In humans, vitamin A (retinol and its esters) is either taken up directly from meat and fish, or, as β-carotenes (provitamin A), from plants. Retinol and its active metabolites, retinoic acid (RA) and retinal, have major bio-logic effects on cell growth and differentiation, reproduction, vision system development, and immune regulation. In particular, RA regulates immune defense against infections, inflammation, epithelial barrier functions, and lymphocyte function and trafficking to the gut.1,,–4 The clinical relevance of these findings is strengthened by the observations that vitamin A deficiency severely compromises host defense, and that vitamin A supplementation can correct these abnormalities.5
The ability of retinol to control growth and development depends on tissue-specific metabolism of retinol to RA, which is then able to function as a ligand for retinoid receptor signaling through the nuclear receptors RAR and RXR. Genetic studies in mice revealed that cytosolic alcohol dehydrogenases (ADH1-4) were involved in the first step (oxidation of retinol to retinaldehyde), and cytosolic retinaldehyde dehydrogenases (RALDH1-3) in the second step (oxidation of retinaldehyde to RA). Metabolism of retinol to retinaldehyde is not tissue restricted; further metabolism of retinaldehyde to RA is the limiting step, and is tissue restricted insofar as the most effective enzymes identified are tissue specific.6
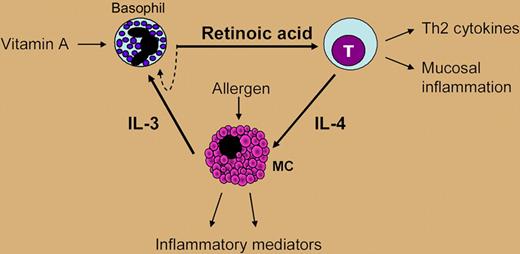
The origin of locally elevated RA levels in post-embryonic mammals is unknown. RALDH1 is abundantly expressed in adult tissues and likely responsible for low RA levels present in tissues throughout the body. RALDH2 and 3 are the more efficient and selective RA-synthesizing enzymes; however, their localization and regulation in adults is largely obscure. Here, Spiegl et al provide new and exciting data by showing that an often-neglected cell type, the basophil granulocyte, expresses high levels of RALDH2 and thus is a major and unique source of immunomodulatory RA among human blood cells. Moreover, preliminary data on the regulation of RALDH2 in basophils are provided. Allergen-dependent basophil activation mimicked by FcϵR1 crosslinking induced low levels of RALDH2, whereas IL-3 not only induced RA receptor activity but also strongly induced the expression of RALDH2 as well as the generation of RA.
However, at particular tissue sites, other cells besides basophils might contribute to RA formation. Recent studies indicate that dendritic cells from mouse mesenteric lymph nodes constitutively express RALDH2 mRNA and produce RA, which regulates the function and guthoming properties of T and B cells and may be involved in intestinal tolerance induction.2,–4 Moreover, intestinal mucosa and Peyer patch dendritic cells express the low-efficiency, RA-synthesizing enzyme RALDH1, suggesting that RA formation at this site may also depend on high local vitamin A concentrations from food. Similar suggestions have been made for vitamin D3, the active form of which is generated locally by dendritic cells and T cells, and induces CCR10 in naive T cells, thereby attracting them to the skin.7 Thus, vitamins might “sense” the local immune system as local concentrations of specific vitamins control the intensity and character of the local immune response. For instance, vitamin D metabolites attract specific populations of lymphocytes to the skin, and vitamin A metabolites direct lymphocytes to the gastrointestinal mucosa. So, locally produced food metabolites program T-cell homing, which offers a new mechanism to explain why hypersensitivity reactions occur at particular body sites.
At present, one can only speculate on the consequences of the findings by Spiegl and colleagues. The authors provide evidence that basophil-derived RA acts not only in an autocrine manner on basophils, but also on neighbor cells such as T cells. Basophil-derived RA polarized naive human T cells to produce Th2 cytokines, namely IL-4, and induced the expression of the adhesion receptors CD38 and α4/β7-integrin, which may guide them to mucosal sites of inflammation. IL-4 induced by basophil-derived RA in T cells promotes mast-cell activation, which in turn enhances their production of IL-3, itself shown to be a major trigger for RA formation in basophils.8 Therefore, RA generation might be an important missing link in the communication between basophils, mast cells, and T cells, between innate and adaptive immunity that results in Th2 immune responses (see figure).
The “vitamin hypothesis.” Local production of RA by basophils at sites of allergic inflammation could enhance Th2 gene-expression programs as well as the phenotype and functions of T cells, B cells, and mast cells, supporting the concept that RA acts as a mediator of mucosal allergy.
The “vitamin hypothesis.” Local production of RA by basophils at sites of allergic inflammation could enhance Th2 gene-expression programs as well as the phenotype and functions of T cells, B cells, and mast cells, supporting the concept that RA acts as a mediator of mucosal allergy.
On the other hand, several important questions need to be addressed in future studies. For example, which other endogenous or exogenous factors, apart from IL-3, might regulate RALDH2 expression in basophils? Is RALDH2 expression enhanced in allergic patients? What are the local factors leading to RALDH2 expression in dendritic cells of mesenteric lymph nodes? Is mucosal RA synthesis dependent on vitamin A status, which needs to be measured by accurate means? Nevertheless, the findings from Spiegl and fellow members of Dahinden's group suggest that basophils are exciting “agent cells” between the food environment and the immune system leading to a down-regulation of Th2 responses in case of vitamin A deficiency, and enhancing the risk of allergy in response to vitamin A excess. Additional epidemiologic and basic studies are needed to further support this attractive vitamin hypothesis, alongside the widely accepted “hygiene hypothesis,” as a new explanation for the rapid increase in allergy and autoimmune diseases in industrial countries.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal