In ischemic stroke, treatment options are limited. Therapeutic thrombolysis is restricted to the first few hours after stroke, and the utility of current platelet aggregation inhibitors, including GPIIb/IIIa receptor antagonists, and anticoagulants is counterbalanced by the risk of intracerebral bleeding complications. Numerous attempts to establish neuroprotection in ischemic stroke have been unfruitful. Thus, there is strong demand for novel treatment strategies. Major advances have been made in understanding the molecular functions of platelet receptors such as glycoprotein Ib (GPIb) and GPVI and their downstream signaling pathways that allow interference with their function. Inhibition of these receptors in the mouse stroke model of transient middle cerebral artery occlusion prevented infarctions without increasing the risk of intracerebral bleeding. Similarly, it is now clear that the intrinsic coagulation factor XII (FXII) and FXI play a functional role in thrombus formation and stabilization during stroke: their deficiency or blockade protects from cerebral ischemia without overtly affecting hemostasis. Based on the accumulating evidence that thrombus formation and hemostasis are not inevitably linked, new concepts for prevention and treatment of ischemic stroke may eventually emerge without the hazard of severe bleeding complications. This review discusses recent advances related to antithrombotic strategies in experimental stroke research.
Introduction
Stroke is the second leading cause of death worldwide.1,2 Approximately 80% of strokes are caused by focal cerebral ischemia due to arterial occlusion, whereas up to 20% are caused by intracerebral hemorrhages.3,4 Extracranial artery stenoses are prone to destabilization and plaque rupture leading to cerebral thromboembolism.5 In approximately one-third of ischemic stroke patients, embolism to the brain originates from the heart, especially in atrial fibrillation.6 Thromboembolic occlusion of major or multiple smaller intracerebral arteries leads to focal impairment of the downstream blood flow, and to secondary thrombus formation within the cerebral microvasculature.
In the center of the ischemic territory, oxygen and glucose deprivation, neuronal depolarization, and Ca2+-mediated excitotoxicity induce necrotic and apoptotic cell death. In the penumbra region surrounding the infarct core, however, tissue is preserved for a certain time span depending on whether blood flow is restored.7 Since numerous agents that proved neuroprotective in experimental stroke failed in subsequent clinical trials,8 the only effective treatment option in acute ischemic stroke remains immediate thrombolysis. In this review, we will focus on the initiating event of stroke development, namely intravascular thrombus formation, and highlight promising novel molecular targets for its prevention and treatment.
Current treatment options in ischemic stroke
Thrombolytic therapy
In acute thromboembolic stroke the principal treatment goal is to rapidly achieve recanalization of occluded intracerebral vessels. In the case of a permanent vessel occlusion, a complete infarct will inevitably develop. At present, early intravenous or intra-arterial thrombolysis are the only established therapeutic options.9,10 Less than 10% of patients are amenable to this treatment due to the limited time window of up to 3 to 6 hours after symptom onset because of the risk of severe intracerebral hemorrhage with later application.11 A trial to extend the therapeutic window up to 9 hours by use of recombinant desmoteplase, a novel plasminogen activator, failed.12 For unknown reasons, thrombolytic treatment leads to the dissolution of the vessel-occluding clots in some cases, but not in others. Moreover, secondary arterial reocclusion may follow a previously successful recanalization.13 Most importantly, patients may develop progressive stroke despite sustained early reperfusion of previously occluded major intracranial arteries, a process referred to as “reperfusion injury.” These observations suggest that reperfusion of occluded major arterial branches is a prerequisite for salvage of tissue, but does not inevitably guarantee prevention of infarct growth and clinical recovery.
Platelet inhibitors
There have been numerous attempts to improve stroke outcome by use of platelet aggregation inhibitors and anticoagulants. The antithrombotic effect of acetylsalicylic acid (ASA) is based on the irreversible inhibition of platelet cyclooxygenases 1 and 2, leading to reduced prostaglandin and thromboxane A2 synthesis. ASA has been evaluated within 48 hours of stroke onset in 2 large trials.14,15 There was a moderate, but statistically significant benefit on stroke outcome. It was assumed, yet not based on solid data, that the primary effect of ASA might be due to prevention of early stroke recurrence rather than limiting the neurologic consequences of the initial stroke per se.16 Although the beneficial role of platelet aggregation inhibitors including ASA, ASA in combination with dipyridamole, and the platelet P2Y12 receptor inhibitor clopidogrel in stroke prevention is well established,17,18 the multifaceted role of platelets in acute stroke development is unclear. This limited understanding extends to the mechanisms by which antiplatelet agents may act in preventing thrombus growth within the brain microvasculature.16,19
The formation of a thrombus requires functional glycoprotein IIb (GPIIb)/GPIIIa, a heterodimeric receptor of the integrin family expressed at high density (50 000-80 000 copies/cell) on the platelet membrane.20 In resting platelets, GPIIb/IIIa exists in a low-affinity state and does not bind its ligands. During platelet activation, intracellular signals are generated that are integrated at defined checkpoints such as CalDAG-GEFI21 and culminate in the activation of talin-122,23 and kindlin-3.24 These bind to the intracellular tails of the integrin β3-subunit (GPIIIa) and induce a conformational change that involves both subunits of the complex. This “final common pathway” of platelet activation results in the exposure of the binding site(s) for a variety of ligands, most notably fibrinogen, von Willebrand factor (VWF), and fibronectin (inside-out signaling), which allows firm adhesion to the extracellular matrix and aggregation. Current strategies to inhibit GPIIb/IIIa include antibodies (abciximab), cyclic peptides adapted from a snake venom disintegrin (eptifibatide), and nonpeptide analogues of an RGD peptide (tirofiban and lamifiban), all of which directly inhibit ligand binding. Although the utility of intravenous GPIIb/IIIa inhibitors in acute coronary syndromes is well established,25 a recent phase 3 trial applying abciximab in acute ischemic stroke was prematurely stopped due to an increased intracranial hemorrhage rate and mortality, as well as lack of efficacy.26 Several studies also evaluated GPIIb/IIIa inhibitors in conjunction with thrombolytic therapy and described some benefit.27,–29 Based on the available data, the use of GPIIb/IIIa inhibitors in acute stroke patients cannot be recommended at present.26,30
Anticoagulants
Anticoagulation with warfarin targets the synthesis of coagulation factors II, VII, IX, and X and is effective in primary and secondary prophylaxis of thromboembolism to the brain in patients with atrial fibrillation.31 Although other anticoagulants, namely unfractionated heparin, low-molecular-weight heparin, or heparinoids that block FXa activity have frequently been used for acute stroke therapy within 48 hours,32,33 several randomized studies have been negative.15,34,35 A most recent trial did not find a significant advantage of low-molecular-weight heparin over ASA.36 The few studies that have addressed the potential benefit of anticoagulation immediately within the first hours after cerebral ischemia gave inconsistent results regarding clinical outcome and stroke recurrence but mostly found a significant increase in intracranial bleeding.36,,–39 Consequently, the recently updated American Heart Association guidelines16 (p1680) state that “urgent anticoagulation with the goal of preventing early recurrent stroke, halting neurologic worsening, or improving outcomes is not recommended.” Because anticoagulation carries a significant risk for intracerebral bleeding in the setting of acute stroke,40,41 it remains a major challenge to develop novel anticoagulants and/or antiplatelet agents with a more favorable safety profile and better efficacy.42
Studies in experimental stroke
Animal models for focal ischemic stroke
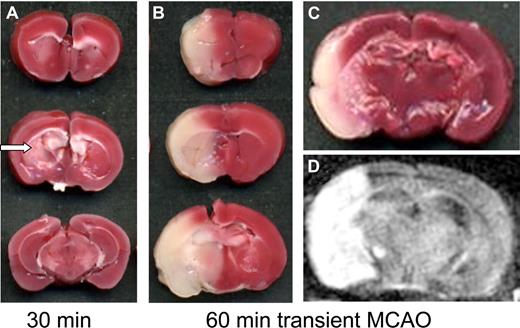
For the study of ischemic stroke, several animal models have been developed.43 Most frequently, occlusion of major extracranial and intracranial arteries is applied in rodents or higher mammals leading to focal cerebral ischemia. Permanent occlusion of the middle cerebral artery (MCAO) at proximal sites, either by a suture or by an intraluminal thread, causes complete infarctions of the middle cerebral artery brain territory involving neocortex and basal ganglia. The clinical situation with vessel occlusion followed by resolution of clots and reperfusion can be mimicked in the MCAO paradigm by withdrawing of the intraluminal thread (so-called transient MCAO model; Figure 1). It has been firmly established that final infarct size depends on the prior occlusion time: ischemic periods less than 30 minutes will lead to infarctions of the caudate and putamen (basal ganglia) and only partly affect the neocortex because ischemic cortical tissue is salvaged by collateral blood supply and reperfusion.44 If reperfusion is delayed further, however, the size of neocortical infarctions will increase, because the surrounding penumbra is subsequently involved in the definite infarct area (Figure 1). One of the unanswered questions is why sufficient reflow does not guarantee salvage of brain tissue. The observation that reperfusion of major intracerebral arteries did not completely prevent further infarct growth led to the concept of a focal “no-reflow” within the brain microvasculature.45 It was shown that thrombus formation continues with accumulation of platelets and fibrin deposition despite removal of the vessel-occluding thread.46,–48 These data indicate that ongoing thrombus formation within the brain during reperfusion is an important pathophysiologic step in stroke development that acts in concert with activation of endothelial cells and adhesion of leukocytes to the vessel wall.47,49
Transient middle cerebral artery occlusion model (tMCAO) in the mouse. (A,B) Three coronal sections through the brain in individual animals. (A) A cerebral infarct at 24 hours after 30 minutes of tMCAO and (B,C) after 1 hour of tMCAO as revealed on tissue sections stained for 2,3,4-triphenyltetarzoliumchloride (TTC), a mitochondrial marker. Red areas represent vital brain tissue; white areas indicate cerebral infarctions. With short occlusion times of 30 minutes, infarcts are restricted to the basal ganglia (arrow in A), whereas prolonged occlusion leads to infarction of the entire MCA territory (B). (D) Infarct development can also be assessed in vivo by magnetic resonance imaging (MRI). Infarcts appear white on T2-w or diffusion-weighed MRI (D) and correspond closely to the extent of infarction seen on tissue sections (C). TTC scans were taken from an Epson Perfection 3200 Photo flatbed scanner (Seiko Epson, Nagano, Japan) at 600 dpi and processed using Epson Scan software. MRI was performed on a 17.6-Tesla ultrahigh field MR unit (Biospin; Bruker BioSpin, Ettlingen, Germany) using a custom-made dual channel surface coil designed for the examination of mouse heads (A063HACG; Rapid Biomedical, Würzburg, Germany). The image protocol comprised a coronal diffusion–weighted sequence (slice thickness, 0.5 mm). MR images were transferred to an external workstation (Leonardo; Siemens, Berlin, Germany) for data processing.
Transient middle cerebral artery occlusion model (tMCAO) in the mouse. (A,B) Three coronal sections through the brain in individual animals. (A) A cerebral infarct at 24 hours after 30 minutes of tMCAO and (B,C) after 1 hour of tMCAO as revealed on tissue sections stained for 2,3,4-triphenyltetarzoliumchloride (TTC), a mitochondrial marker. Red areas represent vital brain tissue; white areas indicate cerebral infarctions. With short occlusion times of 30 minutes, infarcts are restricted to the basal ganglia (arrow in A), whereas prolonged occlusion leads to infarction of the entire MCA territory (B). (D) Infarct development can also be assessed in vivo by magnetic resonance imaging (MRI). Infarcts appear white on T2-w or diffusion-weighed MRI (D) and correspond closely to the extent of infarction seen on tissue sections (C). TTC scans were taken from an Epson Perfection 3200 Photo flatbed scanner (Seiko Epson, Nagano, Japan) at 600 dpi and processed using Epson Scan software. MRI was performed on a 17.6-Tesla ultrahigh field MR unit (Biospin; Bruker BioSpin, Ettlingen, Germany) using a custom-made dual channel surface coil designed for the examination of mouse heads (A063HACG; Rapid Biomedical, Würzburg, Germany). The image protocol comprised a coronal diffusion–weighted sequence (slice thickness, 0.5 mm). MR images were transferred to an external workstation (Leonardo; Siemens, Berlin, Germany) for data processing.
In contrast to focal cerebral ischemia, global ischemia refers to an interruption of the entire brain circulation typically seen during cardiac arrest. If transient, global ischemia causes delayed and selective neuronal death in hypoxia-susceptible brain areas without widespread necrosis. The underlying pathologic mechanisms are quite different between global ischemia and MCAO-induced focal ischemia, and therefore global ischemia is not further considered in this review. Cerebral photothrombosis has often been used as an alternative model to induce focal cerebral lesions, but recent studies have shown that the development of brain lesions after photothrombosis does not require intravascular thrombus formation.50,51 Recently, a promising novel mouse model of thromboembolic stroke was reported based on microinjection of murine thrombin,52 but, as with older similar clot models in mice, no data on the effect of antiplatelet treatment or anticoagulation are available yet. Moreover, embolic models are limited by variable infarct sizes, since it is difficult to anticipate which branch of the middle cerebral artery will finally be occluded by the inserted clot. All experimental studies included in this review were based on the transient MCAO (tMCAO) model in mice, which is the most widely used. Here, brain infarctions are initiated by mechanical, but not thromboembolic, occlusion of a major cerebral artery. Although a single model can cover only some aspects of human stroke, which is a complex and heterogeneous disease, the tMCAO model turned out to be useful in elucidating basic pathomechanisms of thrombus formation in the downstream microvasculature.
The role of platelets in experimental stroke
Pathologic platelet activity and platelet receptor-ligand interactions have been linked to cerebral ischemic events.48 By use of 111In-labeled platelets in a primate model of tMCAO, the del Zoppo group showed that platelets are deposited in the ischemic basal ganglia early during reperfusion, and electron microscopic examination demonstrated aggregates of degranulated platelets together with fibrin and leukocytes (Okada et al53 ). Accordingly, baboons treated with ticlopidine and heparin displayed a significant reduction in platelet deposition and microvascular occlusions in the ischemic basal ganglia.48 The advent of genetic methods that allow targeted manipulations in the mouse genome has paved the way for novel concepts of thrombus formation in mice54,55 that may help to identify important steps in the pathogenesis of human atherothrombosis and ischemic stroke. In the following sections, we will summarize the recent experimental evidence in support of a pathophysiologic role of platelet receptors GPIIb/IIIa, GPIb, and GPVI (Figure 2) and the involvement of the intrinsic coagulation cascade in focal cerebral ischemia. Novel drugs targeting these molecules may help to overcome the current limitations and hazards of conventional anticoagulation and platelet inhibition in acute ischemic stroke.56,57
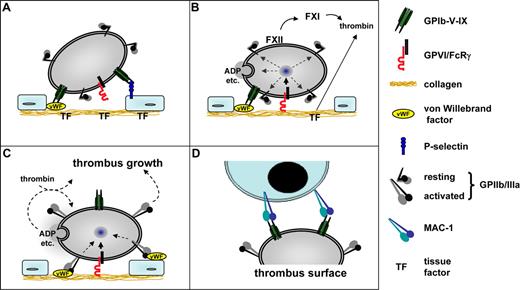
Model of platelet–vessel wall interaction. (A) The initial contact (tethering) of platelets to the extracellular matrix (ECM) is mediated predominantly by GPIbα-VWF interactions. The GPIbα-VWF interaction is essential at high shear rates (> 500 s−1). GPIbα may also interact with P-selectin exposed on activated endothelial cells and thereby contribute to platelet recruitment to the intact vessel wall. (B) At sites of vascular injury, GPVI-collagen interactions initiate cellular activation followed by shifting of integrins to high-affinity state and the release of secondarily acting agonists, most importantly ADP, ATP, and TxA2. GPIb-mediated signaling may amplify GPVI-induced activation pathways. In parallel, exposed tissue factor (TF) locally triggers the formation of thrombin (extrinsic pathway), which in addition to GPVI mediates cellular activation. On the growing thrombus, activation of FXII and FXI also leads to thrombin formation. (C) Activated GPIIb/IIIa (integrin αIIbβ3) together with β1 integrins (not shown) mediates firm adhesion by binding to VWF, fibronectin, and other ligands. Released ADP, ATP, and TxA2 amplify integrin activation on adherent platelets and mediate thrombus growth by activating additional platelets and fibrinogen binding to GPIIb/IIIa. (D) Adherent platelets may recruit leukocytes to the thrombus through GPIbα-MAC1 interactions. This scheme does not exclude the involvement of other receptor-ligand interactions.
Model of platelet–vessel wall interaction. (A) The initial contact (tethering) of platelets to the extracellular matrix (ECM) is mediated predominantly by GPIbα-VWF interactions. The GPIbα-VWF interaction is essential at high shear rates (> 500 s−1). GPIbα may also interact with P-selectin exposed on activated endothelial cells and thereby contribute to platelet recruitment to the intact vessel wall. (B) At sites of vascular injury, GPVI-collagen interactions initiate cellular activation followed by shifting of integrins to high-affinity state and the release of secondarily acting agonists, most importantly ADP, ATP, and TxA2. GPIb-mediated signaling may amplify GPVI-induced activation pathways. In parallel, exposed tissue factor (TF) locally triggers the formation of thrombin (extrinsic pathway), which in addition to GPVI mediates cellular activation. On the growing thrombus, activation of FXII and FXI also leads to thrombin formation. (C) Activated GPIIb/IIIa (integrin αIIbβ3) together with β1 integrins (not shown) mediates firm adhesion by binding to VWF, fibronectin, and other ligands. Released ADP, ATP, and TxA2 amplify integrin activation on adherent platelets and mediate thrombus growth by activating additional platelets and fibrinogen binding to GPIIb/IIIa. (D) Adherent platelets may recruit leukocytes to the thrombus through GPIbα-MAC1 interactions. This scheme does not exclude the involvement of other receptor-ligand interactions.
Glycoprotein IIb/IIIa (integrin αIIbβ3).
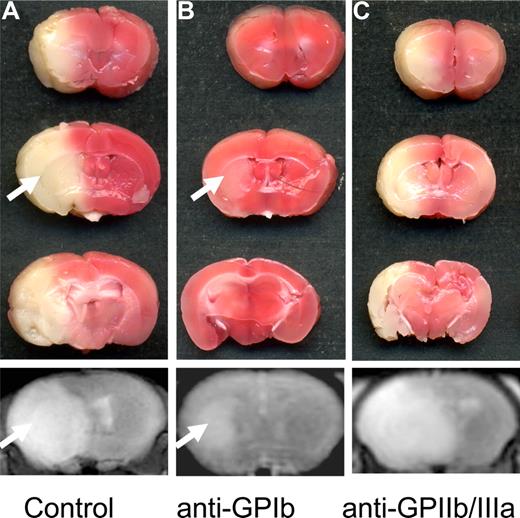
In a seminal paper, Choudhri et al46 used pharmacologic blockade of GPIIb/IIIa in the tMCAO model as proof of concept that even if large arteries are recanalized after thromboembolic stroke, microvascular thrombosis continues to occur at distal sites. When the GPIIb/IIIa antagonist GPI 562 was administered to mice immediately before or after 1 hour of MCAO, platelet and fibrin accumulation as well as cerebral infarct volumes were reduced. This effect was dose-dependent, with an associated significant increase in the rate of intracerebral hemorrhages. Treatment of mice with an anti-GPIIb/IIIa antibody similarly led to reduced endothelial adhesion of leukocytes and platelets during reperfusion after tMCAO.58 In baboons, a 30% inhibition of platelet aggregation by the GPIIb/IIIa antagonist TP9201 was sufficient to regain complete microvascular patency after 3 hours of tMCAO, whereas near complete inhibition of platelet aggregation again led to large intracerebral hemorrhages.59 In a recent study,57 we reassessed efficacy and safety of anti-GPIIb/IIIa treatment in ischemic stroke using Fab2 fragments of the mouse GPIIb/IIIa-blocking mAb, JON/A, which completely inhibits ex vivo platelet aggregation and induces prolonged tail bleeding times.60 Most animals that had received 100 μg anti-GPIIb/IIIa Fab2, leading to a virtually complete receptor blockade, died due to intracerebral hemorrhage, and the few surviving animals exhibited infarct volumes of the same extent as seen in controls (Figure 3). A 78% and 68% receptor blockade improved survival rates, but failed to influence infarct volumes or neurologic outcome. By contrast, in GPIIb-deficient mice, cerebral infarct size was reduced at 24 hours after tMCAO, but no information on bleeding complications is available from this study.61 Excessive GPIIb/IIIa blockade appears to be inevitably associated with major bleeding complications in mice and reflects similar findings in stroke patients.26 Thus, rather than blocking this final common pathway of platelet activation, their aggregation, targeting platelet adhesion, and/or early signaling events may provide a promising therapeutic alternative.
The influence of platelet glycoprotein receptor blockade on stroke outcome. (A) Coronal 2,3,4-triphenyltetarzoliumchloride–stained sections at 24 hours after 1 hour of tMCAO in a sham-treated mouse. Note the large infarction of the entire middle cerebral artery territory, and the corresponding T2-w magnetic resonance image of the infarct in the bottom panel. (B) Blockade with anti-GPIb Fab significantly reduced infarct size. The arrow points to the small infarct within the basal ganglia, whereas the cerebral cortex is protected. The corresponding magnetic resonance image correctly depicts decreased infarct size (arrow in bottom panel). (C) Surprisingly, blockade of GPIIb/IIIa had no influence on the infarct size in surviving animals, and was associated with lethal intracerebral hemorrhage (ICH) in many animals (not shown). Importantly, no areas with signal loss indicating bleeding complications were seen in mice after GPIb-Fab blockade (bottom panel in B; compare with ICH in Figure 2C). TTC scans were taken from an Epson Perfection 3200 Photo flatbed scanner (Seiko Epson) at 600 dpi and processed using Epson Scan software (Seiko Epson). MRI was performed on a 1.5-Tesla MR device (Vision; Siemens) using a custom-made dual channel surface coil designed for the examination of mouse heads (A063HACG; Rapid Biomedical). The image protocol comprised a coronal 3D T2-weighted gradient echo–constructed interference in steady state sequence (slice thickness, 1 mm). MR images were transferred to an external workstation (Leonardo; Siemens) for data processing.
The influence of platelet glycoprotein receptor blockade on stroke outcome. (A) Coronal 2,3,4-triphenyltetarzoliumchloride–stained sections at 24 hours after 1 hour of tMCAO in a sham-treated mouse. Note the large infarction of the entire middle cerebral artery territory, and the corresponding T2-w magnetic resonance image of the infarct in the bottom panel. (B) Blockade with anti-GPIb Fab significantly reduced infarct size. The arrow points to the small infarct within the basal ganglia, whereas the cerebral cortex is protected. The corresponding magnetic resonance image correctly depicts decreased infarct size (arrow in bottom panel). (C) Surprisingly, blockade of GPIIb/IIIa had no influence on the infarct size in surviving animals, and was associated with lethal intracerebral hemorrhage (ICH) in many animals (not shown). Importantly, no areas with signal loss indicating bleeding complications were seen in mice after GPIb-Fab blockade (bottom panel in B; compare with ICH in Figure 2C). TTC scans were taken from an Epson Perfection 3200 Photo flatbed scanner (Seiko Epson) at 600 dpi and processed using Epson Scan software (Seiko Epson). MRI was performed on a 1.5-Tesla MR device (Vision; Siemens) using a custom-made dual channel surface coil designed for the examination of mouse heads (A063HACG; Rapid Biomedical). The image protocol comprised a coronal 3D T2-weighted gradient echo–constructed interference in steady state sequence (slice thickness, 1 mm). MR images were transferred to an external workstation (Leonardo; Siemens) for data processing.
Glycoprotein Ib-V-IX.
The initial tethering of platelets at sites of vascular injury is mediated by GPIb-V-IX, a structurally unique receptor complex exclusively expressed in platelets and megakaryocytes (Figure 2). In humans, lack or dysfunction of this receptor has been associated with the Bernard-Soulier syndrome, a congenital bleeding disorder characterized by mild thrombocytopenia, giant platelets, platelet inability to adhere to subendothelial matrices, and a dramatically prolonged bleeding time.62,63 This phenotype has been reproduced in mice lacking functional GPIb-V-IX.54,64 The binding of GPIbα to the A1 domain of VWF is the principal interaction capable of and necessary for tethering platelets to the vessel wall at high shear flow conditions (> ∼500 s−1), whereas this interaction may not be relevant at lower shear rates.65 Although sufficient to support platelet binding, this adhesive interaction is characterized by a rapid dissociation rate and thus cannot mediate irreversible adhesion by itself. Rather, the interaction keeps platelets in close contact with the matrix, while the cells continuously translocate in the direction of blood flow. The specific requirement for GPIbα for platelet adhesion under conditions of high shear, such as found in diseased arteries, makes this receptor a potentially attractive target for pharmacologic inhibition of pathologic thrombus formation. Inhibition of the VWF-binding site on GPIbα with Fab fragments of the antibody p0p/B in wild-type mice abrogated platelet tethering and adhesion in a model of mechanically induced arterial thrombosis.66 Such mice have prolonged tail bleeding times but do not show signs of spontaneous hemorrhage.57,66 The central role of GPIbα in arterial thrombus formation was later confirmed and extended in a study showing that transgenic mice expressing GPIbα in which the extracellular domain was replaced by that of the human interleukin-4 receptor (GPIb-TG) are completely unable to produce intravascular thrombi.67
A crucial role of GPIb in stroke development has recently been elucidated in experimental focal cerebral ischemia.57 Complete blockade of the VWF-binding site of GPIbα by intravenous injection of 100 μg Fab fragments of p0p/B into mice before tMCAO led to a reduction of stroke volumes of approximately 60%. Importantly, delayed application of anti-GPIb Fab 1 hour after MCAO was likewise effective (Figure 3). Although tail bleeding times were strongly elevated in anti-GPIb Fab–treated mice, no increase in intracerebral hemorrhages was detected. Together, this indicated that GPIbα is critically involved in the pathogenesis of ischemic stroke, but not required to prevent bleeding at sites of ischemia/reperfusion damage in the brain, and supported the previous notion that there is no clear correlation between bleeding time and bleeding risk.68 This surprising result was confirmed shortly afterward by Goerge et al,69 who found that local inflammation in the brain (induced by tMCAO) and other tissues triggers bleeding in the absence of platelets. In the presence of platelets, bleeding was prevented, and this protective effect was unexpectedly also seen in mice lacking functional GPIbα. Thus, it appears that the mechanisms by which platelets contribute to the pathogenesis of ischemic brain injury are different from those required to maintain vascular integrity after ischemia/reperfusion in this organ. These observations not only demonstrate that GPIb is a central player in murine experimental stroke but also raise the intriguing possibility that strong platelet inhibition can be achieved without significantly increasing the risk of (spontaneous) intracerebral bleeding.
Besides its principal ligand, VWF, GPIbα also binds thrombin, high-molecular-weight kininogen, factor XII, Mac-1 (a β2 integrin expressed in neutrophils and monocytes [CD11b/CD18]), and P-selectin.70 The corresponding binding sites are located in the N-terminal region of the receptor, but their precise local arrangement is unknown. It is not entirely clear at present which of the GPIbα interactions are critical for thrombus formation in stroke. However, allelic variants of platelet GPIbα causing enhanced VWF/GPIb interactions are associated with an increased risk of ischemic stroke,71 and increased serum levels of VWF have been recognized as an independent stroke risk factor.72 In support of this, we found in a most recent investigation that VWF-deficient mice are protected against cerebral ischemia, although to a lesser extent than mice treated with anti-GPIbα antibodies (C.K., H. Deckmyn, B.N., and G.S., unpublished observation, June 2008). This indicates that different ligands of GPIb may be involved in the development of infarcts in this model.
Previous studies have shown that mice deficient in Mac-1 are less susceptible to cerebral ischemia/reperfusion injury.73 This protection was associated with reduced neutrophil infiltration after tMCAO, but the exact contribution of Mac-1 to the pathology is unclear. Therefore, it is tempting to speculate that Mac-1–GPIb interactions could mediate platelet-leukocyte adhesion, promoting inflammation at sites of thrombosis after cerebral ischemia. Moreover, GPIbα binds to P-selectin.74 During cerebral ischemia, increased surface P-selectin expression was noted on endothelial cells and platelets as early as 1 hour after reperfusion, and inhibition of P-selectin improved stroke outcome, indicating that this interaction is also functionally relevant.75 Taken together, GPIbα plays a central role as a receptor mediating complex platelet-platelet, platelet-endothelium, and platelet-leukocyte interactions, all of which may be critical in secondary infarct growth after tMCAO in rodents. Fab fragments of the monoclonal antibody 6B4, raised against human GPIbα, exhibited a powerful antithrombotic effect in baboons by blocking the GPIbα-binding site for VWF without significant prolongation of the skin bleeding time.76 This antibody was recently humanized by variable-domain resurfacing guided by computer modeling77 and may provide an important tool to study the role of GPIbα in human thrombotic diseases, including stroke.
Glycoprotein VI.
Although GPIb-VWF interactions can elicit intracellular signals,78 these are generally considered very weak compared with other stimuli, most notably subendothelial collagens, which are exposed to the cells at sites of endothelial damage. Among the numerous collagen receptors expressed in platelets, GPVI is of central importance for cellular activation and subsequent firm arrest.79 GPVI, a 62-kDa type I transmembrane receptor of the Ig superfamily,80 is exclusively expressed in platelets and megakaryocytes.81 It noncovalently associates with the Fc receptor (FcR) γ-chain, and the complex signals through tyrosine phosphorylation cascades leading to calcium mobilization, degranulation, activation of GPIIb/IIIa, and aggregation.79 Platelets in which GPVI has been depleted by in vivo administration of antibodies against the receptor do not respond to collagen.79,81 Several reports have demonstrated a profound antithrombotic effect of such GPVI inhibition after arterial wall injury and collagen-induced thromboembolism,81,–83 which is associated with a very moderate increase in tail bleeding.81 In tMCAO, treatment of mice with the anti-GPVI antibody JAQ1 significantly reduced the brain infarct volumes at day 1 after tMACO57 but did not increase the incidence of intracerebral hemorrhages. This indicates that platelet/collagen interactions via GPVI may also be involved in stroke development in this model. However, GPVI depletion was less effective than GPIb blockade and did not affect clinical outcome variables, suggesting that other platelet agonists contribute to thrombus formation in the tMCAO model. Among these, thrombin is the most powerful initiator of platelet adhesion and thrombus formation sufficient to drive these processes independently of collagen under certain experimental conditions.84
The observation that inhibitors of GPIb or GPVI function provide significant protection from ischemic brain injury suggests that signaling pathways downstream of these receptors could be promising therapeutic targets. This has recently been confirmed by the analysis of mice lacking stromal interaction molecule 1 (STIM1), a key regulator of agonist-induced Ca2+ entry in immune cells and platelets.85,86 Platelets derived from these mice display selective defects in cellular activation downstream of GPVI and shear-resistant adhesion and thrombus formation in vitro and in vivo.87 In contrast, activation in response to G protein–coupled agonists, such as thrombin, is largely preserved, which indicates that STIM1-mediated signaling events are particularly important for the GPIb-GPVI-ITAM pathway in platelets. Mice with STIM1-deficient platelets showed profound reduction in secondary infarct growth after tMCAO, but no increase in intracerebral hemorrhages.87
The intrinsic coagulation pathway as a novel target for stroke prevention
The role of coagulation factors XI (FXI) and XII in thrombus stability and hemostasis.
Hemostasis and pathologic thrombus formation occluding coronary or cerebral arteries in myocardial infarction and stroke, respectively, have long been considered to share identical molecular pathways. According to this concept, treatment of thrombosis would be possible only at the expense of impaired hemostasis. However, this dogma has recently been challenged.55,56,88 Two distinct pathways for initiating plasmatic coagulation exist, triggered either by vessel wall (extrinsic) or blood-borne (intrinsic) factors and converge on a common pathway leading to thrombin and fibrin formation. The extrinsic pathway is initiated by exposure of subendothelial tissue factor upon vessel injury (Figure 2). Tissue factor activates the plasma protease factor VIIa. The intrinsic pathway of coagulation is initiated when coagulation factor XII (FXII, Hageman factor) comes into contact with negatively charged surfaces (contact activation). Because individuals with hereditary FXII deficiency do not show an abnormal bleeding phenotype, FXII has been considered dispensable for proper hemostasis.89 Recent investigations, however, revealed a role of FXII in pathologic thrombus formation and stability.88 FXII-deficient mice, similar to FXII-deficient patients, exhibit a prolonged activated partial thromboplastin time but no bleeding tendency. Importantly, FXII-deficient mice showed impaired formation and stabilization of thrombi in different models of arterial thrombosis.88 These unexpected findings indicate that it is possible to target thrombus formation without ensuing bleeding complications. The molecular mechanisms that activate FXII during pathologic thrombus formation in vivo await elucidation. One possible mechanism has recently been proposed by Kannemeier et al, who demonstrated that extracellular RNA, but not DNA, augments (auto)activation of FXII and FXI and thereby acts as a potent trigger of coagulation in vivo.90 It is at present not clear whether this pathway is the predominant mechanism of FXII activation or whether other, presumably polyanionic, molecules also contribute to this process.
The significance of the intrinsic coagulation system for stroke development.
FXII-deficient mice are also protected from cerebral ischemia.56 After tMCAO, infarct volumes were 50% less in FXII-deficient compared with wild-type mice at 24 hours, and FXII mutants developed significantly less neurologic impairment. Follow-up magnetic resonance imaging (MRI) on days 3 and 7 after tMCAO revealed that this protective effect was sustained over time. Infarcts in FXII-deficient mice were restricted to the basal ganglia, and fibrin deposition in the microvasculature of cortical vessels was markedly reduced. Administration of the FXIIa inhibitor D-Pro-Phe-Arg-chloromethyl ketone (PCK) to wild-type mice similarly conferred protection from stroke. Because FXI-deficient mice were similarly protected from experimental stroke as FXII-deficient mice, the intrinsic coagulation pathway appears to be critically involved in infarct development.56 A recent epidemiologic study disclosed protection against cerebrovascular events in Jewish patients with severe congenital FXI deficiency, indicating that our experimental results in mice may be relevant for the human situation.91 Of note, FXII knockout mice and wild-type mice treated with FXII inhibitors had a prolonged activated partial thromboplastin time, but did not display excessive bleeding during surgery. Moreover, serial MRI using blood-sensitive sequences did not show an increased frequency of intracerebral hemorrhages. Importantly, pharmacologic blockade of factor IX downstream of factors XII and XI was similarly protective after tMCAO.92 However, factor IX deficiency reflects human hemophilia type B, which is associated with a significant bleeding phenotype.93 This can be explained by the fact that factor IX is also activated by the factor VIIa–tissue factor complex and thereby participates in the extrinsic pathway of coagulation.94 Intracerebral hemorrhage is the most feared complication of coumarins, the conventional anticoagulant today. It appears that FXII inhibition could be a novel target for safer anticoagulation and stroke prevention, since FXII is an essential component of pathologic thrombus formation but not of physiologic hemostasis.95
Perspectives for future stroke prevention and treatment
Basic molecular biology has uncovered the functions of several platelet receptors such as GPIb and GPVI and downstream signaling pathways in thrombus formation. The fact that inhibition of these platelet receptors/signaling pathways is not associated with increased intracerebral bleeding after tMCAO may open up new avenues for stroke treatment in the future. Novel therapeutic approaches are eagerly awaited in view of the recent negative experience with GPIIb/IIIa receptor inhibition26 and the limited access of stroke patients to thrombolytic treatment. Therefore, it is very promising that antibodies against human GPIb effectively prevented thrombus formation after peripheral vessel injury in baboons76 and that humanized Fab′ fragments against GPIbα have been generated by molecular engineering.77 Before these can be clinically tested in stroke patients, they await proof of safety and efficacy in a translational primate model. This is important to rule out species-specific effects restricted to rodents, as seen in many other potential therapeutics in the past.8,96
Besides the prevention and treatment of cerebral ischemia resulting from spontaneous thromboembolism, iatrogenic stroke might become another area of application for the novel antithrombotics described. Frequently performed routine procedures such as angiography, angioplasty, or heart surgery are accompanied by a significant incidence of (clinically silent) cerebral thromboemboli, which above a certain threshold can cause severe cognitive dysfunction or even large brain infarction.97 Now, the role of the intrinsic pathway of coagulation in pathologic thrombus formation has been unraveled56,88 and may provide novel options for anticoagulation during these potentially proembolic interventions.95 In balancing risk and benefit of any new stroke prophylaxis or treatment, the reduction of the bleeding risk is as essential as preventing thrombus formation and improving reperfusion. Highly selective FXII inhibitors are currently being developed for the application in humans.98 They may help to control and limit thromboembolic complications with a better safety profile than coumarins or heparins in which the therapeutic benefit has often been neutralized by excess bleeding complications.
Acknowledgments
We thank Profs Wolfgang Müllges, Klaus Toyka, and Ulrich Walter (University of Würzburg) for valuable comments.
Our own work cited in this review was supported by the Deutsche Forschungsgemeinschaft (Bonn, Germany; SFB 688, A1, A3, B1), the Rudolf Virchow Center, and institutional funds of the state of Bavaria, Germany.
Authorship
Contribution: G.S., C.K., and B.N. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Guido Stoll, Department of Neurology, University of Würzburg, Josef-Schneider-Str 11, D-97080 Würzburg, Germany; e-mail: stoll_g@klinik.uni-wuerzburg.de; or Bernhard Nieswandt, Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, Zinklesweg 10; 97080 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.