor, identified as IL-3, selectively induces in basophils strong expression of functional RALDH2. IL-3–activated basophils are able to produce RA at levels sufficient to promote phenotypic and functional cellular alterations in an autocrine and paracrine fashion on basophils and T cells, respectively. Thus, local production of RA by basophils at sites of allergic inflammation could influence gene expression programs, phenotype, and functions of a variety of cells, implying that RA must be considered as a mediator of allergy.
Methods
Cells
Human blood granulocytes and intestinal MCs were isolated as previously described.26,27 Plasmacytoid DCs (pDCs), myeloid DCs (mDCs), monocytes, and B- and T-cell subsets were purified from peripheral blood mononuclear cells (PBMCs) using magnetic selection kits from Miltenyi Biotec (Auburn, CA): Plasmacytoid Dendritic Cell Isolation Kit, for pDCs; CD1c (BDCA-1+) Dendritic Cell Isolation Kit for mDCs; CD14 MicroBeads for monocytes; B-cell Isolation Kit II for B-cells; and Naive CD4+ T-cell Isolation Kit and Memory CD4+ T-cell Isolation Kit for T-cell subsets. pDCs and mDCs were enriched to at least 70%, whereas the purity of all other cell types was more than 95%. All primary cells were cultured in complete RPMI-1640 culture medium containing 2 mM l-glutamine and 25 mM Hepes (Biochrom, Cambridge, United Kingdom), supplemented with 10% heat-inactivated fetal calf serum (FCS; Seromed, Berlin, Germany) and antibiotics (100 IU/mL penicillin, 100 μg/mL streptomycin). Cell density ranged between 0.5 × 106 and 2 × 106 cells/mL. MCs were cultured with 100 ng/mL c-kit ligand (SCF) for 1 week followed by 1 to 3 weeks' expansion with SCF and 20 ng/mL IL-4.
Approval was obtained from the ethical committee of the State of Bern. Informed consent was obtained in accordance with the Declaration of Helsinki.
Reagents
Cell stimuli and reagents and their sources are as follows: rhIL-3 and rhGM-CSF (Novartis, East Hanover, NJ); rhIL-5, rhIFN-α, rhIFN-γ, rhSCF, and anti-FcϵRIα mAb 29C6 (α-IgER) (Roche, Milan, Italy)28 ; rhNGF, rhIL-1β, rhIL-4, rhIL-9, rhIL-10, rhIL-15, rhIL-18, rhMCP-1, rhEotaxin, and rhM-CSF (PeproTech, Rocky Hill, NJ); rhIL-2 (BD Bioscience, San Jose, CA); rhIL-13 (Sanofi, Cambridge, MA); rhIL-7 and rhTSLP (R&D Systems, Minneapolis, MN); rhTNF-α (Bender MedSystems, Burlingame, CA); LPS (Calbiochem, San Diego, CA); LY2940002, U0126, rapamycin, and PDCA (Alexis, Bayport, MN); and PMA, ionomycin, retinol, retinaldehyde, and atRA (Sigma-Aldrich, St Louis, MO). Retinoid stocks were made in DMSO, handled under protection from light, and prediluted in medium prior of use resulting in 0.01% or less DMSO. The pan-RAR antagonist AGN193109 (AGN) was from Basilea Pharmaceutica (Basel, Switzerland). Human C5a was purified as described.29
MC/basophil cocultures
MCs and basophils were cocultured in transwells (0.4 μm; Costar-Corning, Cambridge, MA), 0.5 × 106 cells each/well (48-well plate), in complete RPMI for 24 hours. Before coculture, MCs were activated plus or minus anti-FcϵRIα antibody (100 ng/mL) for 2 hours. MC supernatants used to activate basophils were collected from resting MCs or after stimulation with anti-FcϵRIα antibody (100 ng/mL) for 24 hours. Supernatants were filtered using a 100-kDa cutoff filter unit (ultrafree-0.5 PBHK; Millipore, Billerica, MA) to remove residual anti-FcϵRIα antibody and used 1:2 in basophil cultures. To determine the MC-derived factor inducing RALDH2 in basophils, MC supernatants and recombinant IL-3 (10 ng/mL) were incubated for 2 hours with or without 10 μg/mL neutralizing goat anti–human IL-3 antibody (R&D Systems) or 10 μg/mL control goat IgG (Santa Cruz Biotechnology, Santa Cruz, CA) before addition to the basophil cultures.
Coculture of basophils with autologous naive CD4+ T cells
Freshly isolated naive T-helper cells were cultured alone or with basophils in 96-well plate (5 × 104 cells each/well), stimulated for 48 hours with plate-bound anti-CD3 (1 μg/mL, Okt3; Ortho Biotech, Raritan, NJ) and anti-CD28 (1 μg/mL; BD Bioscience) antibodies, and kept another 8 days in the presence of 100 U/mL IL-2 (BD Bioscience). Medium with IL-2 was replenished every third day. IL-3 and/or the RAR antagonist were added as indicated.
Protein sample preparation and Western blot analysis
Sample preparation and Western blotting were performed as described,27 using the following antibodies at 1:1000 dilution: anti–granzyme B (GzmB) mAb, anti-Pim1 mAb, anti-Trk mAb, and anti–RAR-α rabbit polyclonal antibody (pAb) (Santa Cruz Biotechnology); anti–Phospho-Stat5 rabbit pAb, anti–Phospho-S6 rabbit mAb, anti–Phospho-p44/42 MAPK (P-ERK1/2) rabbit mAb, and anti-p44/42 MAPK (ERK1/2) rabbit pAb (Cell Signaling, Beverly, MA); and anti–β-actin mAb (Sigma-Aldrich). RALDH2 protein expression was analyzed using a polyclonal rabbit anti-RALDH2 antibody that recognizes RALDH2 from several species including human RALDH2 and does not cross-react with ALH1A1 or RALDH3.30 All experiments were repeated at least 3 times with cells from different donors showing identical results.
Immunofluorescence microscopy
Basophils were left to adhere to poly-l-lysine–coated glass coverslips for 30 minutes at 37°C in plain FCS-free medium. Cells were fixed for 10 minutes with 4% PFA/PBS at room temperature (RT), followed by 10-minute incubation in 100% methanol at − 20°C. Cells were blocked (0.5% casein, 10% goat serum, 2.5 mg/mL human systemic intravenous immune globulin in TBS/0.1% Triton X-100) for 2 hours, and anti-RALDH2 rabbit polyclonal antibody was applied for 2 hours at RT (diluted 1:1000). Secondary goat anti–rabbit Alexa Fluor 488 antibody (Molecular Probes, Eugene, OR) was applied for 1 hour at RT (dilution 1:200). DNA was visualized by staining with 0.1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich).
Retinoic acid reporter assay
The RA-responsive reporter cell line F9-RARE-lacZ (Sil-15) carrying the lacZ reporter gene under the control of the RAR-β RA-response element31 was grown overnight to 80% confluence in 96-well plates in complete RPMI-1640 cell culture medium and then subjected for another 18 hours to the potential RA source (basophils or basophil supernatants). RA-driven β-galactosidase expression in reporter cell lysates was measured using a commercial enzyme assay (Promega Catalys; Promega Madison, WI).
Flow cytometry and ELISA
Cell surface staining was performed in PBS/FCS (2%). For intracellular staining, cells were fixed and permeabilized using the Fix-and-Perm Cell Permeabilization kit from Caltag Laboratories (Invitrogen, Frederick, MD). For detection of intracellular cytokines, protein secretion was blocked by brefeldin A (GolgiPlug; BD Bioscience) during the last 4 hours of stimulation. Flow cytometry was performed with a FACSCalibur (BD Bioscience). At least 5 × 104 events were acquired. The following antibodies and corresponding isotype controls (all from BD Bioscience) were used: anti–CD25-FITC and -APC, anti–CD38-PE, anti–β-7 integrin-PE, anti–α-4 integrin-APC, anti–CD4-APC and -PerCP, anti–IL-4-PE, and anti–IFN-γ-FITC. Anti–GzmB-PE was from Serotec (Raleigh, NC) and anti-CCR9-APC from R&D Systems. The induction of GzmB (in the combined supernatants and cell lysates) was also measured by enzyme-linked immunosorbent assay (ELISA) as described.27
Results
Activated tissue MCs induce RALDH2 in blood basophils
To model ex vivo the events following allergen exposure and to investigate the cross talk between MCs and basophils for the development of allergic inflammation, basophils were cocultured with human MCs activated by FcϵRI cross-linking. Mimicking the Th2-type cytokine environment in allergic inflammation, MCs were first expanded in medium containing SCF and IL-4 before using them in coculture experiments because IL-4 has been shown to modulate the cytokine profile of human MCs.26 To examine the effects of soluble MC-derived products on protein expression in basophils, these 2 primary cell types were separated by transwell membranes. Figure 1A shows that coculture with activated MCs induced in basophils a 55-kDa protein visible in the Coomassie-stained gel. The induced protein was identified as RALDH2 by mass spectroscopy (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), in agreement with immunoblotting using RALDH2-specific antibody (Figure 1B). The strong induction of RALDH2 was also supported by the high RALDH2 peptide frequency found by mass spectroscopy (Table S1).
Induction of RALDH2 in human basophils by MC-derived and recombinant IL-3. (A,B) IgE-activated MCs induce RALDH2 in basophils. (A) Resting or IgE-activated MCs (± α-IgER; 100 ng/mL anti-FcϵRIα mAb) were cocultured in transwells with basophils for 24 hours. Basophil (Ba) proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie (left panel). The gel was scanned using Luminescent Image Analyzer (LAS3000; Fuji, Valhalla, NY). The integrated intensities (light arbitrary units [LAU]) of the stained protein bands were evaluated by densitometric measurements using AIDA software (Raytest, Straubenhardt, Germany) (right panel). The profile of noninduced proteins is depicted in blue; the peak corresponding to up-regulated RALDH2, in green. MS analysis of the peptides of the 55-kDa region of the gel is shown in Table S1. (B) Resting or activated MCs (± α-IgER) were cocultured in transwells with basophils for 24 hours (Ba from MC cocultures). Ba alone: Basophils were cultured separately without MCs with or without 10 ng/mL IL-3 and/or α-IgER, as indicated. (C) Neutralizing anti–IL-3 antibody abrogates MC-induced RALDH2 expression in basophils. Basophils were stimulated with IL-3 or with MC supernatants for 24 hours in the absence or presence of neutralizing anti–IL-3 antibody (± α-IL-3; 10 μg/mL), as indicated. MC supernatants were derived from resting or IgE receptor–activated (α-IgER) MCs. Untreated basophils (buffer) are shown as control. (B,C) RALDH2 expression in basophil extracts assessed by Western blotting. (D) Immunofluorescence analysis of RALDH2 expression in basophils. Following culture for 20 hours with or without IL-3, basophils were stained with anti-RALDH2 Abs (green, middle panels) and DNA was stained with DAPI (blue, left panels). Images were acquired using constant settings on a Nikon Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan) equipped with a 60×/1.4 NA oil-immersion objective lens using FITC and DAPI filter sets. A Nikon DXM 1200 digital camera was used to capture images. Merged images (right panels) were created in Adobe Photoshop 8.0.1 (Adobe Systems, San Jose, CA) without any alteration of the original digital images. Priming growth factors (E), chemotactic agonists (F), and immunoregulatory and proinflammatory cytokines (G,H) do not stimulate or modulate RALDH2 expression. In all experiments, basophils were cultured for 24 hours in the absence (buffer) or presence of the stimuli indicated. Stimuli were used as follows: IL-3 and IL-1β (10 ng/mL); IL-2, IL-4, IL-7, IL-9, IL-13, IL-15, and TSLP (20 ng/mL); IL-5, NGF, and GM-CSF (50 ng/mL); IL-10, IL-18, and TNF-α (100 ng/mL); C5a (10 nM); MCP-1 and eotaxin (100 nM); and IFN-α and IFN-γ (1000 U/mL). Data in the different panels were from separate experiments using basophils isolated from different donors. All experiments were repeated at least 3 times showing identical results. However, the weakly immunoreactive bands shown in panel G were not a consistent finding, and we thus can neither confirm nor exclude a marginal RALDH2 induction by some factors.
Induction of RALDH2 in human basophils by MC-derived and recombinant IL-3. (A,B) IgE-activated MCs induce RALDH2 in basophils. (A) Resting or IgE-activated MCs (± α-IgER; 100 ng/mL anti-FcϵRIα mAb) were cocultured in transwells with basophils for 24 hours. Basophil (Ba) proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie (left panel). The gel was scanned using Luminescent Image Analyzer (LAS3000; Fuji, Valhalla, NY). The integrated intensities (light arbitrary units [LAU]) of the stained protein bands were evaluated by densitometric measurements using AIDA software (Raytest, Straubenhardt, Germany) (right panel). The profile of noninduced proteins is depicted in blue; the peak corresponding to up-regulated RALDH2, in green. MS analysis of the peptides of the 55-kDa region of the gel is shown in Table S1. (B) Resting or activated MCs (± α-IgER) were cocultured in transwells with basophils for 24 hours (Ba from MC cocultures). Ba alone: Basophils were cultured separately without MCs with or without 10 ng/mL IL-3 and/or α-IgER, as indicated. (C) Neutralizing anti–IL-3 antibody abrogates MC-induced RALDH2 expression in basophils. Basophils were stimulated with IL-3 or with MC supernatants for 24 hours in the absence or presence of neutralizing anti–IL-3 antibody (± α-IL-3; 10 μg/mL), as indicated. MC supernatants were derived from resting or IgE receptor–activated (α-IgER) MCs. Untreated basophils (buffer) are shown as control. (B,C) RALDH2 expression in basophil extracts assessed by Western blotting. (D) Immunofluorescence analysis of RALDH2 expression in basophils. Following culture for 20 hours with or without IL-3, basophils were stained with anti-RALDH2 Abs (green, middle panels) and DNA was stained with DAPI (blue, left panels). Images were acquired using constant settings on a Nikon Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan) equipped with a 60×/1.4 NA oil-immersion objective lens using FITC and DAPI filter sets. A Nikon DXM 1200 digital camera was used to capture images. Merged images (right panels) were created in Adobe Photoshop 8.0.1 (Adobe Systems, San Jose, CA) without any alteration of the original digital images. Priming growth factors (E), chemotactic agonists (F), and immunoregulatory and proinflammatory cytokines (G,H) do not stimulate or modulate RALDH2 expression. In all experiments, basophils were cultured for 24 hours in the absence (buffer) or presence of the stimuli indicated. Stimuli were used as follows: IL-3 and IL-1β (10 ng/mL); IL-2, IL-4, IL-7, IL-9, IL-13, IL-15, and TSLP (20 ng/mL); IL-5, NGF, and GM-CSF (50 ng/mL); IL-10, IL-18, and TNF-α (100 ng/mL); C5a (10 nM); MCP-1 and eotaxin (100 nM); and IFN-α and IFN-γ (1000 U/mL). Data in the different panels were from separate experiments using basophils isolated from different donors. All experiments were repeated at least 3 times showing identical results. However, the weakly immunoreactive bands shown in panel G were not a consistent finding, and we thus can neither confirm nor exclude a marginal RALDH2 induction by some factors.
De novo induction of RALDH2 in basophils is mediated by MC-derived IL-3
Analysis of soluble factors secreted by FcϵRI-activated MCs revealed a distinct profile of cytokines (not shown), including IL-3, a known key regulator of basophil function. RALDH2 induction in basophils by supernatants derived from activated MCs was abolished in the presence of neutralizing anti–IL-3 antibody (Figure 1C), but not in the presence of control antibody (data not shown). Furthermore, addition of IL-3 to pure basophils induced identical amounts of RALDH2 as observed after coculture of basophils with activated MCs (Figure 1B). Only weak induction was detectable in basophils exposed to an optimal concentration of anti-FcϵRIα antibody in the absence of MCs, or when basophils were cocultured with MCs without activation. Thus, RALDH2 induction is primarily due to MC-derived IL-3 that is released upon FcϵRI activation. RALDH2 induction at the mRNA and protein level was further studied using recombinant IL-3. RALDH2 mRNA was near the detection limit in freshly isolated basophils or following culture with IL-3 for up to 2 hours, highly induced at 6 and 18 hours, and still remaining elevated at 48 hours (Table S2). Consistent with these mRNA data, RALDH2 protein became detectable at around 8 hours and reached maximal expression levels after 48 to 72 hours (Figure S1A). The dose-dependent induction of RALDH2 at 1 to 10 ng/mL IL-3 is shown in Figure S1B and the cytoplasmic distribution of RALDH2 in Figure 1D.
IL-3 is a selective inducer of RALDH2 in human blood basophils
We next studied whether other cytokines and agonists known to activate or modulate basophil functions might induce RALDH2 (Figure 1E,F). Neither the priming growth factors IL-5, GM-CSF, and NGF nor the major basophil agonists C5a, MCP-1, and eotaxin were able to induce RALDH2. Because we previously found that C5a cooperates with IL-3 to induce IL-4 and IL-13 in human basophils,27,32 these agonists were also combined with IL-3, but none of them modulated IL-3–induced RALDH2 expression. The effect of other proinflammatory and immunoregulatory cytokines formed by MCs and/or other immune cells, namely IL-1β, IL-2, IL-4, IL-7, IL-9, IL-10, IL-13, IL-15, IL-18, TSLP, TNF-α, IFN-α, and IFN-γ, is shown in Figure 1G-H, demonstrating that IL-3 is the only efficient inducer of RALDH2.
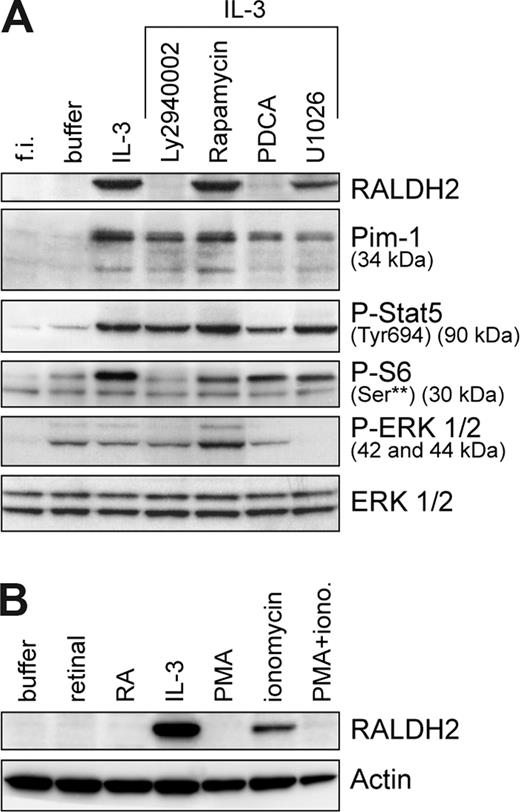
To elucidate signaling events leading to RALDH2 expression, we examined the contribution of PI3-kinase (PI3-K), MAP-kinase, STAT5, and NF-κB transcriptional factors, known to be activated by IL-3. RALDH2 expression was completely blocked by PI3-K–specific inhibitors, LY2940002 (Figure 2A) and wortmannin (not shown), indicating a key role of PI3-kinase. Inhibition of PI3-K–dependent mTOR pathway by rapamycin, as evidenced by the decrease of phosphorylation of the S6 ribosomal protein on Ser235/236, did not affect RALDH2 expression. Blocking of MEK/MAPK pathway with MEK1/2 inhibitor U0126 significantly lowered RALDH2 levels, demonstrating a contribution of ERK activation. Treatment with the NF-κB inhibitor PDCA abrogated RALDH2 expression. The requirement of NF-κB activation was confirmed using lactacystin (a proteasome inhibitor) and IKKIII (an inhibitor of IκB phosphorylation) (not shown). A sustained activation of STAT5 by IL-327 seems not to be sufficient to induce RALDH2, since the inhibitors that block RALDH2 expression did not affect phosphorylation of STAT5 at Tyr694 and only marginally reduced Pim1, a known target of STAT5.
IL-3–induced RALDH2 expression in basophils is dependent on the activation of PI3-kinase and NF-κB signaling pathways. (A) Basophils were cultured for 24 hours in the presence or absence of different inhibitors: PI3-kinase inhibitor, LY2940002 (30 μM); NF-κB signaling pathway inhibitor, PDCA (50 μM); mTOR inhibitor, rapamycin (50 μM); and MEK inhibitor, U1026 (10 μM). Inhibitors were added 30 minutes prior to addition of IL-3 (10 ng/mL). Expression of RALDH2 and Pim1, as well as phosphorylation status of STAT5, S6, and ERK1/2, was analyzed by Western blotting using specific antibodies. Samples of freshly isolated basophils (f.i.) and basophils cultured without IL-3 for 24 hours (buffer) were included in the analysis. (B) Modulation of RALDH2 expression by the xenobiotics PMA and ionomycin. Basophils were cultured for 24 hours with or without IL-3 (10 ng/mL), retinal (5 nM), retinoic acid (RA, 10 nM), PMA (20 nM), and/or ionomycin (iono., 1 μM). RALDH2 expression was analyzed as described for panel A. Actin is shown as a loading control.
IL-3–induced RALDH2 expression in basophils is dependent on the activation of PI3-kinase and NF-κB signaling pathways. (A) Basophils were cultured for 24 hours in the presence or absence of different inhibitors: PI3-kinase inhibitor, LY2940002 (30 μM); NF-κB signaling pathway inhibitor, PDCA (50 μM); mTOR inhibitor, rapamycin (50 μM); and MEK inhibitor, U1026 (10 μM). Inhibitors were added 30 minutes prior to addition of IL-3 (10 ng/mL). Expression of RALDH2 and Pim1, as well as phosphorylation status of STAT5, S6, and ERK1/2, was analyzed by Western blotting using specific antibodies. Samples of freshly isolated basophils (f.i.) and basophils cultured without IL-3 for 24 hours (buffer) were included in the analysis. (B) Modulation of RALDH2 expression by the xenobiotics PMA and ionomycin. Basophils were cultured for 24 hours with or without IL-3 (10 ng/mL), retinal (5 nM), retinoic acid (RA, 10 nM), PMA (20 nM), and/or ionomycin (iono., 1 μM). RALDH2 expression was analyzed as described for panel A. Actin is shown as a loading control.
The effect of the xenobiotics PMA and ionomycin, potent cellular activators known to induce transcription of a large number of genes, is shown in Figure 2B. Interestingly, the calcium ionophore induced some RALDH2, although IL-3 does not elevate intracellular calcium in basophils, whereas PMA, a potent activator of several PKC isoforms that induces MAP-kinase and NF-κB activation downstream of PKC, did not. In fact, PMA even blocked the effect of ionomycin. Finally, retinaldehyde or RA neither induced nor modulated RALDH2 expression, indicating that RALDH2 itself is not a target of RAR-mediated regulation.
RALDH2 induction leads to RA formation by IL-3–activated basophils
To demonstrate that RALDH2 in basophils is functional, we cocultured basophils with an RA-responsive reporter cell line carrying lacZ under the transcriptional control of a RARE31 (Figure 3). β-Galactosidase was indeed induced by IL-3–primed basophils even in medium, and further enhanced in the presence of increasing concentrations of RA precursors (Figure 3A). No β-galactosidase was induced by basophils in the absence of IL-3, and β-galactosidase activity at higher concentrations of retinal or retinol did not differ from controls lacking basophils. The validity of the RA reporter assay was verified using a specific pan-RAR antagonist that completely blocked β-galactosidase expression in reporter cells coincubated with IL-3–stimulated basophils (Figure 3B). The reporter assay also showed that β-galactosidase induction depends on the number of IL-3–treated basophils (Figure 3C). The strong correlation between the levels of RALDH2 expression and the amount of RA generated by basophils is also evident in IL-3 dose-response and time-course experiments (Figure S1). Thus, the data clearly demonstrate that IL-3–induced RALDH2 is functional and that RA is produced by activated basophils under physiological conditions.
Production and release of biologically active RA by IL-3–stimulated basophils. In vitro reporter assay. RA-responsive F9-RARE-lacZ reporter cells were incubated for 24 hours without basophils or cocultured with basophils (± Ba), which were left untreated or stimulated with 10 ng/mL IL-3, as indicated. Reporter cells were harvested and assayed for β-galactosidase activity, which relates to the amounts of RA released by basophils. (A) Reporter cells were incubated with or without basophils in the presence of rising concentrations of retinal or retinol and (B) with or without 10 nM retinal or 30 nM retinol, in the presence or absence of RAR antagonist AGN193109 (AGN, 100 nM). (Inset) As a control, β-galactosidase activity in reporter cells as a function of exogenous RA concentration and its inhibition by AGN193109 (100 nM) is shown. (C) The amount of RA released is proportional to basophil numbers. Different numbers of basophils were cultured for 24 hours with IL-3, and then coincubated with reporter cells in the absence or presence of retinoids (10 nM retinal or 30 nM retinal) for additional 24 hours. Mean values (± SD) of triplicates of representative experiments are shown. Data in the different panels are from separate experiments with basophils from different donors.
Production and release of biologically active RA by IL-3–stimulated basophils. In vitro reporter assay. RA-responsive F9-RARE-lacZ reporter cells were incubated for 24 hours without basophils or cocultured with basophils (± Ba), which were left untreated or stimulated with 10 ng/mL IL-3, as indicated. Reporter cells were harvested and assayed for β-galactosidase activity, which relates to the amounts of RA released by basophils. (A) Reporter cells were incubated with or without basophils in the presence of rising concentrations of retinal or retinol and (B) with or without 10 nM retinal or 30 nM retinol, in the presence or absence of RAR antagonist AGN193109 (AGN, 100 nM). (Inset) As a control, β-galactosidase activity in reporter cells as a function of exogenous RA concentration and its inhibition by AGN193109 (100 nM) is shown. (C) The amount of RA released is proportional to basophil numbers. Different numbers of basophils were cultured for 24 hours with IL-3, and then coincubated with reporter cells in the absence or presence of retinoids (10 nM retinal or 30 nM retinal) for additional 24 hours. Mean values (± SD) of triplicates of representative experiments are shown. Data in the different panels are from separate experiments with basophils from different donors.
The capacity to express RALDH2 is uniquely restricted to blood basophils
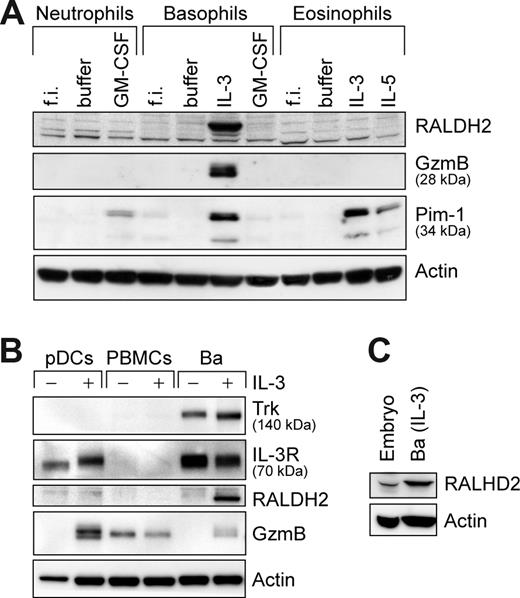
To evaluate whether the ability of basophils to express RALDH2 is shared by other leukocytes, we examined different primary human cells of hematopoietic origin. Figure 4A illustrates that among the members of the granulocyte family only basophils were capable of expressing RALDH2, similar to induction of GzmB reported previously.27 To examine whether the cell type– and IL-3–restricted induction of RALDH2 may be due to a high signaling strength of the IL-3 receptor (CD123), we analyzed RALDH2 expression in pDCs, the only other human cell type expressing CD123 at comparably high densities.33 IL-3 induced GzmB in pDCs but without inducing RALDH2 (Figure 4B), showing different regulation of RALDH2 and GzmB and underscoring the high impact of the cellular background in permitting RALDH2 induction. Furthermore, in mononuclear cells (PBMCs), composed mainly of monocytes and lymphocytes (T cells, B cells, and natural killer [NK] cells), no constitutive or IL-3–inducible RALDH2 expression was detectable.
Among primary blood cells, RALDH2 expression is restricted to basophils. Western blot analysis of RALDH2 expression in different primary human cell subsets. Cells were analyzed directly or after 24 hours of culture with the stimuli indicated. All stimuli were used at a concentration of 50 ng/mL. (A) Freshly isolated (f.i.) or stimulated granulocytes were analyzed for RALDH2, GzmB, and Pim-1 expression. (B) pDCs and peripheral blood mononuclear cells (PBMCs) were probed for RALDH2, the high-affinity NGF receptor Trk, IL-3 receptor-α (IL-3R), and GzmB. Basophils, untreated or stimulated with IL-3, are included as controls. (C) Expression of RALDH2 in leptomeninges of a human embryo (embryo) in comparison with IL-3–stimulated basophils (Ba). Actin is shown as loading control.
Among primary blood cells, RALDH2 expression is restricted to basophils. Western blot analysis of RALDH2 expression in different primary human cell subsets. Cells were analyzed directly or after 24 hours of culture with the stimuli indicated. All stimuli were used at a concentration of 50 ng/mL. (A) Freshly isolated (f.i.) or stimulated granulocytes were analyzed for RALDH2, GzmB, and Pim-1 expression. (B) pDCs and peripheral blood mononuclear cells (PBMCs) were probed for RALDH2, the high-affinity NGF receptor Trk, IL-3 receptor-α (IL-3R), and GzmB. Basophils, untreated or stimulated with IL-3, are included as controls. (C) Expression of RALDH2 in leptomeninges of a human embryo (embryo) in comparison with IL-3–stimulated basophils (Ba). Actin is shown as loading control.
Among cells of the immune system, basophils may indeed represent a unique source of RA as summarized in Table 1 and Figure S2. Neither blood mDCs cultured with or without LPS nor blood monocytes activated by diverse cytokines showed RALDH2 expression. Primary naive or memory T cells, or strongly polarized human Th1 and Th2 T-cell clones, did not express RALDH2 even after activation with anti-CD3/anti-CD28 antibodies. Finally, no RALDH2 was detected in resting or activated human invariant natural killer T (iNKT) cells and in blood B-cell and NK-cell preparations. Because human RALDH2 was cloned from transformed hematopoietic cell lines,34 we also screened several cell lines for RALDH2 protein expression (Table 1, Figure S2E). Consistent with published data,34 a truncated isoform was present in the T-cell lines Jurkat and Molt-4, and the active enzyme was present in Molt-4. All other cells of different lineages, including the human basophilic cell line KU812 and the MC line HMC-1, did not show detectable RALDH2. Curiously, promyelocytic HL60 cells became strongly RALDH2 positive when stably transfected with the BCR-Abl oncogene, although the natural chronic myeloid leukemia (CML)–derived BCR-Abl–transformed cell lines showed no or minimal RALDH2 protein.
RALDH2 expression in primary human cells and transformed human cell lines
| Cell type . | Origin . | Stimuli . | RALDH2 . |
|---|---|---|---|
| Primary human cells | |||
| Basophils | Blood | + IL-3 | + |
| Eosinophils | Blood | ± IL-3, IL-5, GM-CSF | − |
| Neutrophils | Blood | ± GM-CSF | − |
| pDCs | Blood | ± IL-3 | − |
| mDCs | Blood | ± LPS | − |
| Monocytes | Blood | ± IFN-γ, GM-CSF, M-CSF, IL-4, IL-13, IL-3 | − |
| Naive T cells | Blood | ± Anti-CD3/anti-CD28 | − |
| Memory T cells | Blood | ± Anti-CD3/anti-CD28 | − |
| Th1/Th2 clones | Blood | ± Anti-CD3/anti-CD28 | − |
| NKT cells | Blood | ± OCH, aGalCer, PHA | − |
| B cells | Blood | − | − |
| Total PBMCs | Blood | ± IL-3 | − |
| Mast cells | Intestinal tissue | ± SCF | − |
| Embryonic cells | Meninges | − | + |
| Cell lines | Lineage | ||
| HEP-G2 | Human hepatocellular carcinoma | − | |
| K562 | Human chronic myeloid leukemia | (+)? | |
| GM-1 | Human histiocytic lymphoma | − | |
| Jurkat | Human T-cell leukemia | +* | |
| Molt-4 | Human T-cell leukemia | +† | |
| 721.221 | Human B lymphoblastoma | − | |
| HL60 | Human acute myeloid leukemia | − | |
| HL60/Bcr-Abl | HL60 transfected with Bcr-Abl | + | |
| HMC-1 | Human mast cell leukemia | − | |
| KU812 | Human chronic myeloid leukemia (basophilic) | − | |
| NK92 | Human natural killer lymphoma | − | |
| BEAS-2B | Human bronchial epithelium | − | |
| Cell type . | Origin . | Stimuli . | RALDH2 . |
|---|---|---|---|
| Primary human cells | |||
| Basophils | Blood | + IL-3 | + |
| Eosinophils | Blood | ± IL-3, IL-5, GM-CSF | − |
| Neutrophils | Blood | ± GM-CSF | − |
| pDCs | Blood | ± IL-3 | − |
| mDCs | Blood | ± LPS | − |
| Monocytes | Blood | ± IFN-γ, GM-CSF, M-CSF, IL-4, IL-13, IL-3 | − |
| Naive T cells | Blood | ± Anti-CD3/anti-CD28 | − |
| Memory T cells | Blood | ± Anti-CD3/anti-CD28 | − |
| Th1/Th2 clones | Blood | ± Anti-CD3/anti-CD28 | − |
| NKT cells | Blood | ± OCH, aGalCer, PHA | − |
| B cells | Blood | − | − |
| Total PBMCs | Blood | ± IL-3 | − |
| Mast cells | Intestinal tissue | ± SCF | − |
| Embryonic cells | Meninges | − | + |
| Cell lines | Lineage | ||
| HEP-G2 | Human hepatocellular carcinoma | − | |
| K562 | Human chronic myeloid leukemia | (+)? | |
| GM-1 | Human histiocytic lymphoma | − | |
| Jurkat | Human T-cell leukemia | +* | |
| Molt-4 | Human T-cell leukemia | +† | |
| 721.221 | Human B lymphoblastoma | − | |
| HL60 | Human acute myeloid leukemia | − | |
| HL60/Bcr-Abl | HL60 transfected with Bcr-Abl | + | |
| HMC-1 | Human mast cell leukemia | − | |
| KU812 | Human chronic myeloid leukemia (basophilic) | − | |
| NK92 | Human natural killer lymphoma | − | |
| BEAS-2B | Human bronchial epithelium | − | |
Isolated primary human blood cells were stimulated 24 to 72 hours with the stimuli indicated (for concentrations, see corresponding Western blots throughout the paper); MCs were cultured for up to 4 weeks. Cell lines were maintained under standard culture conditions. The presence or induction of RALDH2 protein was determined in cell extracts as analyzed by Western blotting using anti-RALDH2 antibody.
+ indicates clearly detected; (+)?, near detection limit; and −, undetectable. See Figures 1, 4, and S2 for original data.
Truncated transcript only.
Truncated and full-length transcript.
Thus, among primary human leukocytes, the capacity to express RALDH2 appears to be uniquely restricted to basophils. The potential impact of basophil-derived RA is further underscored by the fact that activated basophils contain even higher RALDH2 levels than extracts of meninges of human embryo (Figure 4C), a site of particularly high RA synthesis during fetal development.35
RALDH2 induction leads to an RA-dependent autocrine regulation of IL-3–induced genes
The possible roles of basophil-derived RA are broad since most cells express RA receptors. Because RA acts locally in an autocrine or paracrine fashion, we first examined whether selected IL-3–induced genes in basophils are subject to autocrine regulation by RA (Figure 5). RALDH2 itself, the NGF receptor Trk-A, ST2,27 and CD69 (not shown) are neither induced by RA nor is their IL-3–induced up-regulation affected by endogenously formed or exogenously added RA. However, we find prominent RA-dependent amplification of IL-3–induced up-regulation of the IL-2 receptor α-chain (CD25), as demonstrated by the inhibition with the RAR antagonist and a further increase by addition of RA. In contrast, the IL-3–induced expression of GzmB27 is negatively regulated by the autocrine action of RA. The amount of RAR-α, the major RAR isotype present in basophils, is increased by IL-3 in an RA-independent fashion and rather seems to decrease by autocrine RA despite the presence of a RARE in its promoter region. Thus, the study of only a few IL-3–induced genes clearly revealed the important autocrine regulatory role of RA produced in response to RALDH2 induction.
Basophil-derived RA modulates IL-3–induced gene expression in an autocrine manner. RA-dependent regulation of CD25, GzmB, and RARα expression in IL-3–stimulated basophils. Basophils were cultured ± IL-3 (20 ng/mL) for 24 hours. Retinal (5 nM), RA (10 nM), or AGN (100 nM) was added as indicated. (Top panel) Surface CD25 expression (CD25) was analyzed by flow cytometry. (Middle panel) GzmB expression was analyzed by ELISA. Bar charts represent the mean values (± SD) of triplicates of representative experiments of 3 shown. (Bottom panel) Trk (NFGR), RALDH2, RARα, and GzmB expression was analyzed by Western blotting. Separate panels represent data from individual experiments with cells from different donors.
Basophil-derived RA modulates IL-3–induced gene expression in an autocrine manner. RA-dependent regulation of CD25, GzmB, and RARα expression in IL-3–stimulated basophils. Basophils were cultured ± IL-3 (20 ng/mL) for 24 hours. Retinal (5 nM), RA (10 nM), or AGN (100 nM) was added as indicated. (Top panel) Surface CD25 expression (CD25) was analyzed by flow cytometry. (Middle panel) GzmB expression was analyzed by ELISA. Bar charts represent the mean values (± SD) of triplicates of representative experiments of 3 shown. (Bottom panel) Trk (NFGR), RALDH2, RARα, and GzmB expression was analyzed by Western blotting. Separate panels represent data from individual experiments with cells from different donors.
Basophil-derived RA promotes Th2 polarization and expression of homing receptors and activation markers in naive human T cells
Previous studies indicated that exogenous RA added to T-cell cultures enhances expression of α4/β7 integrin, CCR9, and CD25.13,36 To unravel a possible immunoregulatory role of endogenous basophil-derived RA, purified basophils were coincubated with autologous naive T cells activated by anti-CD3/CD28 antibodies in the presence or absence of IL-3 and/or RAR antagonist. IL-3 itself did not affect T cells (Figure S3), but in the presence of basophils we observed a remarkable IL-3–induced up-regulation of the α4/β7 integrin, a ligand for MadCAM and VCAM (Figure 6A). The dependence of this induction on basophil-derived RA is demonstrated by blocking with RAR antagonist and by a similar enhancement of β7 expression by exogenous RA in T cells cultured in the absence of basophils (Figure S3A). Basophil-derived RA also strongly up-regulated CD38, a known sensitive direct target of RA-mediated transcription (Figure 6A; Figure S3B).37 A weaker AGN-inhibitable CD38 expression observed in the absence of IL-3 may be due to induction of some RALDH2 in basophils by T cell–derived cytokines during coculture. CD25 levels in T cells were not affected by endogenous or exogenous RA, and staining for CCR9 showed no changes under our experimental conditions (Figure 6A; Figure S3C), in contrast to the findings in mouse T cells.13
Basophil-derived RA acts in a paracrine fashion on the phenotype and function of T-helper cells. Purified naive CD4+ T cells cocultured with autologous basophils in a 1:1 ratio were stimulated with plate-bound anti-CD3 and anti-CD28 antibodies (1 μg/mL) in the absence or presence of 10 ng/mL IL-3 and/or the RAR antagonist AGN (100 nM) and cultured for 10 days until analysis. (A) Expression of β7 and α4 integrins, CD38, CD25, and CCR9 in T-helper cells was assessed by flow cytometry. Data are given as delta mean fluorescence intensities (ΔMFI). Mean values of triplicates (± SD) of representative experiments of at least 3 are shown. The different panels represent data from independent experiments with cells from different donors. (B,C) Basophil-derived RA promotes Th2 polarization. Flow cytometric analysis of IL-4 and IFN-γ expression in CD4+ T cells. After coincubation with basophil for 10 days, T cells were restimulated for 4 hours with PMA and ionomycin (20 nM and 1 μM, respectively) in the presence of brefeldin A and double stained for intracellular IL-4 and IFN-γ. (B) (Left panels) Representative 2-parameter dot plots of CD4+ T cells with IFN-γ in the abscissa and IL-4 in the ordinate; percentage of cells in each quadrant is indicated. (Right panel) Ratios of the percentages of IL-4 and IFN-γ single-positive T cells (IL-4/IFN-γ). The mean values (± SD) of duplicates are shown. (C) Pairwise analysis of the different experimental conditions of 4 independent experiments with cells isolated from different donors is shown. Statistical significance was calculated using the one-tailed paired t test for each parameter and each pair of experimental conditions. Statistical significance is shown in the top of each panel. The data (mean of duplicates) from each individual experiment are connected by lines.
Basophil-derived RA acts in a paracrine fashion on the phenotype and function of T-helper cells. Purified naive CD4+ T cells cocultured with autologous basophils in a 1:1 ratio were stimulated with plate-bound anti-CD3 and anti-CD28 antibodies (1 μg/mL) in the absence or presence of 10 ng/mL IL-3 and/or the RAR antagonist AGN (100 nM) and cultured for 10 days until analysis. (A) Expression of β7 and α4 integrins, CD38, CD25, and CCR9 in T-helper cells was assessed by flow cytometry. Data are given as delta mean fluorescence intensities (ΔMFI). Mean values of triplicates (± SD) of representative experiments of at least 3 are shown. The different panels represent data from independent experiments with cells from different donors. (B,C) Basophil-derived RA promotes Th2 polarization. Flow cytometric analysis of IL-4 and IFN-γ expression in CD4+ T cells. After coincubation with basophil for 10 days, T cells were restimulated for 4 hours with PMA and ionomycin (20 nM and 1 μM, respectively) in the presence of brefeldin A and double stained for intracellular IL-4 and IFN-γ. (B) (Left panels) Representative 2-parameter dot plots of CD4+ T cells with IFN-γ in the abscissa and IL-4 in the ordinate; percentage of cells in each quadrant is indicated. (Right panel) Ratios of the percentages of IL-4 and IFN-γ single-positive T cells (IL-4/IFN-γ). The mean values (± SD) of duplicates are shown. (C) Pairwise analysis of the different experimental conditions of 4 independent experiments with cells isolated from different donors is shown. Statistical significance was calculated using the one-tailed paired t test for each parameter and each pair of experimental conditions. Statistical significance is shown in the top of each panel. The data (mean of duplicates) from each individual experiment are connected by lines.
To address the question of whether basophil-derived RA might influence T-cell polarization, we restimulated T cells after coculture with basophils and analyzed their IL-4 and IFN-γ expression by intracellular staining (Figures 6B,C). We found that IL-3–activated basophils skewed T-cell polarization toward Th2, an effect that was blocked by the RAR antagonist, whereas neither IL-3 nor the RAR antagonist affected cytokine expression of T cells in the absence of basophils. In cocultures of T cells with basophils, IL-3 induced a reproducible, RA-dependent enhancement of the IL-4/IFN-γ ratio despite some variability of absolute numbers of IL-4– and IFN-γ–producing T cells in experiments with cells isolated from different donors (Figure 6C). Taken together, our data indicate that RA derived from IL-3–activated basophils can modulate several functions of T cells and may thereby play an important immunoregulatory role.
Discussion
This paper shows that basophils gain the capacity to produce RA when exposed to tissue-derived human MCs activated by FcϵRI cross-linking. The discovery of RA as a novel basophil-derived mediator was initiated by the observation that coculture of basophils with activated MCs induced high levels of RALDH2, one of the most efficient and specific enzymes involved in RA generation. It is well established that the timed local expression of RALDH2 is crucial for the RA gradients needed for proper patterning of the developing embryo. In the adult mouse, however, constitutive RALDH2 expression was found to be largely limited to reproductive organs, and it is believed that retinoid homeostasis is maintained by the widely expressed aldehyde-detoxifying enzyme ALDH1A1 that catabolizes retinaldehyde under conditions of retinol excess.38 In marked contrast to thousands of studies addressing the effects of RA on cell fate, cellular functions, and gene expression programs, the question of whether RALDH2 expression may determine how RA functions in the adult under physiological or pathological conditions is largely unknown. In particular, the possibility that interactions of cells of the immune system could lead to RALDH2 expression has not been considered. Our study now demonstrates that RALDH2 can be induced in human basophils by IL-3 and that basophil-derived RA can regulate adhesion receptor expression and Th2 polarization of T-helper cells. Thus, RA must be viewed as a tightly regulated endogenous immunoregulatory mediator.
The reason for the extraordinarily cell type– and cytokine-restricted induction of RALDH2 in leukocytes remains unknown. Obviously, a high signaling strength due to the particularly high CD123 levels present on basophils is not the sole reason of this IL-3 selectivity since RALDH2 is not induced in pDCs that display an identical density of CD123. The regulation of transcription of the human RALDH2 gene is still unknown. However, the effects of signal transduction inhibitors on RALDH2 protein expression in basophils indicate a requirement of the PI3-kinase and NF-κB pathways and an involvement of ERK activation, whereas the mTOR pathway does not contribute. It is unlikely, however, that PI3-kinase and NF-κB pathways are solely responsible for RALDH2 induction, since they are activated by a variety of stimuli in many cell types. Our data are reminiscent of previous studies demonstrating that IL-3 is a rather unique regulator of the phenotype and function of basophils, for example, by facilitating IL-4 and IL-13 production in synergy with FcϵRI cross-linking or C5a, and by inducing several genes, such as CD25, CD69, ST2, and GzmB.20,27,39 Within the different granulocyte types, the profile of RALDH2 induction is similar to that of GzmB.27 However, in contrast to RALDH2, GzmB is also induced by IL-3 in human pDCs. Thus, among all the IL-3–induced genes studied so far, RALDH2 appears to represent the most IL-3–selective activation signature gene of human basophils.
An autocrine function of RA generated by RALDH2 in basophils is already apparent when studying only a few IL-3–induced proteins: Whereas up-regulation of CD69, Trk A, ST2, and RALDH2 itself is not affected by exogenously added or endogenously produced RA, there is an autocrine amplification of IL-3–induced CD25 up-regulation and an RA-mediated suppression of GzmB and RAR-α induction. The molecular mechanisms responsible for these positive and negative autocrine loops are certainly complex and probably involve the participation of other RA-modulated genes. In addition, there may be cross talk in signaling and transcriptional regulation between IL-3– and RA-mediated pathways. Indeed, the promoter of some genes harbors overlapping STAT/RAR-binding sites, and a physical association of active STAT5 with RA receptors has been demonstrated in an IL-3–dependent cell line.40 This autocrine function of RA in human basophils is reminiscent of that of another active vitamin metabolite, 1,25(OH)vitD3, for LPS-induced cathelicidin expression in human (but not rodent) monocytes reported recently.41 Thus, induction of appropriate vitamin-metabolizing enzymes represents a novel mechanism for the regulation of gene expression programs in cells of the immune system.
Although our study demonstrates a remarkable basophil-restricted expression of RALDH2 among different hematopoietic cell types, endogenous RA may nevertheless play an immunoregulatory role also in other contexts. Indeed, recent studies indicate that gut-derived RA regulates the function and gut-homing properties of T and B cells.12,13 However, it is still unclear to what extent low (ALDH1A1) or high (RALDH2) efficiency enzymes are involved. In contrast to DCs of peripheral sites (and human DCs shown here), mouse mesenteric lymph node (MLN) DCs constitutively express RALDH2 mRNA as demonstrated by reverse-transcription–polymerase chain reaction (RT-PCR).13 Thus, under homeostatic conditions, RALDH2 may be selectively expressed by MLN-DCs, but the level of RALDH2 protein in MLN-DCs (compared with IL-3–activated basophils) and the environmental cues leading to its expression at this anatomic site remain to be determined. Of note, intestinal mucosa and Peyer patches DCs strongly express the low-efficiency RA-synthesizing enzyme ALDH1A1. RA formation at this site may thus depend on high local retinol concentrations thereby sensing vitamin A in food, in analogy to the recently suggested mechanisms for imprinting skin-homing properties on T cells by 1,25(OH)vitD3, which is formed by skin DCs sensing the high local vitamin D3 concentrations formed by exposure to UV light.42
Pharmacologic studies examining the effects of exogenous retinoids on T cells in vitro and on immune responses in vivo collectively suggest that RA can promote a Th2 bias,9,–11,43,–45 but whether this may have a physiological correlate remains unknown. Using cocultures of basophils and naive human T-helper cells, we now show that endogenous RA synthesized by IL-3–activated basophils can indeed promote Th2 polarization since Th2 skewing by basophil-derived RA was completely blocked by a specific RAR antagonist, demonstrating the requirement for RAR activation. Our findings are consistent with recent investigations of effects of relatively low levels of exogenous RA on cytokine expression profiles of purified human T cells.45 This study showed that RA enhanced the secretion of the Th2 cytokines IL-4, IL-5, and IL-13 and inhibited IFN-γ formation, along with a corresponding modulation of the Th2 and Th1 transcription factors, GATA3, c-maf, and t-bet. The Th2 bias in the presence of exogenous RA was observed even under Th1-polarizing conditions with IL-4 neutralization. Thus, the data presented here together with published findings using exogenously added RA strongly indicate that basophil-derived RA acts in a direct paracrine fashion on T cells rather than through an indirect autocrine effect on the basophils themselves. Studies in vitamin A–deficient patients indicate a compromised Th2-type immune response and a cytokine imbalance skewed toward Th1.9 Our data now provide a first potential physiological mechanism for these observations and indicate a novel RA-driven pathway of Th2 skewing. Thus, basophils may regulate Th2-type immune responses in 2 complementary ways: by producing IL-4/IL-13 in response to FcϵRI cross-linking or C5a in synergy with IL-3,19,32,46 and by producing RA in response to IL-3 without the need of a costimulatory signal. Finally, our coculture experiments indicate that basophil-derived RA can also affect other important properties of T cells: it induces CD38, a multifunctional receptor involved in T-cell activation, signaling, and adhesion to endothelial cells (through CD31/PECAM-1) and matrix components (hyaluronic acid),37 and up-regulates α4/β7 integrins. Of note, α4/β7 integrins interact not only with MadCAM but also with VCAM-1, and α4/β7-VCAM interactions were recently found crucial for homing of MC progenitors to the inflamed lung in a mouse model of asthma.47 The coordinated up-regulation of CD38 and α4/b7 integrin may thus regulate trafficking of lymphocytes to sites of inflammation.
Basophil-derived RA may also act on resident cells. For example, RA promotes expression of the mucins MUC2 and MUC5AC, which are increased in human asthma.48,–50 Furthermore, RA and 1,25(OH)vitD3 analogues applied to skin of mice synergistically induce thymic stromal lymphopoietin (TSLP), a master regulator of local and systemic Th2-type inflammatory conditions,51,52 and our study now provides a physiological mechanism by which IgE receptor activation (through MC-basophil interaction) can lead to RA formation. Interestingly, pharmacologic studies of effects of 1,25(OH)vitD3 indicate partially overlapping bioactivities with that of RA, including Th2 polarization (reviewed in Cantorna et al53 ). One may thus speculate that under homeostatic conditions, the active metabolites of vitamins A and D3 have opposing roles (imprinting homing properties to the gut and skin, respectively),12,13,51 whereas in an adaptive immune response they may cooperatively promote different aspects of an allergic phenotype. Our findings suggest that vitamin A deficiency (most prevalent in developing countries) or a particularly high vitamin A intake by vitamin supplements (most frequent in wealthy industrialized countries) may contribute to a lower or higher prevalence of atopic diseases, respectively. In support of such a “vitamin hypothesis of allergy” are also epidemiologic studies in humans54,55 and very recent investigations using a murine asthma model,56 demonstrating that vitamin A deficiency decreased eosinophilic infiltration of the lung and blocked bronchiolar hyperresponsiveness, whereas vitamin A supplementation further enhanced the Th2 response and allergic inflammation.
In conclusion, our study gives the first example of regulated de novo expression of RALDH2 in adults. We describe a new cellular pathway that leads to the generation of RA by cells of the immune system. Thus, RA is an endogenous basophil-derived immunoregulatory mediator with a broad potential to modify the functions of leukocytes and resident cells. Our findings also indicate a novel physiological pathway of Th2 skewing and raise the intriguing hypothesis that vitamin intake may influence the prevalence or manifestation of atopic diseases. Topical RAR antagonism should therefore be considered as a therapeutic strategy for treating allergic diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank M. Wagner for the RA reporter cell line,31 F. Sallusto and M. Messi for extracts of Th1 and Th2 T-cell clones,57 S. Gadola for iNKT cells, and H. R. Widmer for embryonic meninges.
This work was supported by the Swiss National Science Foundation (SSNF) and Stiftung 3R (Münsingen, Switzerland).
Authorship
Contribution: N.S. performed the research, analyzed and interpreted the data, and wrote the paper; S.D. performed the research; P.M. provided the anti-RALDH2–specific antibody; H.L. performed and analyzed mass spectroscopy; C.A.D. designed and supervised all studies and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clemens A. Dahinden, Institute of Immunology, University Hospital Bern, Inselspital, CH-3010 Bern, Switzerland; e-mail: clemens.dahinden@iib.unibe.ch.

![Figure 1. Induction of RALDH2 in human basophils by MC-derived and recombinant IL-3. (A,B) IgE-activated MCs induce RALDH2 in basophils. (A) Resting or IgE-activated MCs (± α-IgER; 100 ng/mL anti-FcϵRIα mAb) were cocultured in transwells with basophils for 24 hours. Basophil (Ba) proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie (left panel). The gel was scanned using Luminescent Image Analyzer (LAS3000; Fuji, Valhalla, NY). The integrated intensities (light arbitrary units [LAU]) of the stained protein bands were evaluated by densitometric measurements using AIDA software (Raytest, Straubenhardt, Germany) (right panel). The profile of noninduced proteins is depicted in blue; the peak corresponding to up-regulated RALDH2, in green. MS analysis of the peptides of the 55-kDa region of the gel is shown in Table S1. (B) Resting or activated MCs (± α-IgER) were cocultured in transwells with basophils for 24 hours (Ba from MC cocultures). Ba alone: Basophils were cultured separately without MCs with or without 10 ng/mL IL-3 and/or α-IgER, as indicated. (C) Neutralizing anti–IL-3 antibody abrogates MC-induced RALDH2 expression in basophils. Basophils were stimulated with IL-3 or with MC supernatants for 24 hours in the absence or presence of neutralizing anti–IL-3 antibody (± α-IL-3; 10 μg/mL), as indicated. MC supernatants were derived from resting or IgE receptor–activated (α-IgER) MCs. Untreated basophils (buffer) are shown as control. (B,C) RALDH2 expression in basophil extracts assessed by Western blotting. (D) Immunofluorescence analysis of RALDH2 expression in basophils. Following culture for 20 hours with or without IL-3, basophils were stained with anti-RALDH2 Abs (green, middle panels) and DNA was stained with DAPI (blue, left panels). Images were acquired using constant settings on a Nikon Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan) equipped with a 60×/1.4 NA oil-immersion objective lens using FITC and DAPI filter sets. A Nikon DXM 1200 digital camera was used to capture images. Merged images (right panels) were created in Adobe Photoshop 8.0.1 (Adobe Systems, San Jose, CA) without any alteration of the original digital images. Priming growth factors (E), chemotactic agonists (F), and immunoregulatory and proinflammatory cytokines (G,H) do not stimulate or modulate RALDH2 expression. In all experiments, basophils were cultured for 24 hours in the absence (buffer) or presence of the stimuli indicated. Stimuli were used as follows: IL-3 and IL-1β (10 ng/mL); IL-2, IL-4, IL-7, IL-9, IL-13, IL-15, and TSLP (20 ng/mL); IL-5, NGF, and GM-CSF (50 ng/mL); IL-10, IL-18, and TNF-α (100 ng/mL); C5a (10 nM); MCP-1 and eotaxin (100 nM); and IFN-α and IFN-γ (1000 U/mL). Data in the different panels were from separate experiments using basophils isolated from different donors. All experiments were repeated at least 3 times showing identical results. However, the weakly immunoreactive bands shown in panel G were not a consistent finding, and we thus can neither confirm nor exclude a marginal RALDH2 induction by some factors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/9/10.1182_blood-2008-01-135251/5/m_zh80150821790001.jpeg?Expires=1769124897&Signature=drVZJvFqXr4-52tKNmO3LM0q3zUtidtr9HvDK83TPkj2NJAviX5utUStcb6PNeMgtw7JOYxK1TNajEZjrQxsQ8krZEIu6orV8gzYB27o3QTj9hgmXyb6EcB3VOl5OYeLxC60xRuiKkvsxRBug7HvEkD9l5PJzWLxJSTZf7nDQOcXTtzC2HwFFe2cVyJcNhFJ1WS3upcLHJvyppBGrEFIffD-AdGURYxXXG9o8CRfF7-QAbLrmQ9Es386MErx9j8WzoRo~uag2SmGgYSjv3MBxYP~uJ~MG5NpUOC2HolAJC8cV~VvUXVw74q-4KzCKOh0itQuICkGRchwler8~tnZ7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)